Abstract
Background
Animal personality has emerged as a key concept in behavioral ecology. While many studies have demonstrated the influence of personality traits on behavioral patterns, its quantification, especially in wild animal populations, remains a challenge. Only a few studies have established a link between personality and recurring movements within home ranges, although these small-scale movements are of key importance for identifying ecological interactions and forming individual niches. In this regard, differences in space use among individuals might reflect different exploration styles between behavioral types along the shy-bold continuum.
Methods
We assessed among-individual differences in behavior in the European hare (Lepus europaeus), a characteristic mammalian herbivore in agricultural landscapes using a standardized box emergence test for captive and wild hares. We determined an individuals’ degree of boldness by measuring the latencies of behavioral responses in repeated emergence tests in captivity. During capture events of wild hares, we conducted a single emergence test and recorded behavioral responses proven to be stable over time in captive hares. Applying repeated novel environment tests in a near-natural enclosure, we further quantified aspects of exploration and activity in captive hares. Finally, we investigated whether and how this among-individual behavioral variation is related to general activity and space use in a wild hare population. Wild and captive hares were treated similarly and GPS-collared with internal accelerometers prior to release to the wild or the outdoor enclosure, respectively. General activity was quantified as overall dynamic body acceleration (ODBA) obtained from accelerometers. Finally, we tested whether boldness explained variation in (i) ODBA in both settings and (ii) variation in home ranges and core areas across different time scales of GPS-collared hares in a wild population.
Results
We found three behavioral responses to be consistent over time in captive hares. ODBA was positively related to boldness (i.e., short latencies to make first contact with the new environment) in both captive and wild hares. Space use in wild hares also varied with boldness, with shy individuals having smaller core areas and larger home ranges than bold conspecifics (yet in some of the parameter space, this association was just marginally significant).
Conclusions
Against our prediction, shy individuals occupied relatively large home ranges but with small core areas. We suggest that this space use pattern is due to them avoiding risky, and energy-demanding competition for valuable resources. Carefully validated, activity measurements (ODBA) from accelerometers provide a valuable tool to quantify aspects of animal personality along the shy-bold continuum remotely. Without directly observing—and possibly disturbing—focal individuals, this approach allows measuring variability in animal personality, especially in species that are difficult to assess with experiments. Considering that accelerometers are often already built into GPS units, we recommend activating them at least during the initial days of tracking to estimate individual variation in general activity and, if possible, match them with a simple novelty experiment. Furthermore, information on individual behavioral types will help to facilitate mechanistic understanding of processes that drive spatial and ecological dynamics in heterogeneous landscapes.
Similar content being viewed by others
Introduction
In the paradigm of movement ecology, an animals' space use is explained as a consequence of the influence of external factors on three movement processes: internal state, navigation, and motion capacity [1]. An increasing number of studies show high among-individual differences in movement characteristics that cannot be explained by the paradigm alone [2, 3]. Spiegel et al. [2] refined this paradigm by including personality-dependent spatial ecology and suggested that among-individual differences in behavioral types are a predictor for space use. Consistent among-individual variation of behavior over time is referred to as animal personality [4], a key concept in behavioral ecology [3, 5,6,7].
Although animal personality is widely recognized as a critical intrinsic component of behavior, its quantification under field conditions remains a challenge for two main reasons. First, extensive species-specific standardized tests need to be developed and performed repeatedly to measure consistency in among-individual differences in behavior [8]. Under such test conditions, restraining individuals could interfere with the procedures and objectives of the intended field study, and direct observation of individuals could be impossible. Second, observed among-individual differences in behavior need to be ecologically validated [4]. In most field studies, this would result in a prolonged handling time of individuals, which contrasts with minimizing effects on trapped animals or maximizing sample size [9].
Réale et al. [4] summarized the fundamental personality traits as activity, boldness, exploration behavior, aggressiveness, and sociability. While most of them are difficult to quantify in the wild, the personality trait activity—defined as an individual's general level of activity—can be recorded using accelerometers. Hence, such derived measurements may allow for a remote classification of behavioral types. The main aim of this study was to assess whether and how aspects of animal personality (quantified in standardized and repeated emergence and novel environment tests) and general activity (quantified via accelerometers) are related and whether they are correlated with space use. Moreover, our novel approach shows a feasible way to combine experiments in a controlled environment with ones in the wild. Thus, we contribute to answering a fundamental question in personality research and movement ecology, namely how behavioral traits covary with movement strategies in the wild [10].
Previous research highlights that among-individual differences in behavior, i.e., animal personality, are a key aspect of variation in "internal states" underlying movement and space use [11] with individuals varying consistently in how, where, and when they move [2, 12]. Variation, particularly along the shy-bold continuum [13, 14], is suggested to affect crucial ecological processes, e.g., predation rates [15] or population structure [12, 16], and to generate spatio-temporal variability that influences individuals' interactions with biotic and abiotic factors [5, 17,18,19,20]. For example, boldness and exploration have been shown to correlate with variation in foraging patterns [21, 22] or habitat use [2, 23]. However, only a few studies focused on local movement types [but see: 32–34], even though small-scale movements are of crucial importance for ecological interactions [24,25,26,27,28], the formation of individual niches [29, 30], and hence the community dynamics and species coexistence [19, 31,32,33,34,35]. In addition to movement data derived from GPS devices, tri-axial accelerometers measure static and dynamic acceleration (i.e., the animals' movement) in three dimensions [36]. These measurements can be used to remotely identify acceleration patterns and assign them to defined behaviors [37, 38] or calculate proxy values for activity levels [39,40,41,42]. One well-established example is the overall dynamic body acceleration (ODBA), allowing us to estimate a free-ranging animals' activity-related energy expenditure after careful validation [39, 43]. Accelerometers are increasingly implemented in studies of animal behavior, ecology, or physiology [44,45,46,47,48,49,50,51,52,53,54] and may be a promising tool to assess animal personalities remotely [22].
