Abstract
Voltage-gated sodium channels (VGSCs) initiate action potentials in electrically excitable cells and tissues. Surprisingly, some VGSC genes are aberrantly expressed in a variety of cancers, derived from “non-excitable” tissues that do not generate classic action potentials, showing potential as a promising pharmacological target for cancer. Most of the previous review articles on this topic are limited in scope, and largely unable to provide researchers with a comprehensive understanding of the role of VGSC in cancers. Here, we review the expression patterns of all nine VGSC α-subunit genes (SCN1A-11A) and their four regulatory β-subunit genes (SCN1B-4B). We reviewed data from the Cancer Genome Atlas (TCGA) database, complemented by an extensive search of the published papers. We summarized and reviewed previous independent studies and analyzed the VGSC genes in the TCGA database regarding the potential impact of VGSC on cancers. A comparison between evidence gathered from independent studies and data review was performed to scrutinize potential biases in prior research and provide insights into future research directions. The review supports the view that VGSCs play an important role in diagnostics as well as therapeutics of some cancer types, such as breast, colon, prostate, and lung cancer. This paper provides an overview of the current knowledge on voltage-gated sodium channels in cancer, as well as potential avenues for further research. While further research is required to fully understand the role of VGSCs in cancer, the potential of VGSCs for clinical diagnosis and treatment is promising.
Similar content being viewed by others
Voltage-gated sodium channels
In recent years, ion channels have emerged as a promising new target for cancer management [1,2,3,4,5,6,7,8,9,10,11,12,13,14]. Many cancer cells and tissues possess a wide range of ion channels and these may be involved in various stages of cancer development, progression, and response to treatment. The membrane potential of cancer and non-cancer cells differ [15, 16]. It has been reported that membrane depolarization facilitates cell proliferation, through actions on initiation of mitosis and DNA synthesis [17, 18]. Interestingly, some tumor tissues have a higher concentration level of sodium ions than their normal tissues, whereas their potassium ion concentrations were similar [10, 19,20,21]. This suggests that intracellular sodium ions may be partially determining the abnormal membrane potentials in cancer cells. Therefore, sodium permeable channels might play a critical role in cancers.
Voltage-gated sodium channels (VGSCs) are transmembrane proteins that increase the permeability of sodium ions across membranes. The permeability of these channels depends on the voltage drop across the membrane. In a typical neuronal action potential, VGSCs remain closed until the membrane potential reaches a threshold, at which point they transiently become permeable to sodium ions. The resulting influx of sodium ions leads to membrane potential depolarization, which regeneratively triggers the opening of more sodium channels, further depolarizing the membrane potential. Within milliseconds, the sodium channels transition to an ion-impermeable inactivated state, while potassium channels are activated. Both events contribute to the restoration of the resting membrane potential [22].
Mammalian Nav channels are formed by a large pseudo-tetrameric pore-forming α-subunit (260 kDa) that can associate with one or more β-subunits (30–40 kDa) (Fig. 1A&B). The α subunit has four homologous domains (DI-IV), each containing six transmembrane helices (S1-6). The S1-4 form the voltage-sensing module, which responds to membrane potential changes, while the S5-6 helices of each of the DI-IV form the pore module (Fig. 1C). To date, a total of nine types of Nav channel α subunit isoforms (Nav1.1, Nav1.2, Nav1.3, Nav1.4, Nav1.5, Nav1.6, Nav1.7, Nav1.8, and Nav1.9) and four types of β subunit isoforms (β1, β2, β3, and β4) have been identified in different human tissues [23, 24] (Fig. 1D). Generally, Nav channel α subunits are divided into two groups, Tetrodotoxin (TTX)-sensitive (Nav1.1, Nav1.2, Nav1.3, Nav1.4, Nav1.6, and Nav1.7) and TTX-resistant (Nav1.5, Nav1.8, and Nav1.9), based on their electrophysiological properties in the presence of the blocker TTX. Neonatal alternative splice variants of the α subunit have been found in some cancer types, such as neonatal Nav1.5 (nNav1.5) which differ from the adult isoform near the S3-S4 linker of Domain I (Fig. 1C). VGSC activation has been suggested to be associated with cancer, leading to an increased interest in this area of research. Despite the potential implications of this connection, the state of research in this field has been disorganized, necessitating an up-to-date summary.
