Abstract
Background
To explore the feasibility, the efficacy, and the mechanism of mandibular advancement devices (MAD) in the treatment of persistent sleep apnea after surgery.
Methods
Nineteen patients who failed uvulopalatopharyngoplasty (UPPP) or UPPP plus genioglossus advancement and hyoid myotomy (GAHM) were given a non-adjustable MAD for treatment. All patients had polysomnography (PSG) at least 6 months post-UPPP with and without the MAD. Seventeen patients had computed tomography (CT) examinations.
Results
After the application of MAD, the apnea hypopnea index (AHI) decreased significantly from 41.2 ± 13.1/h to 10.1 ± 5.6/h in the responder group. The response rate was 57.9% (11/19). During sleep apnea/hypopnea acquired from sedated sleep, the cross-sectional area and anterior-posterior and lateral diameters of the velopharynx enlarged significantly from 4.2 ± 6.0 mm2 to 17.5 ± 15.3 mm2, 1.9 ± 2.3 mm to 6.5 ± 4.1 mm, and 1.1 ± 1.3 mm to 2.6 ± 2.1 mm, respectively (P < 0.01) in the responder group with MAD. The velopharyngeal collapsibility also decreased significantly from 83.3 ± 21.8% to 46.5 ± 27.1%. The glossopharyngeal collapsibility decreased from 39.8 ± 39.1% to −22.9 ± 73.2% (P < 0.05).
Conclusion
MAD can be an effective alternative treatment for patients with moderate and severe OSAHS after surgery. The principal mechanisms underlying the effect of MAD are expansion of the lateral diameter of the velopharynx, the enlargement of the velopharyngeal area, the reduction of velopharyngeal and glossopharyngeal collapsibility, and the stabilization of the upper airway.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Obstructive sleep apnea hypopnea syndrome (OSAHS) is a common condition that is associated with serious adverse health consequences. Its prevalence is about 2 to 4% in adults [1]. Continuous positive airway pressure (CPAP) is the preferred method for treating OSAHS; however, 40% patients with OSAHS cannot tolerate or are unwilling to accept CPAP treatment [2]. They instead choose to undergo other treatments, such as surgery. Uvulopalatopharyngoplasty (UPPP) is the mainstay surgical approach in the treatment of patients with palatopharyngeal obstruction. However, the response rate to this procedure was only 50% in a short-term follow-up [3]. UPPP plus genioglossus advancement and hyoid myotomy (GAHM) has been applied in patients with OSAHS with palatopharyngeal and tongue base obstructions, but the response rate was about 67% [4]. The management of non-responders is difficult. Usually, CPAP and more invasive operations such as mandibular and maxillary advancement (MMA) are recommended, but are rarely accepted. Mandibular advancement devices (MAD) are non-invasive, cheap, and usually used in patients with primary snoring or mild OSAHS. The response rate is 77% [5]. Millman studied 24 patients who failed UPPP with an adjustable MAD [6]. Eighteen patients underwent polysomnography evaluations. Twelve patients had been responders with MAD in place. MAD treatment might be considered for use in non-responders after surgery, but its feasibility and efficacy must be further demonstrated. The mechanism of MAD treatment of sleep apnea is unclear. In this study, 19 non-responders after upper airway surgery were given non-adjustable MAD. The feasibility, the efficacy, and the mechanism of MAD in the treatment of persistent sleep apnea after surgery were evaluated.
Methods
Subjects
The inclusive criteria are: 1. Subjects accepted PSG examination at least 6 months after operation, and post-operation moderate or severe OSAHS were diagnosed. 2. Patients wanted to accept therapy but refused to accept CPAP or reoperation. 3. Patients without occlusal lower respiratory disorders, periodontal disease or the temporomandibular joint (TMJ) diseases. The exclusive criteria are: 1. Patients with central sleep apnea. 2. Patients with severe respiratory disorders. 3. Patients with nasal obstructive diseases. 4. The maximal jaw forward distance is less than 6 mm. 5. Patients with the alveolar bone resorption. 6. The crown root ratio is over 2:1. 111 post-operation OSAHS patients were followed up, only 19 patients (17 men, 2 women) with four surgical procedures met the inclusive criteria, which were Han-uvulopalatopharyngoplasty (HUPPP) (9), HUPPP + GAHM (1), Z-palatopharyngoplasty (ZPPP) [5] (2), and ZPPP + GAHM (7). Subjects with persistent OSAHS had an average age of 42.4 years (23–58 years), average body mass index (BMI) of 28.2 kg/m2 (21.2–33 kg/m2), and average apnea hypopnea index (AHI) of 44.2/h (20.5–83.7/h).