In the present study, we experimentally identify and link behavioral types of a mammalian herbivore (Lepus europeaus) to their general activity recorded by accelerometers. After relating the individuals’ degree of boldness to its’ activity in both captive and wild hares, we ultimately test for their association with space use in a wild population. Recent studies on space use of the European hare found high among-individual variation in movement patterns that remained hitherto largely unexplained [55, 56]. Here, we present a 3-step approach in which we (i) quantify and test the repeatability of among-individual differences in behavior along the bold-shy continuum of captive hares with repeated standardized emergence and novel environment tests in an open-field arena, (ii) link the degree of boldness to a captive individual's general activity in the arena derived from accelerometers and assessed via ODBA, and (iii) investigate the association of wild hares activity and space use with their degree of boldness, estimated from behavioral responses along the shy-bold continuum, proven to be temporarily stable in captive hares. In this final step, we explore the relation of repeatable metrics of behavior linked to animal personality, with space use in a disturbance-mediated agricultural landscape described by Ullmann et al. [55, 56]. More specifically, we relate home range size and the area size to an individual’s position along the shy-bold continuum.
We hypothesize that similar to findings in small [33, 57, 58] and medium sized mammals [59] general activity (i.e., ODBA) and boldness are positively linked in hares. Further, we expect that boldness predicts space use with bolder individuals allocating both a larger home range and a larger core area.
Methods
Step 1-animal personality tests in captive hares
Captive European hares were studied at the field station of the Leibniz Institute for Zoo and Wildlife Research (IZW), located about 40 km north of Berlin (Brandenburg, Germany, 52°51′06.5′′N, 13°54′57.2′′E; WGS84). There, hares are kept and bred for reproduction studies [e.g., 79]. All individuals used in this study are housed singly in small cages of 2 m2 or arenas with concrete floors (5 m x 10 m, where two individuals share the enclosure).
In July and August 2019 and 2020, we conducted repeated novel environment tests with 14 captive hares in an enclosure, that is an open field arena (8 m × 27 m) surrounded by a transparent metal mesh fence with a height of 180 cm. The area was freshly mowed and equipped with a 2 m2 housing box as a familiar retreat site, a small shelter on the opposite side, and two troughs containing food and water ad libitum.
The individual was weighed and transferred to the new enclosure in a wooden box (60 cm x 25 cm x 30 cm). This box was placed in the front right corner inside the enclosure, carefully opened, and remained untouched. Each hare was individually tested while remaining in the enclosure for three consecutive days. In total, we tested 14 individuals in the novel environment test; 12 of them provided GPS/ACC recordings. All experiments were continuously videotaped with a GPS-synced digital camera [60] during periods of sufficient daylight (i.e., 06:00–22:00). Repetition trials were conducted after two weeks. Latencies of specific behaviors (Latency look, Latency leave, and Delta look-leave; definitions in Table 1) were determined from video recordings by one observer (AL).
Step 2-ODBA in captive hares
Prior to testing, each individual was collared with a GPS device with an internal 3-axial accelerometer (ACC) weighing 69 g (< 2% of a hares' body mass, model 1AA, e-obs GmbH). Acceleration was recorded at 33 Hz (byte count 495) every 2 min and ODBA values were calculated with the R package moveACC [61] as \(ODBA=\left|{A}_{x}\right|+\left|{A}_{y}\right|+|{A}_{z}|\), where Ax, Ay, and Az are the derived dynamic accelerations corresponding to the three perpendicular axes of the sensor [40] (Tab. 1).
Step 3—ODBA and home range of free-ranging wild hares
The field study site was located in an agricultural landscape 100 km northeast of Berlin (53°21′22.8′′ N, 13°48′03.0′′ E; WGS84) within the "AgroScapeLab Quillow" catchment, the research platform of the Leibniz Centre for Agricultural Landscape Research (ZALF) and the BioMove research training group. The climate is described as continental/Atlantic transition with long dry phases in spring and cold winters. The mean annual precipitation is 486 mm and the mean annual air temperature is 8.4 °C. The landscape is dominated by loamy soils and intensive cultivation of winter cereals, rape, and maize. The field sizes are on average 27.5 ± 1.1 ha [29, 56].
Wild hares (n = 14, Additional file 1: Table S1) were trapped by chasing them into woolen nets [for details, see 76,83], weighed, sexed, and equipped with GPS/ACC collars (model 1AA, e-obs GmbH [62]). While all hares were adults, the exact age was not determined to reduce the duration of time the animals were exposed to handling stress. An acceleration informed GPS frequency was programmed as follows: GPS locations were recorded every 4 min during normal and high activity. When no activity was recorded, GPS fixes were logged every hour [56]. Tracking data were stored in the Movebank data repository [63]. Acceleration recordings were programmed as for the captive hares. After collar fitting, hares were moved inside a wooden transport box (60 cm × 25 cm × 30 cm) to an open area in the field. At the release point, the top plate of the box was opened, and latencies for looking out of the box and leaving the box were recorded according to the novel environment test (Table 1). From untangling the hare from the net until releasing it from the box, the handling procedure lasted between 30 to 45 min. Remote data download was triggered whenever an individual was within range of a base station (model basis 5, e-obs GmbH) deployed near the trap location. Hares were tracked for a varying duration, depending on the coverage of the receiver antennas and the battery life of the collar (Additional file 1: Table S1).