Voltage-gated sodium channels (VGSC). A An example structure of the VGSC α-subunit/β-subunit protein complex. B Cartoon illustration of VGSC α-subunit and β-subunit proteins within the membrane. C Topology diagram of VGSC α-subunit and β-subunit proteins. D Protein and gene names of VGSC molecules. Images in Figures B and C were created using using the BioRender.com
Overview of previous studies on VGSC in cancers
Following the PRISMA guidelines [25], articles in English in the PubMed database were reviewed up to September 2023 that provided evidence of voltage-gated sodium channels in cancer. Some articles were not reviewed, such as papers studying local anesthetics on cancer cells [26], because they might have additional pharmacological targets [4]. The experimental evidence of VGSC in cancer has been reported since 1995. In the past 10 years, there have been approximately 2–6 research articles published each year in the field except for 2019 which has 11 papers published (S-Fig. 1). It is noteworthy that nearly a quarter of publications on the role of VGSCs in cancer consist of reviews and commentaries [27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47]. This suggests that, while the topic is popular and widely discussed, there are a relatively limited number of experimental studies. Almost all of the previous reviews and commentaries focus on VGSC α-subunits with a few mentioning the β subunits. There are 6 reviews specifically devoted to VGSC in breast cancers [27,28,29,30,31,32], 3 of which focus on Nav1.5 [28, 29, 32]. There are 6 previous reviews that summarized the effect on cancer migration or invasion [27, 29, 32,33,34,35,36], 4 on drugs [30, 37,38,39], and 2 on cancer immune [28, 40]. Notably, commentary on the role of VGSC in cancers [18] was published as early as 1986, predating the first research article on the same topic by nine years. This demonstrates that researchers had an interest and were proposing hypotheses regarding the role of VGSC in cancers long before any experimental data was available. Most of the previous review articles on this topic are limited in scope, and largely unable to provide researchers with a comprehensive understanding of the role of VGSC in cancers. A review of the summary of VGSC overexpression mixed in vivo and in vitro cell lines results [37] overlooked the difference between cancer tissues and cancer cell lines. Other papers have summarized in vivo studies only not including the β subunits [33] or proposed hypotheses without a systematic summary [41], and do not provide an in-depth analysis of the effects of VGSC overexpression in both in vivo and in vitro settings. Furthermore, a systematic review of the VGSC inhibitors for cancer [30] was published 8 years ago and an in-time update is now required to summarize VGSC drugs for cancers in papers since then.
In vitro evidence of VGSCs in cancers
The in vitro studies of VGSCs in cancers collected from the published papers are summarized in Table 1. Breast cancer is the most commonly reported cancer type, followed by prostate cancer, cervical cancer, and colon cancer. Most studies reported the presence of Nav1.5 and Nav1.7 VGSC subtypes, while the β-subunits are rarely reported. Generally, VGSCs were found to promote cancer cell migration and invasion, with a few studies suggesting an effect on cell proliferation.
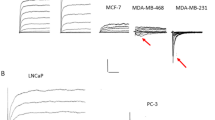
Breast cancer is the most commonly reported cancer type, and numerous studies have demonstrated the expression of Nav1.5 in this type of cancer [32,33,34,35,36,37,38,39,40,41, 48,49,50,51,52,53,54,55,56,57,58,59,60,61]. The MDA-MB-231 cell line is the most extensively studied breast cancer cell line, and evidence suggests that Nav1.5 and α-subunits are involved in the modulation of migration, invasion, proliferation, and chemosensitivity [32,33,34,35,36,37,38,39,40,41, 48,49,50,51,52,53,54,55,56,57,58,59,60,61]. Possible mechanisms of action include alteration in sodium currents, matrix metalloproteinase type 9 activity, Ki67 activity, and repressor element silencing transcription factor and histone deacetylases. Note that the Nav1.5 discovered in breast cancer is the neonatal form. The expression of neonatal Nav1.5 protein in breast cancer has been reported in relation to ERα status [42]. Neonatal Nav1.5 protein was not detected in the human brain, skeletal muscle, cardiac muscle, colon, small intestine, stomach, prostate, or bladder tissues [42]. The reason for the low-level neonatal Nav1.5 immunoreactivity observed in normal breast tissues remains unclear. Notably, neonatal Nav1.5 protein was significantly elevated in breast cancer compared to normal breast tissue, indicated by a two-fold increase in staining intensity and a 20-fold increase in the area of stained ductal structures [42]. These findings significantly build on cell- [32] and tissue-based studies [43]. The difference in the Nav1.5 expression pattern indicates that distinct cancer cells or distinct cell types activate various signaling pathways during growth, leading to the expression of different Nav channels on the cell membrane. Moreover, the pattern of neonatal Nav1.5 immunoreactivity in the plasma membrane became asymmetrical reflected by the increase in the apical/basal ratio value in the breast cancer. This is a noteworthy observation since cancer cells generally lose their polarity during metastasis [62]. Consequently, VGSC may exhibit unique behavior compared to other proteins in the plasma membrane, potentially due to its crucial role in the inherently directional metastatic process.