All patients were given non-adjustable MAD called Snore Guards after polysomnographic recordings and investigation of Epworth sleepiness scale (ESS) with an average of 28.6 months after the surgical procedures. Six months after the application of MAD, all patients again underwent all investigations described above. The efficacy and the side effects of MAD were also judged subjectively by a questionnaire before the last sleep study, including the sleep quality of the previous night, patient satisfaction with the device, and side effects.
To determine the mechanism of MAD and the morphological changes in the upper airway that result from its application, computed tomography (CT) of the upper airway was conducted during a daytime nap induced by intravenous midazolam [7, 8] with and without MAD in place.
Polysomnography
The sleep studies were carried out in the Sleep Disorders Center of the Affiliated Sixth People’s Hospital of Shanghai Jiao Tong University. Apnea was defined as the total cessation of airflow that was >10 s. Hypopnea was defined as an event that met two of the following three criteria: (1) a decrease in airflow of >50%; (2) an EEG arousal as defined by the American Sleep Disorders Association scoring criteria; or (3) oxygen desaturation of >3%. OSAHS was defined as an AHI of more than five episodes per hour during sleep in patients with symptoms of the disorder [9]. Therapeutically effective is defined as a decrease in AHI of >50% of that before treatment with MAD in place. Treatment success was defined as AHI <5/h with MAD in place [10].
Upper airway CT
Upper airway CT (Lightspeed VCT, GE, Finland) was performed in seventeen patients (two patients didn’t accept CT examination because of personal reasons) before and after MAD application under three conditions: quiet breathing, the end of deep inspiration, and apnea. The time of CT acquisition is about one breathing cycle. All CT images were reconstructed from the middle sagittal planes. The planes of the upper airway at the level of the nasopharynx (the extension cord over the lower edge of the hard palate), the velopharynx (the tip of the uvula), the glossopharynx (the narrowest part of the glossopharynx), and the epiglotopharynx (5-mm below the tip of the epiglottis) were obtained during the phase of quiet tidal breathing, end of deep inspiration, and sleep apnea/hypopnea. The cross-sectional area, anterior-posterior diameter, and lateral diameter of each plane of the upper airway were manually measured by using electronic calipers by the same technician. The degree of collapse was calculated as follows: (quiet breathing cross-sectional area at the end of deep breathing or sleep apnea) / cross-sectional area during quiet breathing [9]. CT scans were evaluated by the same investigator who was blinded to both responders and non-responders. We informed all patients about the purposes of the CT examination, as well as the short-term potential hazards or long-term complications caused by frequent radiation examinations, after which all patients signed an informed consent. With 55 months of follow-up, no side effects or adverse reactions were seen to be from radiation exposure.
MAD
Before the intervention of MAD, a clinical examination of the stomatognathic system, which included measurement of mandibular mobility, palpation of the temporomandibular joints and masticatory muscles, and recording of pain when the mandibular moved forward, was performed. In the present study, the same dentist took care of all patients, and the same dental technician was responsible for the preparation of all appliances.
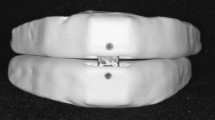
We used a MAD called ‘Snore Guard’ (Xintai Ltd., Beijing) for the advancement of the mandible (Figs. 1 and 2). The degree of mandibular advancement induced by MAD was adjusted according to the anatomy and tolerance of the individual patient. Patients tried only one position during therapy. In former studies [11–18], physicians measured the maximum distance of mandibular advancement, then choose the position which is about 75% of the maximum distance and did the MAD moulding at the position following the product instruction. The goal was to minimize nocturnal apnea and snoring while maintaining a comfortable fit. In our study, the mandibular protrusive maneuver of each subject was measured. The averaged distances were 5 to 7 mm, which varied from 51 to 100% of patients’ maximum protrusive maneuver. After the last fitting, patients were asked to wear the device every night during sleep. The patients were encouraged to wear the device regularly by telephone as well as when they returned for visits at our office [11–14].
