Abstract
Background
The prevalence of allergic diseases is approximately 10 % in infants whose parents and siblings do not have allergic diseases and 20–30 % in those with an allergic first-degree relative. Vitamin D is involved in the regulation of the immune system and it may play a role in the development, severity and course of asthma and other allergic diseases.
Objective
The World Allergy Organization (WAO) convened a guideline panel to develop evidence-based recommendations addressing the use of vitamin D in primary prevention of allergic diseases.
Methods
Our WAO guideline panel identified the most relevant clinical questions and performed a systematic review of randomized controlled trials and non-randomized studies (NRS), specifically cohort and case-control studies, of vitamin D supplementation for the prevention of allergic diseases. We also reviewed the evidence about values and preferences, and resource requirements (up to January 2015, with an update on January 30, 2016). We followed the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to develop recommendations.
Results
Having reviewed the currently available evidence, the WAO guideline panel found no support for the hypothesis that vitamin D supplementation reduces the risk of developing allergic diseases in children. The WAO guideline panel suggest not using vitamin D in pregnant women, breastfeeding mothers, or healthy term infants as a means of preventing the development of allergic diseases. This recommendation does not apply to those mothers and infants who have other indications for prophylactic or therapeutic use of vitamin D. The panel’s recommendations are conditional and supported by very low certainty evidence.
Conclusions
WAO recommendations about vitamin D supplementation for the prevention of allergic diseases support parents, clinicians and other health care professionals in their decisions whether or not to use vitamin D in preventing allergic diseases in healthy, term infants.
Similar content being viewed by others
Executive summary
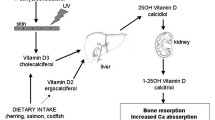
Approximately 10 % of children without an allergic parent or sibling, and 20 to 30 % of those with allergies in their first-degree relatives experience allergic diseases in infancy. As demonstrated in cell culture and animal model experiments, the active form of vitamin D, calcitriol, is a modulator of both adaptive and innate immune responses [1]. Nevertheless, its role is complex and not completely understood. Additional studies relating to vitamin D’s immunomodulating function suggest it may impact the development of sensitization and allergy [2] and therefore, if administered in sufficient doses, may provide health benefits to humans by reducing a predisposition to allergic diseases.
Methodology
The WAO-McMaster University guideline panel included allergists, paediatricians, primary care physicians, researchers in allergic diseases, and methodologists. We used the GRADEpro (www.gradepro.org) software to develop recommendations in this document following the GIN-McMaster Guideline Development Checklist (https://cebgrade.mcmaster.ca/guidecheck.html) and the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach as applied to other WAO guidelines [3–10]. Potential conflicts of interests were managed as suggested by the World Health Organization (WHO).
The guideline panel developed and graded the recommendations, and assessed the certainty of the supporting evidence (also called confidence in the estimates of effects or quality of evidence). The certainty of the evidence is categorized as high, moderate, low or very low based on consideration of risk of bias, directness of evidence, consistency and precision of the estimates, and other considerations. Low and very low certainty evidence indicates that the estimated effects of interventions are very uncertain and any further research is very likely to influence current recommendations.
Interpretation of strong and conditional recommendations
The strength of recommendations is expressed as either strong (guideline panel recommends…) or conditional (guideline panel suggests…) and has the following interpretation:
Strong recommendation
-
For patients: most individuals in this situation would want the recommended course of action, and only a small proportion would not.
-
For clinicians: most individuals should receive the intervention. Adherence to this recommendation according to the guideline could be used as a quality criterion or performance indicator. Formal decision aids are not likely to be needed to help individuals make decisions consistent with their values and preferences.
-
For policy makers: the recommendation can be adopted as policy in most situations.
Conditional recommendation
-
For patients: the majority of individuals in this situation would want the suggested course of action, but many would not.
-
For clinicians: recognize that different choices will be appropriate for individual patients and that you must help each patient arrive at a management decision consistent with his or her values and preferences. Decision aids may be useful in helping individuals to make decisions consistent with their values and preferences.
-
For policy makers: policy-making will require substantial debate and involvement of various stakeholders.
How to use these guidelines
The GLAD-P guidelines about the use of vitamin D provide the basis for rational, informed decisions for clinicians, parents and other decision makers. Clinicians, patients, third-party payers, institutional review committees, other stakeholders, or the courts should not view these recommendations as dictates. No recommendation can take into account all of the often-compelling unique individual circumstances but provides guidance for typical patients. Thus, no one charged with evaluating health care professionals´ actions should apply the recommendations from these guidelines by rote or in a blanket fashion.
Note: statements regarding the underlying values and preferences as well as qualifying remarks accompanying each recommendation are integral to the recommendations and serve to facilitate more accurate interpretation; they should never be omitted when quoting recommendations from these guidelines.
Recommendations
Question 1: Should vitamin D be used in pregnant women?
Recommendation
The WAO guideline panel suggests that clinicians, parents and other decision makers do not use vitamin D supplementation in pregnant women with the intention of preventing the development of allergic diseases in their children (conditional recommendation, very low certainty of evidence).
Values and preferences
The recommendation not to use vitamin D in pregnant women with the intention of preventing the development of allergic diseases in their children places a relatively higher value on avoiding additional cost and burden and a relatively lower value on very uncertain, if any, effect on prevention of allergic diseases.
Explanations and other considerations
Evidence does not support vitamin D supplementation in pregnant women prevents the development of allergic diseases in their children. This recommendation does not apply to those pregnant women who have other indications for prophylactic or therapeutic use of vitamin D.
Question 2: Should vitamin D be used in breastfeeding women?
Recommendation
The WAO guideline panel suggests that clinicians, parents and other decision makers do not use vitamin D supplementation in breastfeeding mothers with the intention of preventing allergic diseases their children (conditional recommendation, very low certainty evidence).
Values and preferences
The recommendation to not use vitamin D in breastfeeding mothers with the intention of preventing the development of allergic diseases in their children places a relatively higher value on avoiding additional cost and burden and a relatively lower value on very uncertain, if any, effect on prevention of allergic diseases.