In the case of colon cancer, HCT116, HT29, SW480, and SW620 cell lines have been used, and they all express the Nav1.5 subtype [45,46,47,48,49]. It has been found that this subtype mediates cancer invasion by increasing sodium currents. Additionally, in SW480 and DLD1 cell lines, it has been suggested that Nav1.5 mediates proliferation, migration, and invasion, and increases chemosensitivity through the cell cycle, epithelial-mesenchymal transition, and Ras signaling pathways [45,46,47,48,49]. For ovarian cancer, the α-subunits may mediate proliferation and enhance chemosensitivity [50,51,52]. For endometrial cancer, primary cancer cells have been studied, and it has been found that Nav1.7 could mediate invasion and reduce apoptosis [53]. In prostate cancer cells PC3 α-subunits may mediate proliferation, migration, and invasion through the cell cycle and glucose uptake [54,55,56,57,58,59,60, 63]. Additionally, for gastric cancer, it has been found that Nav1.7 in BGC-823 and MKN-28 cell lines could mediate proliferation and invasion by regulating extracellular and intracellular pH via increased Na + /H + exchanger-1 [64]. Research into cervical cancer has suggested that Nav1.5, Nav1.6, Nav1.7, and β1-4 aid in the proliferation, migration, and invasion of SiHa, CaSki, and HeLa cell lines [51, 65,66,67]. Lung cancer studies have determined that Nav1.6 and Nav1.7 mediate invasion, and β1 and β3 are expressed in cancer cells using H23, H460, and Calu-1 cell lines [68,69,70]. NCI-H146 cell line has been used to investigate the effects of α-subunits on cancer cells. Interestingly, Nav1.7 has been found in thyroid cancer cell lines MZ-CRC-1 and TT, which mediates migration by its sodium currents [71, 72].
In vivo evidence of VGSCs in cancers
Preclinical in vivo studies bearing on VGSCs in cancers collected from the published papers were summarized in Table 2. A number of different animal models have been used to study VGSCs in cancers, including mice and rat models. Both allograft and xenograft models have been applied, with most interference achieved either by VGSC knockdown in the cells used for modeling or by using VGSC inhibitors or activators [34, 46, 48, 51, 54, 60, 61, 64, 75,76,77,78,79,80]. Notably, a study using Pulsed Magnetic Field Stimulation to interfere with VGSC currents achieved quite promising results [81]. Similar to the in vitro studies, the most commonly studied cancer type is breast cancer [34, 48, 60, 61, 76, 77, 81], followed by prostate cancer [54, 78,79,80]. Most studies reported the expression of Nav1.5 and Nav1.7 VGSC subtypes in cancers, while the β-subunits were only reported in two studies, for β1 [34] and β3 [75] respectively. Most of the animal model studies implicated that the VGSCs mediated tumor growth with some suggesting also metastasis.
In breast cancer models such as 4T1-BALB/c mice and MDA-MB-231-J/Nu mice, interventions like intravenous administration of Anti-Neonatal Nav1.5 Antibodies [48] and Pulsed Magnetic Field Stimulation [81], directed respectively at Nav1.5 or pan-α-subunits, have emerged as strategies. These interventions have consistently demonstrated effects on tumor growth and metastasis, underscoring the significance of VGSCs in breast cancer progression [34, 60, 61, 76, 77]. Similarly, in prostate cancer models such as Mat-LyLu-Copenhagen rats and PC3-BALB/c mice, interventions including gavage ranolazine and subcutaneous injection of tetrodotoxin have shown promise in altering cancer progression by targeting Nav1.7 or pan-α-subunits, respectively [54, 78,79,80]. Furthermore, investigations into colon, gastric, ovarian, and liver cancer have utilized various interventions, from intratumoral injections of Nav1.5 antibodies to knockdown of β3 Nav1.7 in cells, reflecting the diverse approaches employed to modulate VGSCs across different cancer types [46, 51, 64, 75]. Overall, these experiments highlighted crucial insights into the therapeutic potential of targeting VGSCs in cancer treatment, underscoring their role in driving cancer metastasis and growth across a spectrum of malignancies.