Statistical analysis
Statistical analysis was performed using SPSS version 18.0. The paired-samples t-test was used to compare variables of PSG results and CT parameters pre- and post-treatment with MAD. One-way analysis of variance (ANOVA) was used for comparisons of PSG results and CT parameters between the responder and non-responder groups. All parametric results were expressed as means ± standard deviation (SD). A statistically significant difference was defined as P < 0.05 for all parameters.
Results
Polysomnographic results
All-night polysomnographic data of 19 patients were available. Patients were grouped into responders and non-responders according to criteria described in the Method portion. The AHI in both groups was 44.2 ± 19.2/h, and the LSaO2 was 77.2% ± 7.6% post-surgically; i.e., before the application of MAD. The AHI decreased significantly to 28.2 ± 24.6/h and the LSaO2 increased to 79.3 ± 10.4% with MAD (Table 1). While in the responder group, the AHI decreased significantly from 41.2 ± 13.1/h to 10.1 ± 5.6/h, and the LSaO2 increased significantly from 78.1 ± 8.9% to 85.4 ± 6.5% with MAD (Tables 2 and 3).
Eleven patients were classified as responders,two of whom were complete response with MAD as shown by an AHI of less than five, and eight as non-responders; the response rate was 57.9% (11/19). The ESS decreased from 8.6 to 6.1 after the application of MAD. None of the patients had a substantial change in body measurements during the study (Tables 2 and 3).
Upper airway CT results
In this study, cephalometric examinations were taken both without and with MAD in place, but the result showed no significant difference. As a three-dimensional imaging examination, CT showed more accurate changes in the upper airway (Fig. 3). Upper airway CT was performed in 17 patients before and after the MAD application (Table 4). In non-responders, the change was not significance before and after the MAD application, which is not explained in text.
During the phase of quiet tidal breathing, the velopharyngeal cross-sectional area and the lateral diameters of the velopharynx and nasopharynx were much larger with MAD in place than without MAD in the responder group (46.4 ± 26.4 mm2 vs. 26.8 ± 19.0 mm2, 12.4 ± 4.7 mm vs. 9.7 ± 6.3 mm, and 16.5 ± 5.1 mm vs.11.8 ± 6.6 mm, respectively; P < 0.05).
During the state of deep inspiration, the changes in the upper airway did not reach statistical significance in both the responder and non-responder groups.
During sleep apnea/hypopnea, the cross-sectional area, anterior-posterior diameter, and lateral diameter of the velopharynx were significantly enlarged at 5.0 ± 6.4 mm2 to 21.2 ± 10.5 mm2, 1.3 ± 1.2 mm to 2.9 ± 0.9 mm, and 2.2 ± 2.3 mm to 8.2 ± 3.8 mm, respectively (P < 0.01) in the responder group with MAD in place (Table 4). The velopharyngeal collapsibility of the responder group decreased significantly from 83.3 ± 21.8% to 46.5 ± 27.1% with MAD in place (P < 0.01), and glossopharyngeal collapsibility decreased from 39.8 ± 39.1% to −22.9 ± 73.2% (P < 0.05) (Table 5).
Side effects
After wearing MAD all night for 1 week, various side effects of different degrees occurred in all patients, including weak occlusion (1/19), excessive saliva (6/19), dry mouth (2/19), temporomandibular joint aches (1/19), dental soreness (9/19), toothache (1/19), loose lower incisors (2/19), mucosal ulceration (2/19), and masseter muscle pain (3/19). However, all patients were able to continue the treatment, and all symptoms were relieved in 1 month with timely and appropriate care.