Statistical analyses
First, we estimated adjusted repeatability of behaviors in captive hares (Latency look, Latency leave, Delta look-leave, Exploring first 3 m, Exploring enclosure; Table 1) using linear mixed effect models and bootstrapping (number of parametric bootstraps for interval estimation: 10000; number of permutations to calculate asymptotic p-values: 10,000; p-values shown refer to repeatability) with the individual as a random factor and adjusting for housing type as a fixed effect with the R package rptR [64, 65].
Second, we tested whether repeatable among-individual differences in behavior explain variation in recorded ODBA. As "Delta look leave" (Table 1) is derived from both Latency leave and Latency look and the latter correlated among each other (Pearson correlation coefficient > 0.7), we considered Latency look and Latency leave in separate models for subsequent analyses. We calculated a linear mixed effect model with the latency as a response variable, housing type and number of the respective trial (i.e., first or second measure of the latency) as fixed effects and individual as a random effect. Following Hertel et al. [66], we derived the best linear unbiased predictor (BLUP) by extracting the conditional modes of the random effect (individual) from the fitted model. Then, we calculated a generalized linear model with activity (mean ODBA over the 3 days in the exclosure) as a response variable, Gamma error distribution and predictor variables BLUP, body mass and housing type. Then, we related latencies of wild hares to the mean ODBA during the first 3, 10, 20 and 30 days of tracking per individual (Additional file 1: Table S1).
Body mass was included in both models as a fixed effect. As captive hares experienced two types of housing conditions (small cages of 2 m2 with one individual; small arenas of 50 m2with two individuals), we additionally included housing as a fixed effect in the models. Subsequently, for captive hares we performed a step-wise backward model selection based on the information criterion Akaike (AICc, corrected for small sample size) using the dredge function implemented in the R package MuMIn [67]. Following the studies of Anderson and Burnham [68] and Pinheiro and Bates [69], we selected the model with the highest Akaike score (lowest AICc value) to best explain our data. All models within 2 AICc units were considered as competing models (Additional file 1: Tables S2 and S3).
Third, we tested if among-individual differences in behavior, i.e., boldness expressed as short latencies, predict space use in wild hares. We first calculated the cumulative home range sizes of consecutive days (day 1 to 32, Additional file 1: Fig. S1) to assess how many tracking days are needed to reach home range size saturation. After visual inspection (Additional file 1: Fig. S1), we decided to calculate home range sizes of the initial 20 and 30 (n = 12, Additional file 1: Table S1) days after releasing the captured hares. Then, we calculated home range sizes based on 95% (home range) and 50% (core area) of the kernel utilization distribution while considering autocorrelation for continuous time (akde; R package move [70] and ctmm [71]). Finally, we related the logarithmized home range and core area sizes to the predictor variables “body mass” and Latency look or “Latency leave” with Gaussian error distribution (quantile residuals were checked using the DHARMa package [72]). Due to the low sample size of captive hares (repeatability: n = 14, 4 females, 10 males; ODBA: n = 12, 4 females, 8 males) and wild hares (see Additional file 1: Table S1), we did not further analyze the effects of sex. All analyses were performed in R version 4.0.2 [57] and R Studio version 1.2.5019 [59].
Results
Among-individual differences in behavior and their linkage to acceleration data
We found temporal consistency in three of the five behavioral variables (n = 14, all R ≥ 0.5, all p < 0.05): Latency look (R = 0.62 ± 0.18, p = 0.014), Latency leave (R = 0.59 ± 0.18, p = 0.021) and Delta look-leave (R = 0.49 ± 0.20, p = 0.021). All other behavioral variables were not repeatable over time (Table 1, Fig. 1).
Adjusted repeatability (i.e., fixed effect for enclosure type, 10 hares were kept in cages, 4 hares were kept in arenas) of behavioral variables quantified in repeated novel environment tests of 14 captive European hares (Lepus europaeus) in an open field arena. Latency look: p (LRT) = 0.006, p (permutation) = 0.009; Latency leave: p (LRT) = 0.010, p (permutation) = 0.014; Delta look-leave: p (LRT) = 0.026, p (permutation) = 0.030; Exploring first 3 m: p (LRT) = 0.093, p (permutation) = 0.124; Exploring enclosure: p (LRT) = 0.135, p (permutation) = 0.183. Shown are repeatability estimates (red dots) and their 95% confidence intervals (lines) estimated via parametric bootstraps (n = 10,000 simulations); p-values are based on permutations (n = 10,000)
Consequently, we considered hares that took a comparatively long time to look out or leave the box as shy and hares that left the box quickly as bold individuals. The bolder an individual (i.e., the shorter Latency look and Latence leave), the higher the individual's activity (ODBA) during the first three successive days it roamed freely in the novel environment without effects of body mass (Fig. 2A, B, Table 2).
Observed values (circles and triangles), predicted values (connected by the black line), and SE (gray shading) for A The latency to first look out of a safe retreat; p = 0.003 and B the latency to leave a safe retreat in relation to general activity (mean ODBA measured in \(\left[\frac{m}{{s}^{2}}\right]\)) during the three consecutive days in a novel environment in 12 captive hares; p = 0.045. The behavioral trait C Latency-look and D Latency leave (small latencies equal to a high score on the bold-shy continuum) of wild hares (Lepus europaeus) and their relationship with general activity (mean ODBA) for the first 3 (n = 14), 10 (n = 13), 20 (n = 12) and 30 (n = 12) days of tracking; all p < 0.026
We found a similar pattern for free-ranging hares (single measurement of the latencies while releasing the individual). Hares that quickly looked out of the release box (Latency look) or left it (Latency leave) were also more active throughout the first 3, 10, 20 and 30 tracking days (Fig. 2C, D, Table 2).