Clinical in vivo evidence of VGSCs in cancers
Clinical in vivo studies of VGSCs in cancers collected from the published papers are summarized in Table 3. In the clinical studies, colon cancer was the most prevalent [45, 46, 82,83,84,85,86,87], followed by breast cancer [32, 34, 42, 61, 82, 83] and prostate cancer [56, 60, 82, 83, 88]. Most studies focused on the α-subunits [32, 42, 45, 46, 52, 53, 56, 61, 64, 66, 73, 82,83,84,85,86,87,88], with only two reported β subunits [34] [60], where Nav1.5 was the most reported subtype [32, 42, 45, 52, 61, 73, 84, 85, 88]. For studies that determined the expression of VGSC in cancer tissue and compared it with normal tissue, almost all of them suggested overexpression of the α-subunits in cancers [32, 34, 42, 45, 52, 53, 56, 61, 64, 66, 73, 84,85,86, 88]. Eight studies suggested that VGSC was a risk factor for cancer [45, 53, 56, 64, 82, 83, 86], with four suggesting it was not associated with survival [34, 60, 61, 66]. Three of the studies involved VGSC inhibitors [82, 83, 87]. Despite the non-specificity, these studies interpreted the effects of VGSC inhibitors on cancer by proposing the role of VGSCs in cancers. These studies mostly did not specify the exact effect of VGSCs. The other studies suggested that VGSCs mediate metastasis or chemosensitivity.
Specifically, in breast, prostate, and colon cancer, VGSC-specific drugs have been associated with improved survival [45, 53, 56, 64, 82, 83, 86], suggesting that VGSCs may be involved in these cancers. In endometrial, cervical, and ovarian cancers, VGSCs have been found to increase metastasis [32, 53, 66, 85]. In addition, in breast and colon cancer, Nav1.5 has been found to be overexpressed [32, 42, 45, 61, 84], and this has been associated with an increased risk for cancer patients in colon cancer [45, 84, 86]. Lastly, in breast cancer, Nav1.5 has been found to mediate lymph node metastasis [32] and to associate with estrogen receptor-β expression [42].
Insight from data reviewing
The data review included 9 α-subunit and 4 β-subunit of VGSC as shown in Fig. 1D and 33 types of cancers in The Cancer Genome Atlas (TCGA) as shown in S-Table 1 where the abbreviations were also listed. Data includes TCGA, Genotype-Tissue Expression (GTEx), and the Human Protein Atlas (HPA). In the transcriptomic analysis, a limitation is that several studies have reported the presence of the neonatal splice variant of Nav1.5 in cancer [32, 89, 90]. There is a difference of six amino acids between the neonatal Nav1.5 and normal Nav1.5, and the two splice variants have been shown to be pharmacologically distinct [89]. However, given that both variants are over 2000 amino acids long, using the adult Nav1.5 as a reference for sequencing and quantifying gene expression levels in this study might not result in any major differences.
Single nucleotide variant (SNV) profile of VGSC in TCGA
Based on analysis of TCGA data, the top five cancer types with mutations in VGSC genes (average rank in each cancer type) were skin cutaneous melanoma (SKCM), uterine corpus endometrial carcinoma (UCEC), colon adenocarcinoma (COAD), lung squamous cell carcinoma (LUSC), and stomach adenocarcinoma (STAD). The top 5 mutated VGSC genes in cancers are SCN1A, SCN9A, SCN11A, SCN2A, and SCN3A (S-Fig. 2A). The overall gene alteration frequency of both SCN1A and SCN10A is 22%, while that of SCN11A, SCN2A, and SCN5A is 20%. The most frequent variant classification is a missense mutation followed by a nonsense mutation and frameshift deletion. The most frequent variant type is a single nucleotide polymorphism (SNP). The commonest SNP class is C > T followed by C > A and T > C (S-Fig. 2B). However, all of the mutations occurred at a lower than 25% mutation frequency (S-Fig. 2A). In addition, survival analysis revealed that only a few VGSC mutations had a significant effect on overall survival, such as SCN3B in breast cancer (BRCA); however, this result should be interpreted with caution due to the low case number of the BRCA cohort, with only three cases of SCN3B mutation in BRCA (S- Fig. 2C).