Explanations and other considerations
Evidence does not support vitamin D supplementation in breastfeeding mothers prevents the development of allergic diseases in infants. This recommendation does not apply to those breastfeeding women who have other indications for prophylactic or therapeutic vitamin D supplementation. This recommendation does not address vitamin D supplementation for secondary prevention of allergic diseases.
Question 3: Should vitamin D be used in infants?
Recommendation
The WAO guideline panel suggests that clinicians, parents and other decision makers do not use vitamin D supplementation in infants with the intention of preventing the development of allergic diseases (conditional recommendation, very low certainty evidence).
Values and preferences
The recommendation to not use vitamin D in healthy term infants with the intention of preventing the development of allergic diseases places a relatively higher value on avoiding additional cost and burden and a relatively lower value on very uncertain, if any, effect on prevention of allergic diseases.
Explanations and other considerations
Evidence does not support vitamin D supplementation in infants prevents the development of allergic diseases. This recommendation does not apply to infants who have other indications for prophylactic or therapeutic vitamin D supplementation. This recommendation does not address vitamin D supplementation for secondary prevention of allergic diseases.
Scope and purpose
The purpose of this document is to evaluate the current evidence and provide guidance on the use of vitamin D for the primary prevention of allergic diseases focusing on asthma and/or wheezing, allergic rhinitis, atopic eczema (dermatitis), and food allergy. The target audiences for these guidelines are general practitioners, pediatricians, specialists in allergic disease and immunology, respiratory medicine specialists, obstetrician/gynaecologists, and dermatologists managing pregnant women and infants at risk of developing allergic diseases. General internists, and other health care professionals and policy makers may also benefit from these guidelines. Policy makers interested in these guidelines include those involved in developing local, national or international policies that have the goal of reducing the incidence of allergic diseases, and limiting the direct and indirect costs of allergic diseases [11]. This document may also serve as the basis for development and implementation of locally adapted guidelines.
Introduction
Allergic diseases represent a spectrum of health conditions with a large worldwide burden. In infants, its incidence is highly influenced by the allergic status of their parents: approximately 10 % in infants without an allergic parent or sibling, versus approximately 20 to 30 % in those with an atopic history in their first-degree relatives [12].
A growing body of literature has addressed associations between vitamin D concentrations and various conditions. Observational studies and randomized trials have addressed the possible effectiveness of vitamin D supplementation in the prevention or treatment of a variety of disorders and adverse health outcomes. Emerging evidence indicates that vitamin D may play a role in the immune system. In particular, the active form of vitamin D, calcitriol, has been shown to modulate immune functioning in cell culture and animal models [13]. Nevertheless, understanding of the complex role of vitamin D in immune function remains limited [14].
The Guidelines for Atopic Disease Prevention (GLAD-P) is a joint effort of the World Allergy Organization (WAO) and the Department of Clinical Epidemiology & Biostatistics at McMaster University to evaluate the current evidence addressing the preventive effect of probiotics, prebiotics, and vitamin D on allergic diseases and related patient-important outcomes. This document provides recommendations on the rationale for use of vitamin D.
We use the following definitions throughout the document:
-
Vitamin D supplement: any formulation of vitamin D, either alone or in multi-vitamin products, including products available by prescription or over the counter, and food supplements available from pharmacies and retail outlets [15].
-
Family history influencing risk for allergic diseases in a child: biological parent or sibling with existing or history of allergic rhinitis, asthma, eczema, or food allergy [16].
Methods
Panel composition and meetings
We followed the procedures and methodology using the GIN-McMaster Guideline Development Checklist, the GRADE approach and methods we had previously applied to WAO guidelines [3–9]. Under the auspices of the organizations, we assembled a team of experts including allergists, pediatricians, and family physicians and representatives of the general public. The guideline panel included methodologists who helped to prepare systematic reviews and evidence summaries.
A face-to-face meeting was held in January 2015 coinciding with the WAO Symposium in Rome, Italy. During the meeting the guideline panel discussed specific questions in the context of existing research evidence to make recommendations.
Disclosure of potential conflicts of interest
Guideline panel members disclosed all potential conflicts of interest according to the World Health Organization policies. The chairs (AF, RP and HJS) reviewed and resolved all potential conflicts of interest of panel members (see Additional file 1 for the list of declared conflicts of interest for all panel members). During all deliberations, panel members with potential conflicts of interest abstained from decisions about recommendations related to their potential conflict of interest.
WAO provided meeting facilities during the Symposium and financial support to perform systematic reviews. The views and interests of the WAO as well as any commercial entity that provided external funding to WAO had no influence on the final recommendations.
Formulating specific clinical questions and determining outcomes of interest
We used the electronic tools: GRADEpro Guideline Development Tool (www.gradepro.org) [17] and SurveyMonkey (https://www.surveymonkey.com/) to brainstorm and subsequently prioritize questions related to the use of vitamin D for the prevention of allergic diseases.
The following questions were prioritized and addressed in this document:
-
1.
Should vitamin D versus no vitamin D be used in pregnant women?
-
2.
Should vitamin D versus no vitamin D be used in women who are breastfeeding?
-
3.
Should vitamin D versus no vitamin D be used in infants?
The guideline selected outcomes of interest for each question, following the approach suggested by the GRADE Working Group [3]. All outcomes were identified a priori and the panel explicitly rated their relative importance for decision-making. Ranking outcomes by their relative importance can help focus attention on the outcomes that are considered most important and help to resolve or clarify potential disagreements.
Evidence review and development of clinical recommendations
Evidence summaries for each question were prepared by the methodologists (JJYN, CCG, JB and HJS) using GRADEpro GDT (www.gradepro.org). All guideline panel members reviewed the summaries of evidence and made corrections when appropriate. We based the evidence summaries on a systematic review of the literature performed specifically for these guidelines. (Reference. in preparation). An updated search strategy (presented in the online Additional file 2) was performed on January 30, 2016 that provided two additional studies. We followed the methods of the Cochrane Collaboration (handbook.cochrane.org) and assessed the risk of bias at the outcome level using the Cochrane Collaboration’s risk of bias tool [18], and version 1.0 of the Cochrane Risk of Bias Assessment Tool for Non-Randomized Studies of Interventions, now called ROBINS-I [19], for RCTs and NRSs, respectively. Subsequently, we assessed the certainty of the body of evidence (confidence in the estimated effects) for each of the outcomes of interest using the GRADE approach based on the consideration of risk of bias, directness of evidence, consistency and precision of the estimates, and other factors such as publication bias.