Compliance
With reference to the definition of CPAP compliance [15], we considered wearing the MAD for >70% of nights and wearing it for >4 h per night as good MAD treatment compliance. In this study, 63.2% (12/19) of patients had good compliance; in fact, these patients wore the MAD all night. Among the patients with poor compliance, three wore the MAD <3 days per week because of loose lower incisors in two patients after UPPP (HUPPP or ZPPP) combined with GAHM, and gum pain caused by MAD deformation in one patient. One patient wore the MAD for <4 h per night because of dry mouth. The survey results had no difference between pre- and post-MAD treatment for both responders and non-responders. The pre- and post-MAD treatment ESS scores of responders were 7.4 ± 6.1 and 5.4 ± 5.8 (P = 0.215), and the quality of life scores were 5.5 ± 1.5 and 5.6 ± 1.7 (P = 0.772). There was no difference before and after treatment, which maybe related with the low compliance of MAD at home.
Discussion
MADs are the most commonly used oral appliances and recommended in the treatment of snoring and mild to moderate OSAHS patients, but they were rarely used in patients with severe OSAHS, especially in those with persistent OSAHS condition after surgical treatment. There are multiple MAD designs. Physicians used those devices intending to protrude the mandible, increase tension of soft tissues as the soft palate, lateral pharyngeal walls and the tongue, and improve the airway patency [16]. Many studies were done in past decades, but the mechanism underlying the efficacy of MAD for the treatment of OSAHS was still controversial in terms of whether it helped enlarge the volume of the pharynx cavity or increased the airway stability. Zhao et al. used MAD to treat 11 patients with moderate to severe OSAHS without surgery [12]. In their study, magnetic resonance imaging (MRI) scans were performed at each time when the lower jaw were moved forward by 0, 2, 4, 6, or 8 mm while patients were wakeful or during sleep apnea/hypopnea. They found that during sleep apnea/hypopnea, the velopharyngeal cross-sectional area with MAD in place was larger than that without MAD, especially the coronal axis. No statistically significant changes in sagittal diameter were found between the various mandibular advancements. In our study, the velopharyngeal cross-sectional area, the coronal axis, and the sagittal diameter with MAD in place were all larger than those without MAD, and the difference was statistically significant. Upper airway CT imaging showed that the velopharyngeal cross-sectional area and lateral diameter of the velopharynx were significantly enlarged in the MAD responders, but not non-responders, during quiet tidal breathing and sleep apnea/hypopnea. The result was consistent with previous reports [13, 17, 18].
According to Schwartz et al’s study [19], a negative pressure of about −13 cm H2O under normal circumstances could cause airway collapse. Huang et al. [20] also measured airway pressures in patients with OSAHS after various treatments. The pressure was −21 cm H2O with MAD in place, −18 cm H2O after UPPP, and −17 cm H2O after the soft palate implant surgery. These results showed that the airway would not collapse as easily if the patients were wearing a MAD. In our study, The velopharyngeal and the glossopharyngeal collapsibility of the responder group was significantly decreased from 83.3 ± 21.8% to 46.5 ± 27.1% and from 39.8 ± 39.1% to −22.9 ± 73.2%, respectively (P < 0.05). These results are consistent with those of previous studies too [13, 17, 20–22].
The results of our study showed that MAD could expand the velopharyngeal coronal axis, enlarge the velopharyngeal cross-sectional area by mandibular advancement, and reduce velopharyngeal and glossopharyngeal collapsibility, resulting in stabilization of the upper airway. During the phase of quiet tidal breathing, the cross-sectional area of nasopharyngeal in the responder group without MAD in place were less than the non-responder group. In further study, we would do some research to validate whether it could be used as a predictor of respond on MAD.
Thus, MAD treatment might be considered for patients with persistent VP or GP, especially VP obstruction after surgery if they are unable to tolerate CPAP and refuse more invasive surgery. Thus, MAD might be an effective and feasible treatment for non-responding patients after upper airway surgery.