VGSC α-subunits are indeed lengthy proteins with over 2,000 amino acid residues, which means they encompass a substantial number of structural elements where random mutations can occur. As a result, generally, they are more susceptible to a higher overall mutation rate compared to shorter proteins. The TCGA data suggested that the overall mutation rate of VGSC in cancers was relatively low. Uterine corpus endometrial carcinoma (UCEC) and skin cutaneous melanoma (SKCM) are the two cancer types with a few gene alteration frequencies greater than 20%. Despite this, the data from TCGA does not provide evidence to support the critical role of VGSC mutation in cancers. To date, no studies have reported a significant association between VGSC mutation and cancer, which is in line with the TCGA mining results.
Expression profiles of VGSC in TCGA
This study compared the expression of VGSC in cancer and normal tissues across 33 cancer types from TCGA data. In the analysis of cancer-noncancer differences, two approaches were employed: (1) an unpaired t-test was used to compare cancer samples from TCGA with normal samples from both TCGA and the GTEx databases (S-Fig. 3); (2) a paired t-test was used to compare TCGA-paired cancer-normal samples from the same patient (S-Fig. 4). MESO and UVM were excluded from the first approach because there are no corresponding normal tissues for comparison. Thus, only the expression of cancer was displayed for MESO and UVM. The second approach excluded many cancer types due to the lack of paired normal samples. Additionally, a few cancer types had extremely low case numbers, such as SARC, SKCM, and THYM, and were only included for reference. The results of this analysis provide a systematic profile and important insights into the differences between cancer and normal tissue at the molecular level.
The Human Protein Atlas (HPA) database provides protein-staining data for SCN2A, SCN3A, SCN9A, SCN11A, and SCN4B. The reliability of the staining results was found to be limited due to the non-optimized experimental conditions and the undesirable properties of the antibodies. However, these data have been included in the present study for reference purposes. Representative images were displayed in S-Fig. 5. It should be noted that only the relevant gene-cancer data reported from literature studies have been presented, which facilitated for comparison at the end of this review.
Distinct cancer cells activate various signaling pathways during growth, leading to the expression of different Nav channels on the cell membrane. Currently, the field is focused on demonstrating the impact of Nav channels on cancer, rather than investigating the regulation of Nav channel gene expression. Therefore, the pathways or mechanisms regulating Nav channel gene expression in cancer cells remain unclear. To understand the regulation of VGSC in cancers, this study conducted an in-depth analysis to provide a systematic profile of the three expression-related aspects: copy number, methylation, and microRNAs (miRNAs). Specifically, the copy number variant (CNV) profiles of VGSC in cancers were displayed, presenting the percentage of different types of CNV and data of both heterozygous CNV and homozygous CNV (S-Fig. 6). Furthermore, the correlation of CNV/expression and correlation of methylation/expression were evaluated to explore the potential impact of CNV and methylation on expression (S-Fig. 7). In addition, miRNAs play a significant role in the regulation of cancer cells, influencing various aspects of cancer progression such as proliferation, invasion, and metastasis, potentially through the mediation of Nav channel expression. Here, we constructed the regulatory relationships and presented the miRNA-gene expression correlations in a network plot, based on data collected from databases. (S-Fig. 8). These profiles help to understand how VGSC expression is regulated in cancers and may be of great utility for future studies.
Clinical association of VGSC in TCGA
The clinical association of each of the VGSC genes across 33 cancer types in TCGA was systematically analyzed in terms of survival association, immune subtype association, and molecular subtype association. The survival analysis, compared patients with high (above median) and low (below median) expression of the gene in the respective cancer type and calculated hazard ratios. Investigations of the immune and molecular subtype associations compared the expression levels of VGSCs between different subtypes. Detailed analysis results are presented in S-Fig. 9.
Evidence comparison for key questions on VGSC in cancers
Bioinformatic evidence derived from high-throughput data and evidence from published data both offers insights, though both sources have their limitations. Therefore, it is prudent to combine and compare both to obtain a comprehensive understanding of the topic. A previous study applied similar principles to investigate the role of TRPM7 in cancer [1].
Expression of VGSCs in cancers
VGSCs have been observed in many cancer cell lines (Table 4 column 14), providing a foundation for in vitro studies to explore the role of VGSCs in cancers. Although cell line models are very helpful for studying VGSC function in cancers, results from cancer and normal cell lines may not be directly comparable with tumor cells from clinical patients. Much as VGSCs have not been reported in some cancer types, the available studies suggest a greater VGSCs expression in cancer compared to normal tissues (Table 4 column 4). However, the TCGA data suggested a VGSC underexpression in tumor tissues of many cancer types, including colon cancer, prostate cancer, and breast cancer (Table 4 columns 6 and 7). The HPA protein staining data is generally in line with the TCGA data (Table 4 column 8).