We searched for evidence about values and preferences and cost of vitamin D supplementation. We prepared the evidence-to decision frameworks based on the estimates of the health effects, values and preferences, and resource use.
During the meeting, the guideline panel developed recommendations based on the evidence summaries and the evidence-to-decision frameworks. For each recommendation, the guideline panel considered and agreed on the following: the certainty of the evidence, the balance of desirable and undesirable consequences of compared management options, the feasibility, acceptability and impact on health inequities for each recommendation, as well as the assessment of the values and preferences associated with the decision. The guideline panel also explicitly took into account the possible extent of resource use associated with alternative management options.
Recommendations and their strength were developed through consensus and no recommendation required voting. The panel agreed on the final wording of recommendations and remarks with further qualifications for each recommendation.
We labelled the recommendations as either “strong” or “conditional” according to the GRADE approach. We used the words “the panel members recommend” for strong recommendations and “suggest” for conditional recommendations. Table 1 provides suggested interpretation of strong and conditional recommendations.
Document review
Each member of the guideline panel reviewed the final draft document and approved the document, which was then submitted to the WAO for peer review. The document was revised to incorporate the pertinent comments suggested by the external reviewers.
How to use these guidelines
The WAO-McMaster University GLAD-P guidelines about the use of vitamin D in the primary prevention of allergic diseases in children are not intended to impose a standard of care. They provide the basis for rational decisions. Clinicians, patients, third-party payers, institutional review committees, other stakeholders, or the courts should never view these recommendations as dictates. No recommendation can take into account all of the often-compelling unique circumstances of each individual patient. Therefore, no one charged with evaluating health care professionals’ actions should apply the recommendations from these guidelines as rote or in a blanket fashion.
Statements regarding the underlying values and preferences as well as qualifying remarks accompanying each recommendation are integral parts and serve to facilitate more accurate interpretation. They should never be omitted when quoting recommendations from these guidelines.
Recommendations
Question 1. Should vitamin D versus no vitamin D be used in pregnant women?
Summary of the evidence
We found no systematic review addressing this question. We found seven publications [reporting six randomized control trials (RCTs)] that investigated the effects of vitamin D supplementation in pregnant women. Only one RCT [20] measured the risk of developing allergic diseases in children, and the remaining trials [21–27] reported nutritional status, adverse effects, and development of rickets. No study reported quality of life and development of a composite of “any allergy”. We also found two observational studies, one case-control and one cohort study, which reported the effect of vitamin D supplementation in pregnant women on the development of food allergy and wheezing in their infants respectively [28, 29].
The randomized trial failed to detect an effect of vitamin D supplementation on the risk of developing allergic diseases in children: atopic dermatitis (RR 0.96, 95 % CI 0.57 to 1.61), allergic rhinitis (RR 0.76, 95 % CI 0.31 to 1.85), asthma and/or wheezing (RR 1.12, 95 % CI 0.50 to 2.54), and food allergy (RR 1.92, 95 % CI 0.57 to 6.50). Vitamin D supplementation in pregnant women was not associated with a lower birth weight in infants compared to no vitamin D (mean difference 52.78 g more, 95 % CI -64.34 to 169.90). Adverse effects as measured by infants being born small for gestational age, gestational age, symptomatic hypocalcaemia, as well as severe adverse effects in a newborn and in the mother were very infrequent and any estimates are very imprecise. There was no difference between the groups.
The findings of the case-control study were consistent with the findings of the randomized trial for food allergy (OR 1.50, 95 % CI 0.78 to 2.88). In the cohort study, vitamin D was associated with low risk of developing wheezing (OR 0.65, 95 % CI 0.46 to 0.93). The overall certainty of the body of evidence was very low owing to the risk of bias and imprecision. Online Additional file 3 presents the characteristics of all included studies for all questions.
Benefits
Thus far, there is no direct evidence from clinical studies suggesting that vitamin D supplementation during pregnancy reduces the risk of developing allergic diseases in children (see evidence profile for question 1 in the Additional file 4).
Harms and burden
Adverse effects in pregnant women were well defined in all studies. Abnormal glucose challenge test was reported as an adverse effect in one study [22]. Other adverse effects included nausea and vomiting [26]. Maternal serious adverse effects included gastroenteritis, preterm delivery with premature rupture of membranes, injury, and pre-eclampsia. For pregnant women with adverse effects and serious adverse effects, there was no difference among those who received vitamin D and those who did not receive vitamin D (RR 1.46, 95 % CI 0.31 to 6.78; RR 1.55, 95 % CI 0.65 to 3.69 respectively). The certainty in the estimate of the risk of adverse events in pregnant mothers ranged between low to very low due to the development of few events in both arms.
Adverse effects in infants were well documented and included low birth weight (assessed in grams), gestational age (preterm labour), small for gestational age (assessed as weight lower than 10th centile or less than 2.5 kg) (RR 0.67, 95 % CI 0.37 to 1.19), hypocalcaemia (RR 0.10, 95 % CI 0.01 to 1.82), any and serious adverse effects (RR 0.79, 95 % CI 0.33 to 1.90). No adverse effects related to the administration of vitamin D in infants were reported in two studies [20, 21]. Serious adverse effects included hypoxic-ischemic encephalopathy, meconium aspiration syndrome, jaundice, neonatal seizures, sepsis, sub-acute intestinal obstruction, pneumonia, intrauterine, and neonatal death. In total, three intrauterine and four neonatal deaths (two and one deaths in the vitamin D group respectively) were reported. Reasons for intrauterine deaths were not specified. A preterm infant with very low birth weight, died within 5 min of birth at home (placebo group). The reasons for other neonatal deaths were sepsis and multi-organ failure, severe hypoxic-ischemic encephalopathy and respiratory failure, cardio-respiratory failure of unknown etiology. Adverse effects were not different among infants who received vitamin D and those who did not receive vitamin D. The certainty of the evidence of adverse effects in infants ranged between moderate (gestational age, birth weight), low (weight at 1 year, any, and serious adverse events), and very low (small for gestational age, symptomatic hypocalcaemia). The certainty in the estimates was assessed as moderate and low confidence due to imprecision in the estimates. Very low certainty in the estimates was downgraded due to imprecision and risk of bias.