The small number of patients and lack of a control group or randomization probably decrease the power of the study. The multiple different pharyngeal and hypopharygneal procedures that were performed decrease the internal and external validity of the study. In our study, we followed 111 post-operational patients in total, and only 19 patients matched the inclusion criteria. Because of the limited number of patients and four types different surgical procedures, we were not able to separate all subjects into groups. Therefore, the correlation between specific surgeries and changes on CT imaging could haven’t been investigated. We have compared the physical examination results and AHIs of both responders and non-responders, and the results showed no significant different. We will conduct a blinded, controlled, larger prospective study, try to include more subjects and use adjustable MAD in the treatment. Hopefully better efficacy could be seen.
Conclusions
Post-operational MAD treatment can be an effective alternative for patients with moderate and severe OSAHS after surgery. The principal mechanisms underlying the effect of MAD treatment are the expansion of the lateral diameter of the velopharynx, the enlargement of the velopharyngeal area, the reduction of the velopharyngeal and glossopharyngeal collapsibility, and the stabilization of the upper airway.
Abbreviations
- AHI:
-
The apnea hypopnea index
- ANOVA:
-
One-way analysis of variance
- A-P:
-
Anterior-posterior diameter
- BMI:
-
Average body mass index
- CPAP:
-
Continuous positive airway pressure
- CSA:
-
Cross-sectional area
- CT:
-
Computed tomography
- EP:
-
Epiglopharynx
- ESS:
-
Epworth sleepiness scale
- GAHM:
-
Genioglossus advancement and hyoid myotomy
- GP:
-
Glossopharynx
- MAD:
-
Mandibular advancement devices
- MMA:
-
Mandibular and maxillary advancement
- NP:
-
Nasopharynx
- OSAHS:
-
Obstructive sleep apnea hypopnea syndrome
- PSG:
-
Polysomnography
- SD:
-
Standard deviation
- TMJ:
-
The temporomandibular joint
- UPPP:
-
Uvulopalatopharyngoplasty
- VP:
-
Velopharynx
- ZPPP:
-
Z-palatopharyngoplasty
References
Seemann RJ, DiToppa C, Holm MA, et al. Combination surgical and mechanical therapy for refractory cases of obstructive sleep apnea. J Otolaryngol. 2002;31:85–8.
Abdelghani A, Slama S, Hayouni A, et al. Acceptance and long-term compliance to continuous positive airway pressure in obstructive sleep apnea. A prospective study on 72 patients treated between 2004 and 2007. Rev Pneumol Clin. 2009;65:147–52.
Lowe AA. Oral appliances for sleep breathing disorders. In: Kryger MH, Roth T, Dement WE, editors. Principles and practice of sleep medicine. 3rd ed. Philadelphia: WB Saunders; 2000. p. 929–39.
Yin SK, Yi HL, Lu WY, et al. Genioglossus and advancement hyoid suspension plus uvulopalatopharygoplasty for severe OSAHS. Otolaryngol Head Neck Surg. 2007;136:626–31.
Yi HL, Yin SK, Zhang YJ, et al. Z-palatopharyngoplasty for obstructive sleep apnea/hypopnea syndrome. Otolaryngol Head Neck Surg. 2009;140:640–45.
Millman RP, Rosenberg CL, Carlisle CC, et al. The efficacy of oral appliances in the treatment of persistent sleep apnea after uvulopalatopharyngoplasty. Chest. 1998;113:992–6.
Ferguson KA, Cartwright R, Rogers R, et al. Oral appliances for snoring and obstructive sleep apnea: a review. Sleep. 2006;29:244–62.
Gentil B, Tehindrazanarivelo A, Lienhart A, et al. Respiratory effects of midazolam in patients with obstructive sleep apnea syndromes. Ann Fr Anesth Reanimb. 1994;13:275–9.
Tang XL, Yi HL, Luo HP, et al. The application of CT to localize the upper airway obstruction plane in patients with OSAHS. Otolaryngol Head Neck Surg. 2012;147:1148–53.
The Atlas Task Force, American Sleep Disoders Association. ASDA report: EEG arousals: scoring rules and examples. Sleep. 1992;15:173-84
Hoffstein V. Review of oral appliances for treatment of sleep-disordered breathing. Sleep Breath. 2007;11:1–22.