Personality effects on space use of wild hares
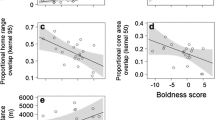
Behavioral responses were shorter in wild hares (Latency look: 27 s ± 18 s and 26 s ± 18 s, Median ± SD; Latency leave: 37 ± 18 s and 26 s ± 19 s, Median ± SD) compared to captive hares (72 s ± 1017 s and 135 s ± 1276 s; Median ± SD). Bold behavioral types (i.e., individuals with fast behavioral respones) had smaller 20-day home ranges with larger 20-day core areas (Fig. 3, Table 3). The same pattern was found for 30 days and Latency look, whereas the link with Latency leave was almost marginally significant (Fig. 3, Table 3).
Observed values (circles) with low–high error bars (95% CI), predicted values (connected by lines, dashed lines show non-significant regressions), and SE (gray shading) of wild hares (Lepus europaeus) for relationships between behavioral variables A Latency look and B Latency leave with home range size (akde95) of the first 20- and 30 days after release; C Latency Look and D Latency leave with core area size (akde50) of the first 20- and 30 days after release. n = 12
Discussion
By combining standardized behavioral tests under open field arena conditions and biologging of activity and space use of free-ranging individuals, we found that among-individual differences along the shy-bold continuum are consistent over time and related to overall activity and space use in European hares. Furthermore, we demonstrated how remote assessment of personality types without additional disturbance of the focal individual is achievable.
The variation in boldness of the hares was associated with short- and long-term differences in overall activity (ODBA) calculated from accelerometer measurements, with shy individuals having lower activity scores than bold individuals. Interestingly, this pattern was observed for both, hares under experimental conditions in an open field arena and hares in a wild population in an agricultural landscape in north-eastern Germany. In wild hares, heavier individuals were also less active. We can not exclude that this might also be partly related to an individual’s age, which was not determined during handling; however, all individuals were adults (> 3800 g, roughly above 2 years).
Notably, wild hares generally responded faster than captive hares. We suspect that this is due to captive hares being more used to handling and are therefore not as naive to humans as wild hares. Due to the relatively small sample sizes, we did not follow a covariance partitioning approach and did not account for differing habitat prerequisites (e.g., multivariate mixed models to study correlations between behavioral traits on various hierarchical levels [58]). Despite the limited sample size, the repeatability of behaviors compares well with findings from other taxa [e.g., meta-analysis: 91]. Further, we showed how behavioral traits covary with movement behavior leading to the dichotomy in space use patterns in a wild hare population. The positive correlation between general activity and boldness is consistent with previous findings using standardized behavioral tests, for example, in common voles, Microtus arvalis [73], bank voles Myodes glareolus [29], gray mouse lemurs, Microcebus murinus [74], and Siberian chipmunks, Tamias sibiricus [75]. This consistency highlights that the correlation also persists beyond short behavioral tests to longer-term and natural conditions. Our approach extends existing observations and shows that activity in relation to animal personality can be measured using animal-borne accelerometers in combination with simple novelty experiments.
Similarly, in eastern chipmunks, dawn activity and percentual activity per day (measured as ODBA via accelerometers) were positively related to exploration speed, although overall activity patterns varied according to temporal variability in food availability [22]. Behavioral variation along the shy-bold continuum could reduce intraspecific competition as individuals with varying levels of boldness likely also differ in their risk-taking or exploration behavior [30, 74]. Particularly in agricultural landscapes, bolder individuals may also be more resilient to disturbance from agricultural measures like soil tillage or harvest.
Boldness (and associated activity) was correlated with space use of hares in the wild population. Previous studies have shown that bolder individuals occupy more extensive home ranges, as found for bank voles (Myodes glareolus) [29], sleepy lizards (Tiliqua rugosa) [34], or common brushtail possums (Trichosurus vulpecula) [76]. Contrary to our expectation, bold hares had smaller home ranges during the initial 20 and 30 days after release, but with larger core areas.
Dissimilarity in space use and movement of bold and shy individuals might reflect different exploration styles between behavioral types along the shy-bold continuum. In general, resources are dispersed across a landscape [77, 78], but parts of a home range with higher resource density should be more important than those with fewer. Particularly bold individuals might successfully defend larger portions of these high-value areas, whereas shy individuals might be more likely to roam in search of less contested habitats, resulting in larger home ranges with smaller core areas. Hence, we suggest that bold hares are more successful in competing for valuable areas against shy individuals, forcing them to continue moving to find an unoccupied, suitable habitat.
This interpretation is tentatively supported by the energy expenditure of hares in our study, being negatively associated with body mass (non-significant trend only, Additional File 1: Fig. S2), which is consistent with findings of great tits Parus major [79] and Asian particolored bats Vespertilio sinensis [80]. Since bold animals tend to take more risks [81,82,83] and have higher energy costs, we suggest that occupying and defending a smaller area of higher forage quality might further allow bolder individuals to outweigh an increased risk of being detected by predators. This signisuggestion is in line with home range sizes varying largely among individuals. Although shy individuals in the present study presumably avoided risky situations, they occupied large home ranges with low habitat quality, i.e., they needed to move further to meet their energy demands and had lower feeding rates, possibly negatively affecting their fitness [81, 83, 84]. However, boldness has been shown to scale positively with (basal) metabolic rate in many species, and individuals with faster rates require more or higher-quality resources to meet their energetic demands [85,86,87,88,89]. Thus, for bold hares, dealing with risky situations and defending their home range against competitors could also be seen as a trade-off between energy expenditure versus habitat quality. Nevertheless, such behavioral variation might eventually facilitate the coexistence of individuals with varying behavioral phenotypes within the same population [5].
The link between movement ecology and animal personality is still in its infancy [2, 11], and the vast majority of studies on animal space use have been conducted without the inclusion of personalities, interpreting their variability mainly in terms of external factors or simple, measurable state variables, such as differences in sex or age. As wild animal populations are naturally composed of individuals differing in behavioral traits [17, 90], these inter-individual differences equip populations with a set of variable behavioral responses that could increase their resilience to fluctuating environmental conditions [53, 91, 92].