Decision criteria and considerations
The values and preferences of women regarding the use of vitamin D during pregnancy are likely dependent on cultural and socioeconomic background. We explicitly considered the required resources. Prices of vitamin D are likely to vary depending on the factors such as country, region or formulation. The literature review did not identify any cost-effectiveness analysis related to the preventive use of vitamin D for allergic diseases.
Conclusions and research needs
The guideline panel determined that it is unlikely that there is a net benefit from using vitamin D in pregnant women who have no specific indications for prophylactic or therapeutic vitamin D supplementation and the only purpose would be to primary prevention of allergic diseases in their children. We found no impact of vitamin D on the development of allergic diseases. There is a need for a rigorously designed and executed randomized trial of vitamin D supplementation in pregnant women that would properly measure and report patient-important outcomes, including development of allergic diseases, quality of life, and adverse effects. Long-term follow-up of such studies to evaluate long-term effects is also needed.
What others are saying
We found no guidelines that made specific recommendations about the use of vitamin D in pregnancy with the intention of preventing allergic diseases in children. The World Health Organization (WHO) states “Vitamin D supplementation is not recommended during pregnancy to prevent the development of pre-eclampsia and its complications (strong recommendation). In addition, due to the limited evidence currently available to directly assess the benefits and harms of the use of vitamin D supplementation alone in pregnancy for improving maternal and infant health outcomes, the use of this intervention during pregnancy as part of routine antenatal care is also not recommended (conditional recommendation) [30]”.
The National Institute for Health and Care Excellence (NICE) recommends increasing the access to vitamin D supplements in high risk groups that have a low vitamin D status such as pregnant and breastfeeding women, particularly teenagers and young women [15]. The American Academy of Pediatrics (AAP) [31] recommends, on an individual basis, pregnant women should receive adequate amounts of vitamin D3 to ensure that her 25-OH-D levels are sufficiently high (>80 nmol/L). Similarly, the Endocrine Society Clinical Practice Guideline [32] states that pregnant women require adequate supplementation of vitamin D so that vitamin D levels are not insufficient.
The European Society for Paediatric Gastroenterology, Hepatology, and Nutrition Committee on Nutrition [33], the European Academy of Allergy and Clinical Immunology [34], Food Allergy and Anaphylaxis Guidelines [35] and the Guidelines from the US National Institute of Allergy and Infectious Diseases [16], make no specific recommendations regarding the use of vitamin D in pregnant women.
Recommendation 1
The WAO guideline panel suggests that clinicians, parents and other decision makers do not use vitamin D supplementation in pregnant women with an intention to prevent development of allergic diseases in their children (conditional recommendation, very low certainty of evidence).
Values and preferences
The recommendation not to use vitamin D in pregnant women with the intention of preventing the development of allergic diseases in their children places a relatively higher value on avoiding additional cost and burden and a relatively lower value on very uncertain, if any, effect on prevention of allergic diseases.
Explanations and other considerations
Evidence does not support vitamin D supplementation in pregnant women prevents the development of allergic diseases in their children. This recommendation does not apply to those pregnant women who have other indications for prophylactic or therapeutic use of vitamin D. See the evidence to recommendation table for question 1 in online Additional file 5.
Question 2. Should vitamin D versus no vitamin D be used in breastfeeding mothers?
Summary of the evidence
We found no systematic review or randomized trial of vitamin D supplementation in breastfeeding mothers that reported on the development of allergic diseases in children. We identified one case-control study which addressed this question and found no effect of vitamin D supplementation in breastfeeding mothers on the risk of developing asthma and/or wheezing in children: (OR 1.09, 95 % CI 0.84 to 1.40) [36].
One RCT [37] reported no cases of rickets among 60 children of breastfeeding mothers that either used or didn’t use vitamin D supplements. The time of follow-up was 6 weeks, which was considered too short to develop this outcome. No experimental or observational study with an independent control group reported other outcomes of interest. Adverse effects were not reported in the included studies. The overall certainty of the body of evidence was very low.
Benefits
There is no evidence from clinical studies suggesting an effect of vitamin D supplementation in breastfeeding mothers on the risk of developing allergic diseases in children (see evidence profile for question 2 in the Additional file 4).
Harms and burden
Any estimate of potential adverse effects is very uncertain due to the unavailability of reports of adverse effects.
Decision criteria and considerations
The panel agreed that the considerations of values and preferences, resource implications, and equity are likely similar to those in pregnant women.
Conclusions and research needs
The guideline panel determined that it is not possible to determine whether there is any benefit from using vitamin D supplementation in breastfeeding women who have no specific indications for prophylactic or therapeutic vitamin D supplementation for the sole purpose of reducing the risk of developing allergic diseases in otherwise healthy term children. Given the paucity of information, it is not possible to exclude an appreciable benefit, no effect or appreciable harm.
There is a need for a rigorously designed and well-executed randomized trial of vitamin D in breastfeeding women that would properly measure and report patient-important outcomes, including quality of life and adverse effects. Long-term follow-up of such studies to evaluate long-term effects is also needed.
What others are saying
We found no guidelines that made specific recommendations about the use of vitamin D in breastfeeding mothers with the intention of preventing allergic diseases in children. The AAP [31] does not recommend supplementing breastfeeding mothers with high doses of vitamin D in order to increase the 25-OH-D concentrations in their breastfed infants. However, they recommend, “a supplement of 400 IU/day of vitamin D should begin within the first few days of life and continue throughout childhood”. NICE [15] recommends increasing the access to vitamin D supplements in high risk groups of low vitamin D status such as pregnant and breastfeeding women, particularly teenagers and young women. ESPGHAN [33], considering the prevalence of vitamin D deficiency in pregnant mothers, recommends that a higher vitamin D supply in preterm infants could be necessary in order to correct the foetal low plasma level. This statement holds for both premature infants fed mother’s milk and those fed formula milk.