Okawara Y, Tsuiki S, Hiyama S, et al. Oral appliance titration and nasal resistance in nonapneic subjects. Am J Orthod Dentofacial Orthop. 2004;126:620–2.
Zhao XG, Liu YH, Gao Y. Three-dimensional upper-airway changes associated with various amounts of mandibular advancement in awake apnea patients. Am J Orthod Dentofacial Orthop. 2008;133:661–8.
Martínez-Gomis J, Willaert E, Nogues L, et al. Five years of sleep apnea treatment with a mandibular advancement device. Side effects and technical complications. Angle Orthod. 2010;80:30–6.
Chan AS, Cistulli PA. Oral appliance treatment of obstructive sleep apnea: an update. Curr Opin Pulm Med. 2009;15:591–6.
Cistulli OA, Gotsopoulos H, Marklund M, Lowe AA. Treatment of snoring and obstructive sleep apnea with mandibular repositioning appliances. Sleep Med Rev. 2004;8:443–57.
Gao XM, Zeng XL, Fu MK, et al. Magnetic resonance imaging of the upper airway in obstructive sleep apnea before and after oral appliance therapy. Chin J Dent Res. 1999;2:27–35.
Gale DJ, Sawyer RH, Woodcock A, et al. Do oral appliances enlarge the airway in patients with obstructive sleep apnoea? A prospective computerized tomographic study. Eur J Orthod. 2000;22:159–68.
Morris LG, Setlur J, Burschtin OE, et al. Acoustic rhinometry predicts tolerance of nasal continous positive airway pressure: a pilot study. Am J Rhinol. 2006;20:133–7.
Schwartz AR, Schubert N, Rothman W, et al. Effect of uvulopalatopharyngoplasty on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis. 1992;145:527–32.
Ng AT, Gotsopoulos H, Qian J, et al. Effect of oral appliance therapy on upper airway collapsibility in obstructive sleep apnea. Am J Respir Crit Care Med. 2003;168:238–41.
Battagel JM, Johal A, Kotecha BT, et al. Sleep nasendoscopy as a predictor of treatment success in snorers using mandibular advancement splints. J Laryngol Otol. 2005;119:106–12.
Acknowledgments
Thanks to Dr Bin Chen, Dr. Jian Guan and postgraduates of the OSAHS team in department of Otolaryngology for all their efforts on following up OSAHS patients, doing surgical procedures and collecting physical examination data. Thanks to technicians of Radiology for accompanying and assisting Dr. Luo in CT examinations till midnight. Thanks to Dr. Ting Chen and technicians of Dental Department for the X-ray cephalometrics. Thanks to the nursing team with Mrs. Fang Fang as the head nurse for instructions of PSG examinations to patients with great patience and manipulation for data acquisition.
Funding
The study is supported by Program for Fitting and Proper Technology of Shanghai Shenkang Center (SHDC12010209), including the inspection charges of PSG, CT, MAD and labor costs.
Availability of data and materials
The data will not be shared because we may use the data in further research.
Authors’ contributions
Dr. HL designed the study, carried out all the experiments, performed the statistical analysis and drafted the manuscript. Dr. XT and Dr. YX participated in acquiring CT data. Dr. LM carried out the analysis of all the PSG reports. Dr. HY participated in the study design as program coordinator, and also modified the manuscript. Dr. SY provided the idea of this study. All authors read and approved the final manuscript.
Competing interest
Our research is supported by Program for Fitting and Proper Technology of Shanghai Shenkang Center (SHDC12015101). The authors declare that they have no competing interests.
Consent for publication
Written informed consent was obtained from the patient ('s legal guardian or next of kin) for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Ethics approval and consent to participate
The research was approved by the Institutional Review Board of the research unit.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Luo, H., Tang, X., Xiong, Y. et al. Efficacy and mechanism of mandibular advancement devices for persistent sleep apnea after surgery: a prospective study. J of Otolaryngol - Head & Neck Surg 45, 56 (2016). https://doi.org/10.1186/s40463-016-0167-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40463-016-0167-x