Considering animal personalities in space use studies might be crucial, as there is increasing evidence that sampling bias may inevitably influence the composition of animal personalities within a drawn sample and therefore the results of the respective study [4, 93]. Behavioral and ecological studies of various species may be affected, as the test subjects may not represent larger populations whose ecological patterns the researchers seek to understand. For example, due to well-established sampling protocols, bolder individuals are more likely to be trapped. In a study with pumpkinseed sunfish, Lepomis gibbosus, Wilson and others [94] noted that some fish were so shy that it was impossible to catch them even once, whereas bolder specimens were caught repeatedly [94]. Further studies have drawn attention to this personality-related sampling bias [95,96,97,98,99], suggesting that the assumption of random sampling might have been violated in many studies [100]. Although we do not know to what extent we could represent the extent of the shy-bold continuum in hares, we found substantial variation along its axis and demonstrated related differences in activity and space use.
Conclusions
Carefully validated under standardized conditions, activity measurements via accelerometers, such as ODBA, could be a valuable tool to contribute to assessing behavioral types remotely. Considering that accelerometers are often already built into GPS units, we recommend activating them at least during the initial days of tracking to estimate individual variation in general activity and, if possible, match them with a simple novelty experiment. This additional information on individual behavioral types will help to explain variation in state-dependent behavior (e.g., risk-taking) and space use and further facilitate mechanistic understanding of processes that drive spatial and ecological dynamics in heterogeneous environments.
Availability of the data and materials
GPS and acceleration data sets generated and analyzed during the current study are stored in the Movebank Data Repository (study IDs: 933567888, 1138520346). Together with the measured behavioral responses, these are available upon reasonable request from the corresponding author.
References
Nathan R, Getz WM, Revilla E, Holyoak M, Kadmon R, Saltz D, et al. A movement ecology paradigm for unifying organismal movement research. Proc Natl Acad Sci USA. 2008;105:19052–9.
Spiegel O, Leu ST, Bull CM, Sih A. What’s your move? Movement as a link between personality and spatial dynamics in animal populations. Ecol Lett. 2017;20:3–18.
Shaw AK. Causes and consequences of individual variation in animal movement. Mov Ecol Move Ecol. 2020;8:1–12.
Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ. Integrating animal temperament within ecology and evolution. Biol Rev. 2007;82:291–318.
Wolf M, Weissing FJ. Animal personalities: consequences for ecology and evolution. Trends Ecol Evol. 2012;27:452–61.
Dingemanse NJ, Dochtermann NA. Quantifying individual variation in behaviour: mixed-effect modelling approaches. J Anim Ecol. 2013;82:39–54.
Koski SE. Broader horizons for animal personality research. Front Ecol Evol. 2014;2:1–6.
Uher J, Addessi E, Visalberghi E. Contextualised behavioural measurements of personality differences obtained in behavioural tests and social observations in adult capuchin monkeys (Cebus apella). J Res Personal. 2013;47(4):427–44. https://doi.org/10.1016/j.jrp.2013.01.013.
Webster MM, Rutz C. How strange are your study animals? Nature. 2020;582:337–40.
Nilsson J-Å, Brönmark C, Hansson L-A, Chapman BB. Individuality in movement: the role of animal personality. Oxford: Oxford University Press; 2014.
Hertel AG, Hertel AG, Niemelä PT, Dingemanse NJ, Mueller T, Mueller T. A guide for studying among-individual behavioral variation from movement data in the wild. Mov Ecol Mov Ecol. 2020;8:1–18.
Found R, Clair CC. Behavioural syndromes predict loss of migration in wild elk. Anim Behav. 2016;115:35–46. https://doi.org/10.1016/j.anbehav.2016.02.007.
Archer J. Tests for emotionality in rats and mice: a review. Anim Behav. 1973;21:205–35.
Gosling SD. From mice to men: what can we learn about personality from animal research? Psychol Bull. 2001;127(1):45–86. https://doi.org/10.1037/0033-2909.127.1.45.
Leclerc M, Zedrosser A, Pelletier F. Harvesting as a potential selective pressure on behavioural traits. J Appl Ecol. 2017;54:1941–5.
Clobert J, Le Galliard JF, Cote J, Meylan S, Massot M. Informed dispersal, heterogeneity in animal dispersal syndromes and the dynamics of spatially structured populations. Ecol Lett. 2009;12:197–209.
Bolnick DI, Amarasekare P, Araújo MS, Bürger R, Levine JM, Novak M, et al. Why intraspecific trait variation matters in community ecology. Trends Ecol Evol. 2011;26:183–92.
Chapman BB, Hulthén K, Blomqvist DR, Hansson LA, Nilsson JÅ, Brodersen J, et al. To boldly go: individual differences in boldness influence migratory tendency. Ecol Lett. 2011;14:871–6.
Best EC, Blomberg SP, Goldizen AW. Shy female kangaroos seek safety in numbers and have fewer preferred friendships. Behav Ecol. 2015;26:639–46.
Holtmann B, Santos ESA, Lara CE, Nakagawa S. Personality-matching habitat choice, rather than behavioural plasticity, is a likely driver of a phenotype–environment covariance. Proc R Soc B Biol Sci. 2017;284(1864):20170943. https://doi.org/10.1098/rspb.2017.0943.
Patrick SC, Pinaud D, Weimerskirch H. Boldness predicts an individual’s position along an exploration–exploitation foraging trade-off. J Anim Ecol. 2017;86:1257–68.