WHO [30], the Endocrine Society Clinical Practice Guideline [32], the European Academy of Allergy and Clinical Immunology [34] Food Allergy and Anaphylaxis Guidelines [35] and the Guidelines from the US National Institute of Allergy and Infectious Diseases [16], make no specific recommendations about the use of vitamin D in breastfeeding women.
Recommendation 2
The WAO guideline panel suggests that clinicians, parents and other decision makers do not use vitamin D supplementation in breastfeeding mothers with the intention of preventing allergic diseases in their children (conditional recommendation, very low certainty evidence).
Values and preferences
This recommendation not to use vitamin D in breastfeeding mothers with an intention of preventing the development of allergic diseases in their children places a relatively higher value on avoiding additional cost and burden and a relatively lower value on very uncertain, if any, effect on prevention of allergic diseases.
Explanations and other considerations
Evidence does not support that vitamin D supplementation in breastfeeding mothers prevents the development of allergic diseases in infants. This recommendation does not apply to those breastfeeding women who have other indications for prophylactic or therapeutic vitamin D supplementation. This recommendation does not address vitamin D supplementation for secondary prevention of allergic diseases. See the evidence to recommendation table for question 2 in online Additional file 5.
Question 3. Should vitamin D vs. no vitamin D be used in healthy infants?
Summary of the evidence
We found no systematic review addressing this question. We found 5 randomized trials that investigated vitamin D supplementation in infants but none reported allergy outcomes [38–42]. One RCT [42] could not be meta-analyzed because it reported mean values without measures of dispersion. We identified three NRS. Two cohort studies reported the risk of developing allergic rhinitis and asthma [43], and wheezing/asthma [29]. The case control study measured development of food allergy [28]. No study measured quality of life and development of eczema or a composite of “any allergy”.
In the first cohort study [43], regular vitamin D supplementation during the first year of life increased the risk of developing allergic rhinitis (RR 1.95, 95 % CI 0.69 to 5.54) but there were only three events among 20 children in the control group, which makes these results very fragile and very imprecise. Study authors combined the group that never received vitamin D with a group that used it irregularly to reduce fragility of the results – if those using vitamin D regularly were compared with those who either did not use it, or used it irregularly, the RR would be 1.31 (95 % CI 1.15 to 1.49). Irrespective of the choice of control group, there is a high risk of bias in these estimates, because results were not adjusted for confounding factors despite the fact that “cohort members with a family history of asthma were less likely to receive supplementation according to recommendations (…) and many of the same characteristics that were predictive of worse compliance were associated with reduced risk of allergies” [43]. In the same study, the risk of developing asthma and/or wheezing was estimated too imprecisely to make any conclusion about the effect (RR 3.07, 95 % CI 0.19 to 50.88). If those using vitamin D regularly were compared with those who either did not use it or used it irregularly, the RR would be 1.36 (95 % CI 1.00 to 1.85). The certainty of the evidence for these two outcomes was very low.
The second cohort study [29] did not show an effect in the primary prevention of asthma/wheezing in infants if they were exposed to vitamin D supplementation during their chilhood (OR 1.00, 95 % CI 0.81 to 1.23).
In the case-control study the risk of developing food allergy during the first year of life was reduced in infants who received vitamin D compared to those who did not (OR 0.49, 95 % CI 0.27 to 0.88) [28]. However, the confidence in this estimate is also very low owing to indirectness of the evidence and risk of bias.
Four RCTs reported the estimation of developing rickets. None of the infants in these four studies developed rickets. One RCT reported weight at one year of age without significant differences between those infants who received vitamin D and those who did not receive vitamin D. Information about weight was available only graphically. The certainty of evidence ranged between low to very low owing to imprecision, risk of bias, and indirectness.
Adverse effects were reported in three RCTs. These adverse effects included inter-current acute diseases, and urinary tract infection. As a serious adverse effect, one study reported a sudden infant death syndrome, which was not related to the trial. Adverse effects and serious adverse effects were not different among infants who received vitamin D and those who did not receive vitamin D (RR 0.85, 95 % CI 0.23 to 3.14; RR 0.17, 95 % CI 0.01 to 3.70 respectively). The certainty of the evidence was very low due to serious risk of bias and imprecision.
No RCT or NRS addressed the efficacy or association of primary prevention of allergic diseases in children (over 2 years old) after vitamin D supplementation.
Benefits
There is a probable reduction in the risk of developing food allergy in infants during the first year of life (RR: 0.49, 95 % CI: 0.27 to 0.88) with vitamin D supplementation. There is no evidence supporting a reduction in the risk of developing any other allergic disease in infants (see evidence profile for question 3 in the Additional file 4).
Harms and burden
Regular vitamin D supplementation during the first year of life increased the risk of developing allergic rhinitis (RR 1.95, 95 % CI 0.69 to 5.54), and asthma and/or wheezing (RR 3.07, 95 % CI 0.19 to 50.88; OR 1.00, 95 % CI 0.81 to 1.23) but the certainty of the evidence is very low.
Decision criteria and considerations
If vitamin D is used in infants, it is not clear when it should be started and how long it should be used and there is an uncertainty about the dosage.
The previous considerations concern otherwise healthy infants in whom vitamin D would be used for primary prevention of allergic diseases. They do not concern using vitamin D for specific indications, e.g., preterm infants, especially with birth weight <1800 to 2000 g [35].
Conclusions and research needs
The guideline panel determined that net benefit from using vitamin D in infants is uncertain. There is a need for rigorously designed and well executed randomized trials of vitamin D in infants that would measure and adequately report patient-important outcomes, including adverse effects.
What others are saying
We found no guidelines that made specific recommendations about the use of vitamin D in infants with the intention of preventing development of allergic diseases. NICE [15] recommends increasing the access to vitamin D supplements in high risk groups of low vitamin D status such as infants and children aged under 5. AAP [31] recommends that “infants who receive a mixture of human milk and formula also should get a vitamin D supplement of 400 IU/day to ensure an adequate intake. Additionally, “any infant who receives <1 L or 1 qt of formula per day needs an alternative way to get 400 IU/day of vitamin D, such as through vitamin supplements”. The Endocrine Society Clinical Practice Guideline [32] suggests that infants and children age 0-1 year require an intake of vitamin D of at least 400 IU/d, and children 1 year and older, at least 600 IU/d to maximize bone health. ESPGHAN [39] recommends that, considering the prevalence of vitamin D deficiency in pregnant mothers, higher vitamin D supply in preterm infants could be necessary to rapidly correct the foetal low plasma level. A vitamin D intake of 800 to 1000 IU/day (and not per kilogram) during the first months of life is recommended. WHO [30] and the European Academy of Allergy and Clinical Immunology Food Allergy and Anaphylaxis Guideline [35] make no specific recommendations about the use of vitamin D in infants.
Recommendation 3
The WAO guideline panel suggests that clinicians, parents and other decision makers do not use vitamin D supplementation in infants with an intention to prevent development of allergic diseases (conditional recommendation, very low certainty evidence).
Values and preferences
This recommendation to not use vitamin D in healthy term infants with the intention of preventing the development of allergic diseases places a relatively higher value on avoiding additional cost and burden and a relatively lower value on very uncertain, if any, effect on prevention of allergic diseases.
Explanations and other considerations
Evidence does not support that vitamin D supplementation in infants prevents the development of allergic diseases. This recommendation does not apply to infants who have other indication for prophylactic or therapeutic vitamin D supplementation. This recommendation does not address vitamin D supplementation for secondary prevention of allergic diseases. See the evidence to recommendation table for question 3 in online Additional file 5.
Priorities for revision of the guidelines
Plans for updating these guidelines
To remain useful, guidelines need to be updated regularly as new information accumulates. A revision of this document will be needed, because there was limited evidence for the three clinical questions.
This document will be updated when major new research is published. As it was stated before, this guideline was focused on primary prevention. However we will look at secondary prevention in the next update of this guideline. The need for updates will be determined no later than in 2019.
Updating or adapting recommendations locally
The methods used to develop these guidelines are transparent. The recommendations have been developed to be as specific and detailed as possible without losing sight of the desirability of simplicity. Since GLAD-P guidelines are meant as international guidelines, the panel encourages feedback on all aspects including their applicability in individual countries. The panel will consider this feedback when revising the document.
Adaptation of these guidelines will be necessary in many circumstances. Depending on when such a process takes place, the following steps should be taken:
-
Appointing a guideline committee comprised of clinicians and methodologists.
-
Determining the scope of the localized guidelines.
-
Defining the clinical questions to be addressed.
-
Updating the evidence profiles and evidence-to decision frameworks, if necessary.
-
Reviewing the recommendations in the GLAD-P guidelines (the recommendations may need to be modified at a local level, depending on the local values and preferences, availability of medications, costs, etc.).
-
Disseminating the guidelines, with a clear “use by” date.
-
Developing a method to obtain feedback and plans for review and update.
Priorities for research
During the guideline development process we identified a need for more data on specific topics. This results in the following recommendations for research. We summarize these gaps in the evidence as research recommendations, to assist those in a position to provide such information by the design and execution of specific research projects.
Specific research needs to be addressed:
-
Development of clinical prediction guides for evaluating the risk of allergic diseases in children (the family history predicts only about 30 % of the population risk).
-
Evaluation of effects of using vitamin D in breastfeeding mothers specifically in that period (as opposed to intervention administered during pregnancy and to children).
-
Evaluation of the effects of different ways of administering vitamin D, e.g., as milk or dairy supplements, stand-alone supplements, etc.
-
Performance of rigorously designed, adequately powered, and well executed randomized trials of vitamin D in infants who did not receive vitamin D prenatally and/or during breastfeeding; studies should include infants considered to be at high and low/average risk for allergic diseases and should properly report patient-important outcomes, including adverse effects. The estimated optimal information size for this question is from approximately 2500 participants (for eczema) to 27,000 participants (for food allergy). However, for the evaluation of adverse effects, a large compilation of RCTs as well as observational studies might be necessary with thousands of observations.
-
Evaluation addressing which of the 3 populations (pregnant women, breastfeeding mothers, and infants) should receive vitamin D – whether there is a larger benefit with supplementation in one or a combination of these populations and, if so, which populations to target.
References
Della Giustina A, Landi M, Bellini F, Bosoni M, Ferrante G, Onorari M, et al. Vitamin D, allergies and asthma: focus on pediatric patients. World Allergy Organ J. 2014;7(1):27.
Reinholz M, Ruzicka T, Schauber J. Vitamin D and its role in allergic disease. Clin Exp Allergy. 2012;42(6):817–26.
Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol. 2011;64(4):395–400.
Fiocchi A, Pawankar R, Cuello-Garcia C, Ahn K, Al-Hammadi S, Agarwal A, et al. World Allergy Organization-McMaster University Guidelines for Allergic Disease Prevention (GLAD-P): Probiotics. World Allergy Organ J. 2015;8(1):4.
Fiocchi A, Brozek J, Schunemann H, Bahna SL, von Berg A, Beyer K, et al. World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow's Milk Allergy (DRACMA) Guidelines. Pediatr Allergy Immunol. 2010;21 Suppl 21:1–125.
Brozek JL, Akl EA, Compalati E, Kreis J, Terracciano L, Fiocchi A, et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines part 3 of 3. The GRADE approach to developing recommendations. Allergy. 2011;66(5):588–95.
Brozek JL, Akl EA, Jaeschke R, Lang DM, Bossuyt P, Glasziou P, et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines: Part 2 of 3. The GRADE approach to grading quality of evidence about diagnostic tests and strategies. Allergy. 2009;64(8):1109–16.
Brozek JL, Akl EA, Alonso-Coello P, Lang D, Jaeschke R, Williams JW, et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines. Part 1 of 3. An overview of the GRADE approach and grading quality of evidence about interventions. Allergy. 2009;64(5):669–77.
Schunemann HJ, Wiercioch W, Etxeandia I, Falavigna M, Santesso N, Mustafa R, et al. Guidelines 2.0: systematic development of a comprehensive checklist for a successful guideline enterprise. CMAJ. 2014;186(3):E123–42.