Gharnit E, Bergeron P, Garant D, Reále D. Exploration profiles drive activity patterns and temporal niche specialization in a wild rodent. Behav Ecol. 2020;31:772–83.
Hertel AG, Leclerc M, Warren D, Pelletier F, Zedrosser A, Mueller T. Don’t poke the bear: using tracking data to quantify behavioural syndromes in elusive wildlife. Anim Behav Elsevier Ltd. 2019;147:91–104. https://doi.org/10.1016/j.anbehav.2018.11.008.
Conner M, Plowman B, Leopold BD, Lovell C. Influence of time-in-residence on home range and habitat use of bobcats. J Wildli Manag. 1999;63(1):261. https://doi.org/10.2307/3802508.
Stamps J. Motor learning and the value of familiar space. Am Nat. 1995;146:41–58.
Powell RA. Animal home ranges and territories and home range estimators. In: Boitani L, Fuller TK, editors. Res Tech Anim Ecol Controv consequences. Columbia University Press: New York; 2000. p. 65–110.
Mettke-Hofmann C, Wink M, Winkler H, Leisler B. Exploration of environmental changes relates to lifestyle. Behav Ecol. 2005;16:247–54.
Morris DW. Habitat-dependent foraging in a classic predator-prey system: a fable from snowshoe hares. Oikos. 2005;109:239–54.
Schirmer A, Herde A, Eccard JA, Dammhahn M. Individuals in space: personality-dependent space use, movement and microhabitat use facilitate individual spatial niche specialization. Oecologia. 2019;189(3):647–60. https://doi.org/10.1007/s00442-019-04365-5.
Schirmer A, Hoffmann J, Eccard JA, Dammhahn M. My niche: individual spatial niche specialization affects within-and between-species interactions. Proc R Soc B. 2020;287(1918):20192211.
Kobler A, Klefoth T, Mehner T, Arlinghaus R. Coexistence of behavioural types in an aquatic top predator: a response to resource limitation? Oecologia. 2009;161:837–47.
Pearish S, Hostert L, Bell AM. Behavioral type-environment correlations in the field: A study of three-spined stickleback. Behav Ecol Sociobiol. 2013;67:765–74.
Farine DR, Whitehead H. Constructing, conducting and interpreting animal social network analysis. J Anim Ecol. 2015;84:1144–63.
Spiegel O, Leu ST, Sih A, Godfrey SS, Bull CM. When the going gets tough: behavioural type-dependent space use in the sleepy lizard changes as the season dries. Proc R Soc B Biol Sci. 2015;282(1819):20151768.
Schlägel UE, Grimm V, Blaum N, Colangeli P, Dammhahn M, Eccard JA, et al. Movement-mediated community assembly and coexistence. Biol Rev. 2020;95:1073–96.
Hughey LF, Hein AM, Strandburg-Peshkin A, Jensen FH. Challenges and solutions for studying collective animal behaviour in the wild. Philos Trans R Soc B Biol Sci. 2018;373:1–13.
Scheibe KM, Gromann C. Application testing of a new three-dimensional acceleration measuring system with wireless data transfer (WAS) for behavior analysis. Behav Res Methods. 2006;38:427–33.
Watanabe N, Sakanoue S, Kawamura K, Kozakai T. Development of an automatic classification system for eating, ruminating and resting behavior of cattle using an accelerometer. Grassl Sci. 2008;54:231–7.
Wilson RP, White CR, Quintana F, Halsey LG, Liebsch N, Martin GR, et al. Moving towards acceleration for estimates of activity-specific metabolic rate in free-living animals: the case of the cormorant. J Anim Ecol. 2006;75:1081–90.
Qasem L, Cardew A, Wilson A, Griffiths I, Halsey LG, Shepard ELC, Gleiss AC, Wilson R. Tri-axial dynamic acceleration as a proxy for animal energy expenditure; should we be summing values or calculating the vector? PLoS ONE. 2012;7(2):e31187. https://doi.org/10.1371/journal.pone.0031187.
López LMM, Miller PJO, De Soto NA, Johnson M. Gait switches in deep-diving beaked whales: biomechanical strategies for long-duration dives. J Exp Biol. 2015;218:1325–38.
Gunner RM, Wilson RP, Holton MD, Scott R, Hopkins P, Duarte CM. A new direction for differentiating animal activity based on measuring angular velocity about the yaw axis. Ecol Evol. 2020;10:7872–86.
Gleiss AC, Wilson RP, Shepard ELC. Making overall dynamic body acceleration work: on the theory of acceleration as a proxy for energy expenditure. Methods Ecol Evol. 2011;2:23–33.
Fahlman A, Wilson R, Svärd C, Rosen DAS, Trites AW. Activity and diving metabolism correlate in steller sea lion eumetopias jubatus. Aquat Biol. 2008;2:75–84.
Shepard ELC, Wilson RP, Halsey LG, Quintana F, Laich AG, Gleiss AC, et al. Derivation of body motion via appropriate smoothing of acceleration data. Aquat Biol. 2008;4:235–41.
Green JA, Halsey LG, Wilson RP, Frappell PB. Estimating energy expenditure of animals using the accelerometry technique: activity, inactivity and comparison with the heart-rate technique. J Exp Biol. 2009;212:745–6.
Halsey LG, Shepard ELC, Quintana F, Gomez Laich A, Green JA, Wilson RP. The relationship between oxygen consumption and body acceleration in a range of species. Comp Biochem Physiol A Mol Integr Physiol. 2009;152:197–202.
Halsey LG, Shepard ELC, Wilson RP. Assessing the development and application of the accelerometry technique for estimating energy expenditure. Comp Biochem Physiol A Mol Integr Physiol. 2011;158:305–14. https://doi.org/10.1016/j.cbpa.2010.09.002.