Cuello-Garcia CA, Fiocchi A, Pawankar R, Yepes-Nuñez JJ, Morgano GP, Zhang Y, et al. World Allergy Organization-McMaster University Guidelines for Allergic Disease Prevention (GLAD-P): Prebiotics. World Allergy Organ J. 2015. In press.
Haahtela T, von Hertzen L, Makela M, Hannuksela M, Allergy Programme Working G. Finnish Allergy Programme 2008-2018--time to act and change the course. Allergy. 2008;63(6):634–45.
Arshad SH, Kurukulaaratchy RJ, Fenn M, Matthews S. Early life risk factors for current wheeze, asthma, and bronchial hyperresponsiveness at 10 years of age. Chest. 2005;127(2):502–8.
Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. 2014;348:g2035.
Peroni DG, Boner AL. Food allergy: the perspectives of prevention using vitamin D. Curr Opin Allergy Clin Immunol. 2013;13(3):287–92.
NICE. Vitamin D: increasing supplement use among at-risk groups. National Institute for Health and Care Excellence; 2014. [updated January 30, 2016]. Available from: https://www.nice.org.uk/guidance/ph56.
Panel NI-SE, Boyce JA, Assa'ad A, Burks AW, Jones SM, Sampson HA, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6 Suppl):S1–58.
GRADEpro GDT. GRADEpro Guideline Development Tool [Software]. McMaster University, 2015 (developed by Evidence Prime, Inc.). Available from gradepro.org.
Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. http://handbook.cochrane.org/. Accessed 30 Jan 2016.
Sterne JAC, Higgins JPT, Reeves BC on behalf of the development group for ACROBAT-NRSI. A Cochrane Risk Of Bias Assessment Tool: for Non-Randomized Studies of Interventions (ACROBAT-NRSI), Version 1.0.0, 24 September 2014. Available from https://sites.google.com/site/riskofbiastool/. [01 December 2014].
Goldring ST, Griffiths CJ, Martineau AR, Robinson S, Yu C, Poulton S, et al. Prenatal vitamin d supplementation and child respiratory health: a randomised controlled trial. PLoS One. 2013;8(6):e66627.
Brooke OG, Brown IR, Bone CD, Carter ND, Cleeve HJ, Maxwell JD, et al. Vitamin D supplements in pregnant Asian women: effects on calcium status and fetal growth. Br Med J. 1980;280(6216):751–4.
Hossain N, Kanani FH, Ramzan S, Kausar R, Ayaz S, Khanani R, et al. Obstetric and neonatal outcomes of maternal vitamin D supplementation: results of an open-label, randomized controlled trial of antenatal vitamin D supplementation in Pakistani women. J Clin Endocrinol Metab. 2014;99(7):2448–55.
Roth DE, Al Mahmud A, Raqib R, Akhtar E, Perumal N, Pezzack B, et al. Randomized placebo-controlled trial of high-dose prenatal third-trimester vitamin D3 supplementation in Bangladesh: the AViDD trial. Nutr J. 2013;12:47.
Mallet E, Gugi B, Brunelle P, Henocq A, Basuyau JP, Lemeur H. Vitamin D supplementation in pregnancy: a controlled trial of two methods. Obstet Gynecol. 1986;68(3):300–4.
Roth DE, Perumal N, Al Mahmud A, Baqui AH. Maternal vitamin D3 supplementation during the third trimester of pregnancy: effects on infant growth in a longitudinal follow-up study in Bangladesh. J Pediatr. 2013;163(6):1605–11. e3.
Yu CK, Sykes L, Sethi M, Teoh TG, Robinson S. Vitamin D deficiency and supplementation during pregnancy. Clin Endocrinol (Oxf). 2009;70(5):685–90.
Sablok A, Thariani K, Batra A, Bharti R, Aggarwal AR, Kabi BC, et al. Supplementation of Vitamin D in pregnancy and its correlation with feto-maternal outcome. Clin Endocrinol (Oxf). 2015;83(4):536–41.
Allen KJ, Koplin JJ, Ponsonby AL, Gurrin LC, Wake M, Vuillermin P, et al. Vitamin D insufficiency is associated with challenge-proven food allergy in infants. J Allergy Clin Immunol. 2013;131(4):1109–16, 16 e1–6.
Anderson LN, Chen Y, Omand JA, Birken CS, Parkin PC, To T, et al. Vitamin D exposure during pregnancy, but not early childhood, is associated with risk of childhood wheezing. J Dev Origins Health Dis. 2015;6(4):308–16.
WHO. Guideline: Vitamin D supplementation in pregnant women. Geneva: World Health Organization; 2012.
Wagner CL, Greer FR, American Academy of Pediatrics Section on B, American Academy of Pediatrics Committee on N. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122(5):1142–52.
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–30.
Agostoni C, Buonocore G, Carnielli VP, De Curtis M, Darmaun D, Decsi T, et al. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2010;50(1):85–91.
Muraro A, Halken S, Arshad SH, Beyer K, Dubois AE, Du Toit G, et al. EAACI food allergy and anaphylaxis guidelines. Primary prevention of food allergy. Allergy. 2014;69(5):590–601.
Abrams SA, Committee on N. Calcium and vitamin d requirements of enterally fed preterm infants. Pediatrics. 2013;131(5):e1676–83.
Bener A, Ehlayel MS, Tulic MK, Hamid Q. Vitamin D deficiency as a strong predictor of asthma in children. Int Arch Allergy Immunol. 2012;157(2):168–75.
Rothberg AD, Pettifor JM, Cohen DF, Sonnendecker EW, Ross FP. Maternal-infant vitamin D relationships during breast-feeding. J Pediatr. 1982;101(4):500–3.
Alonso A, Rodriguez J, Carvajal I, Prieto MA, Rodriguez RM, Perez AM, et al. Prophylactic vitamin D in healthy infants: assessing the need. Metabolism. 2011;60(12):1719–25.
Greer FR, Searcy JE, Levin RS, Steichen JJ, Steichen-Asche PS, Tsang RC. Bone mineral content and serum 25-hydroxyvitamin D concentrations in breast-fed infants with and without supplemental vitamin D: one-year follow-up. J Pediatr. 1982;100(6):919–22.