Halsey LG, White CR. Measuring energetics and behaviour using accelerometry in cane toads Bufo marinus. PLoS ONE. 2010;5(4):e10170. https://doi.org/10.1371/journal.pone.0010170.
Nathan R, Spiegel O, Fortmann-Roe S, Harel R, Wikelski M, Getz WM. Using tri-axial acceleration data to identify behavioral modes of free-ranging animals: general concepts and tools illustrated for griffon vultures. J Exp Biol. 2012;215:986–96. https://doi.org/10.1242/jeb.058602.
Mosser AA, Avgar T, Brown GS, Walker CS, Fryxell JM. Towards an energetic landscape: Broad-scale accelerometry in woodland caribou. J Anim Ecol. 2014;83:916–22.
Noonan MJ, Markham A, Newman C, Trigoni N, Buesching CD, Ellwood SA, et al. Climate and the individual: Inter-annual variation in the autumnal activity of the European badger (Meles meles). PLoS ONE. 2014;9(1):e83156. https://doi.org/10.1371/journal.pone.0083156.
Chimienti M, Cornulier T, Owen E, Bolton M, Davies IM, Travis JMJ, et al. The use of an unsupervised learning approach for characterizing latent behaviors in accelerometer data. Ecol Evol. 2016;6:727–41.
Hicks O, Burthe S, Daunt F, Butler A, Bishop C, Green JA. Validating accelerometry estimates of energy expenditure across behaviours using heart rate data in a free-living seabird. J Exp Biol. 2017;220:1875–81.
Ullmann W, Fischer C, Pirhofer-Walzl K, Kramer-Schadt S, Blaum N. Spatiotemporal variability in resources affects herbivore home range formation in structurally contrasting and unpredictable agricultural landscapes. Landscape Ecol. 2018;33(9):1505–17. https://doi.org/10.1007/s10980-018-0676-2.
Ullmann W, Fischer C, Kramer-Schadt S, Pirhofer-Walzl K, Glemnitz M, Blaum N. How do agricultural practices affect the movement behaviour of European brown hares (Lepus europaeus)? Agric Ecosyst Environ. Elsevier. 2020;292: 106819. https://doi.org/10.1016/j.agee.2020.106819.
R Core Team. R: A language and environment for statistical computing. Vienna, Austria; 2020. https://www.r-project.org/
Brommer JE. On between-individual and residual (co)variances in the study of animal personality: are you willing to take the “individual gambit”? Behav Ecol Sociobiol. 2013;67:1027–32.
R Studio Team. RStudio: integrated development environment for R. Boston, MA; 2019. http://www.rstudio.com/
Woodmann N, Bates AJ, Lazar JR, Richardson N. GoPro Inc.. San Meteo, California, USA: GoPro Incorporation; 2002. http://www.gopro.com
Scharf A. moveACC: Visualitation and Analysis of Acceleration Data (Mainly for eObs Tags). 2018. https://gitlab.com/anneks/moveACC/
e-obs GmbH. München, Germany; 2020. www.e-obs.de.
Wikelski M, Davidson SC, Kays R. The movebank data repository. Movebank Arch. Anal. Shar. Anim. Mov. data. Hosted by Max Planck Inst. Anim. Behav. 2020 [cited 2020 Dec 11]. www.movebank.org
Stoffel MA, Nakagawa S, Schielzeth H. rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol Evol. 2017;8:1639–44.
Nakagawa S, Schielzeth H. Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol Rev. 2010;85:935–56.
Hertel AG, Hertel AG, Niemelä PT, Dingemanse NJ, Mueller T, Mueller T. A guide for studying among-individual behavioral variation from movement data in the wild. Mov Ecol. 2020;8:1–36.
Barton K. MuMIn: Multi-model inference. R Packag version 1156. 2016;1–45. http://cran.r-project.org/package=MuMIn
Burnham KP, Anderson DR, editors. Model Selection and multimodel inference. New York: Springer; 2004. https://doi.org/10.1007/b97636.
Pinheiro JC, Bates DM. Mixed-Effects Models in Sand S-PLUS. New York: Springer; 2000.
Kranstauber B, Smolla M, Scharf A.: Move: visualizing and analyzing animal track data. 2020. https://cran.r-project.org/package=move
Fleming CH, Calabrese JM. ctmm: Continuous-time movement modeling. 2020. https://cran.r-project.org/package=ctmm
Hartig F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level / Mixed) Regression Models. 2022. http://florianhartig.github.io/DHARMa/
Lantová P, Šíchová K, Sedláček F, Lanta V. Determining behavioural syndromes in voles: the effects of social environment. Ethology. 2011;117:124–32.
Dammhahn M, Almeling L. Is risk taking during foraging a personality trait? A field test for cross-context consistency in boldness. Anim Behav Elsevier Ltd. 2012;84:1131–9. https://doi.org/10.1016/j.anbehav.2012.08.014.
Boyer N, Réale D, Marmet J, Pisanu B, Chapuis JL. Personality, space use and tick load in an introduced population of Siberian chipmunks Tamias sibiricus. J Anim Ecol. 2010;79:538–47.
Wat KKY, Herath APHM, Rus AI, Banks PB, McArthur C. Space use by animals on the urban fringe: interactive effects of sex and personality. Behav Ecol. 2021;31:330–9.
Macdonald DW, Johnson DDP. Patchwork planet: The resource dispersion hypothesis, society, and the ecology of life. J Zool. 2015;295:75–107.
Silver P, Cooper JK, Palmer MA, Davis EJ. The arrangement of resources in patchy landscapes: effects on distribution, survival, and resource acquisition of chironomids. Oecologia. 2000;124:216–24.
Moiron M, Araya-Ajoy YG, Mathot KJ, Mouchet A, Dingemanse NJ. Functional relations between body mass and risk-taking behavior in wild great tits. Behav Ecol. 2019;30:617–23.