Ponnapakkam T, Bradford E, Gensure R. A treatment trial of vitamin D supplementation in breast-fed infants: universal supplementation is not necessary for rickets prevention in Southern Louisiana. Clin Pediatr. 2010;49(11):1053–60.
Madar AA, Klepp KI, Meyer HE. Effect of free vitamin D(2) drops on serum 25-hydroxyvitamin D in infants with immigrant origin: a cluster randomized controlled trial. Eur J Clin Nutr. 2009;63(4):478–84.
Chan GM, Roberts CC, Folland D, Jackson R. Growth and bone mineralization of normal breast-fed infants and the effects of lactation on maternal bone mineral status. Am J Clin Nutr. 1982;36(3):438–43.
Hypponen E, Sovio U, Wjst M, Patel S, Pekkanen J, Hartikainen AL, et al. Infant vitamin d supplementation and allergic conditions in adulthood: northern Finland birth cohort 1966. Ann N Y Acad Sci. 2004;1037:84–95.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The following are potential conflicts of interest (within last 4 years) for the guideline panel members and authors of this work.
Kangmo Ahn; declares no competing interest related to this guideline.
Suleiman Al-Hammadi; received honoraria for speakers bureau, giving talks, and sponsorship at meetings from Danone Nutrition.
Kirsten Beyer; consult as technical advisor for DuPont, Unilever and Danone.
Jan Brożek; received research support from WAO for development of DRACMA and GLAD-P guidelines.
Wesley Burks (not present during the meeting); current consulting agreements with: Dynavax Technologies Corp., Genalyte, GLG Research, Perrigo Company, Regeneron Pharmaceuticals, NIH Grant support c. Abbott Laboratories, Levine's Children's Hospital, Mylan Speciality, Perosphere, Inc. Past consulting agreements: ActoGeniX, Curalogic, Dow AgroSciences, ExploraMed Development, McNeill Nutritionals, Merck, Novartis Pharma AG, Sanofi-Aventis US, Schering Plough, Unilver.
Giorgio Walter Canonica (not present); declares no competing interest related to this guideline.
Carlos Cuello-García; declares no competing interest related to prebiotics. Part of his PhD scholarship and travel support for meetings is supported by WAO.
Motohiro Ebisawa (not present during the meeting); declares no competing interest related to this guideline.
Alessandro Fiocchi; received research support from Danone. Has received travel support for meetings for presentation of DRACMA guidelines in South East Asia from Danone Malaysia, and support for travel and honorarium for speaking from Ordesa Group.
Rose Kamenwa; declares no competing interest related to this guideline.
Bee Wah Lee (not present during meeting); received research support from Abbott Nutrition, Danone Nutrition, Nestle Nutrition Honoraria for speakers bureau, giving talks, sponsorship at meetings.
Haiqi Li (not present during meeting); declares no conflicts of interest related to this guideline.
Ruby Pawankar; received support from Danone Asia Pacific for act as a speaker at a Food allergy Asia Pacific symposium 2012.
Susan Prescott; Advisory Board - Nestle Nutrition Institute (Australasia), Advisory Board for Danone (Asia Pacific), investigator initiated trial for which Danone provided prebiotic.
John Riva; declares no competing interest related to this guideline.
Lanny Rosenwasser; received research Grant from Novartis/Genentech Roche (finished in 2011). Advisory board and speakers board for Astra Zeneca and Novartis/Genentech (last applicable in 2012), 5 patents on the biology of IL-1 and it's uses.
Hugh Sampson; Consultant– Danone Research for Specialized Nutrition Scientific Advisory Committee.
Holger Schünemann; received research support from WAO for development of DRACMA and GLAD-P guidelines.
Michael D. Spigler (not present during meeting); declares no competing interest related to this guideline.
Luigi Terracciano (not present during meeting); Heinz-Plada Italy Medical consultant for website. Travel support from World Allergy Organization.
Andrea Vereda; declares no competing interest related to this guideline.
Susan Waserman; declares no competing interest related to this guideline.
Juan José Yepes-Nuñez; declares no competing interest related to this guideline. Part of scholarship paid by WAO funding received by HJS and JLB for developing these guidelines.
Yuan Zhang; declares no competing interest.
Gian Paolo Morgano; declares no competing interest.
Arnav Agarwal; declares no competing interest.
Shreyas Gandhi; declares no competing interest.
Authors’ contributions
JJYN was involved in the methodological design of the guideline, conducted the acquisition of data, analyses, and interpretation; contributed in drafting the article and modified according to substantial feedback from the guideline panel members (authors). AF RP and HJS as Chair of the Guideline Panel, directed and organized guideline panel meetings, was involved in the initial concept and methodological design of the guideline, conducted data interpretation, and provided substantial feedback on the drafted manuscript. CACG was involved in the initial concept and methodological design of the guideline. Conducted the acquisition of data, analyses, and interpretation; drafted the article and modified according to substantial feedback from the guideline panel members (authors). GPM, YZ, AA and SG conducted data acquisition, analyses, and interpretation; provided sign6ificant feedback for the draft of the guideline. KA, SAH, KB, WB, GWC, ME, RK, BWL, HL, SP, JJR, LR, HS, MS, LT, AV and SW was involved in the analysis and interpretation of the evidence, drafting of the recommendations, and provided significant feedback for the final manuscript. JLB directed and organized guideline panel meetings and the methodologist team; was involved in the initial concept and methodological design of the guideline, conducted data acquisition and interpretation, and helped writing the final manuscript. All authors read and approved the final manuscript.
Additional files
Additional file 1:
Declaration of potential conflicts of interest (within last 4 years). (DOCX 500 kb)
Additional file 2:
Search strategies. (DOC 152 kb)
Additional file 3:
Characteristics of included studies. (DOC 124 kb)
Additional file 4:
Evidence profiles. (DOCX 79 kb)
Additional file 5:
Evidence to Decision table. (DOC 196 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Yepes-Nuñez, J.J., Fiocchi, A., Pawankar, R. et al. World Allergy Organization-McMaster University Guidelines for Allergic Disease Prevention (GLAD-P): Vitamin D. World Allergy Organ J 9, 17 (2016). https://doi.org/10.1186/s40413-016-0108-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40413-016-0108-1