Wang Y, Shi B, Zhao X, Feng J, Jiang T. Morphological correlates of personality in female asian particolored bats (Vespertilio sinensis). Animals. 2020;10:1–15.
Sih A, Bell AM, Johnson JC, Ziemba RE. Behavioral syndromes: An integrative overview. Q Rev Biol. 2004;79:241–77.
Carter AJ, Feeney WE, Marshall HH, Cowlishaw G, Heinsohn R. Animal personality: what are behavioural ecologists measuring? Biol Rev. 2013;88:465–75.
Zwolak R, Sih A. Animal personalities and seed dispersal: a conceptual review. Funct Ecol. 2020;34:1294–310.
Luttbeg B, Sih A. Risk, resources and state-dependent adaptive behavioural syndromes. Philos Trans R Soc B Biol Sci. 2010;365:3977–90.
Biro PA, Stamps JA. Are animal personality traits linked to life-history productivity? Trends Ecol Evol. 2008;23:361–8.
Careau V, Thomas D, Humphries MM, Réale D. Energy metabolism and animal personality. Oikos. 2008;117:641–53.
Careau V, Bininda-Emonds ORP, Thomas DW, Réale D, Humphries MM. Exploration strategies map along fast-slow metabolic and life-history continua in muroid rodents. Funct Ecol. 2009;23:150–6.
Réale D, Dingemanse NJ, Kazem AJN, Wright J. Evolutionary and ecological approaches to the study of personality. Philos Trans R Soc B Biol Sci. 2010;365:3937–46.
Mathot KJ, Dingemanse NJ. Energetics and behavior: Unrequited needs and new directions. Trends Ecol Evol Elsevier Ltd. 2015;30:199–206. https://doi.org/10.1016/j.tree.2015.01.010.
Bolnick DI, Svanbäck R, Fordyce JA, Yang LH, Davis JM, Hulsey CD, et al. The Ecology of Individuals: Incidence and Implications of Individual Specialization. Am Nat. 2003;161:1–28. https://doi.org/10.1086/343878.
Atwell JW, Cardoso GC, Whittaker DJ, Campbell-Nelson S, Robertson KW, Ketterson ED. Boldness behavior and stress physiology in a novel urban environment suggest rapid correlated evolutionary adaptation. Behav Ecol. 2012;23:960–9.
Merrick MJ, Koprowski JL. Should we consider individual behavior differences in applied wildlife conservation studies? Biol Conserv Elsevier Ltd. 2017;209:34–44. https://doi.org/10.1016/j.biocon.2017.01.021.
Morton FB, Lee PC, Buchanan-Smith HM. Taking personality selection bias seriously in animal cognition research: a case study in capuchin monkeys (Sapajus apella). Anim Cogn. 2013;16:677–84.
Wilson DS, Coleman K, Clark AB, Biederman L. Shy-bold continuum in pumpkinseed sunfish (Lepomis gibbosus): an ecological study of a psychological trait. J Comp Psychol. 1993;107(3):250–60. https://doi.org/10.1037/0735-7036.107.3.250.
Tuyttens FAM, Macdonald DW, Delahay R, Rogers LM, Mallinson FJ, Donnelly CA, et al. Differences in trappability of European badgers Meles meles in three populations in England. J Appl Ecol. 1999;36:1051–62.
Réale D, Gallant BY, Leblanc M, Festa-Bianchet M. Consistency of temperament in bighorn ewes and correlates with behaviour and life history. Anim Behav. 2000;60:589–97.
Cooke SJ, Suski CD, Ostrand KG, Wahl DH, Philipp DP. Physiological and behavioral consequences of long-term artificial selection for vulnerability to recreational angling in a teleost fish. Physiol Biochem Zool. 2007;80:480–90.
Biro PA, Post JR. Rapid depletion of genotypes with fast growth and bold personality traits from harvested fish populations. Proc Natl Acad Sci USA. 2008;105:2919–22.
Boon AK, Réale D, Boutin S. Personality, habitat use, and their consequences for survival in North American red squirrels Tamiasciurus hudsonicus. Oikos. 2008;117:1321–8.
Biro PA. Are most samples of animals systematically biased? Consistent individual trait differences bias samples despite random sampling. Oecologia. 2013;171:339–45.
Acknowledgements
We thank all employees of the field station of the Leibniz Centre for Agricultural Research (ZALF) in Dedelow and the field station of the Leibniz Institute for Zoo and Wildlife Research Berlin (IZW), especially Irina Kasprzak for their support and commitment to the study. Further, we would like to thank Anne Hertel and one anonymous reviewer for thorough comments and substantial improvements to the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the DFG funded research training group "BioMove" (DFG-GRK 2118/1). During manuscript preparation, MD was supported by a grant from the German Science Foundation (DA 1377/4-1).
Author information
Authors and Affiliations
Contributions
JS, MD and NB conceived the idea. JS carried out most of the field work, analyzed the data and led the manuscript writing. AL participated substantially in the field work. N.B. and MD led the revision of the manuscript. SO and SKS participated in manuscript revision and supervised throughout the process. All authors gave final approval for the publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All experiments were conducted in accordance with the German Federal Nature Conservation Act (§ 45 Abs. 7 Nr. 3) and approved by the animal ethics committee of the University of Potsdam and by the local nature conservation authority (AZ: 2347-6-2019).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Contains supplementary figures and tables, including details of several of the methods used here, as well as model selection tables.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Stiegler, J., Lins, A., Dammhahn, M. et al. Personality drives activity and space use in a mammalian herbivore. Mov Ecol 10, 33 (2022). https://doi.org/10.1186/s40462-022-00333-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40462-022-00333-6