Such inconsistencies might result from biases in pre-hypothesis studies from the experiments or inherent biases in the TCGA bulk sequencing data. A major issue with much of the TCGA data is that it relies on bulk RNAseq, while the tumor microenvironment is highly heterogeneous. For example, VGSCs may only be expressed in a small subpopulation of cells within the tumor microenvironment, resulting in 'low' overall expression in the bulk sample. In certain cancer types, a notable limitation arises due to the potential incongruence between the origins of normal tissue and the tumor tissue. For example, in the context of glioma, the comparison between cancer and normal tissues in this study might be inaccurately portrayed. The normal bulk sample utilized encompasses brain tissue more broadly, rather than specifically targeting glial cells where glioma is derived. It is essential to emphasize that the majority of cells in brain tissue are neurons, and these neurons may express high levels of VGSCs. This expression pattern within neurons could potentially introduce a confounding factor, influencing the accuracy and interpretation of the analysis.
Another issue with TCGA data is that it only provides an indication of transcript levels, missing any post-transcriptional or post-translational regulation of these ion channels. Therefore, the channels may be present and functional even if bulk transcript levels are relatively low. Emerging technologies, such as single-cell RNAseq and spatial transcriptomics, will hopefully help resolve these contradictions in the future. Nevertheless, the aberrant expression levels of VGSCs in some cancer types in the RNA sequencing data, whether overexpressed or underexpressed, support their potentially different roles in cancer and normal tissues, and they could still be potential clinical diagnostic biomarkers with RNA sequencing.
Survival associations of VGSCs in cancers
Expression and survival are two of the most fundamental pieces of information provided by TCGA. Despite a few studies that have reported the risky prognostic effects of VGSCs in cancer(Table 4 column 5), the TCGA data suggests that the most commonly studied VGSC genes do not associate with patient survival (Table 4 column 9). The inconsistency between the published papers and TCGA reflects the potential bias in the VGSC-cancer studies. Notably, the SCN5A gene, the most studied VGSC in breast cancer, was reported as a risk factor in one study and not associated with altered survival in another study. However, the TCGA data suggests that SCN5A is associated with improved survival in TCGA breast cancer. As shown in the Kaplan–Meier Plots, TCGA data suggests that SCN5A is slightly associated with better overall survival, disease-specific survival, and progress-free interval. The GEO data (accessed from the Kaplan–Meier Plotter) validated that SCN5A is associated with better overall survival and progress-free interval [91]. The GEO data also suggested that better post-progression survival is also associated with high SCN5A [91]. However, the higher SCN5A expression is associated with distant metastasis-free survival in breast cancer [91]. This clinical data indicates that SCN5A might contribute to breast cancer metastasis, and this conclusion aligns with many in vitro studies.
Functional impact of VGSCs on cancer cells
Preclinical studies in vitro and in vivo have provided functional evidence for the involvement of VGSCs in cancer cell proliferation, migration, and invasion (Table 4 columns 15, 16, and 17). The most studied VGSC, Nav1.5, has been studied in the context of breast and colon cancers. More than 12 papers with in vitro studies on Nav1.5 in breast cancers suggested the channel could facilitate cell migration and invasion, with four studies additionally reporting increased cancer cell proliferation. The role of Nav1.5 in breast cancer cell migration and invasion in vitro has been observed to correlate with the results of a clinical distant metastasis-free survival analysis in breast cancer from GEO data (accessed from the Kaplan–Meier Plotter) (S-Fig. 10). In colon cancer, Nav1.5 was also shown to enhance the invasion of cancer cells. Although most in vitro studies suggested that the VGSC α-subunit functions in cell migration, preclinical in vivo studies have suggested that the α-subunit of VGSC can facilitate tumor growth, with a few also suggesting it could facilitate metastasis. Fewer studies have been conducted on VGSC β-subunits; these have indicated that the β1 subunit could affect cervical cancer cell proliferation and that the β4 subunit could affect invasion. Additionally, the β3 subunits were reported to potentially affect cancer cell apoptosis in liver cancer. However, despite preclinical evidence suggesting a promising role of VGSCs in cancer, caution should be exercised in interpreting the implications of these data, because, as mentioned, large-scale datasets TCGA indicate that VGSC expression is generally lower in cancer than in normal tissues and that the expression of VGSCs generally does not associate with patient survival. Given the bias in RNA sequencing data, it remains unclear if these functional impacts of VGSCs on cancer cells could make a clinically meaningful difference in cancer patients. Nevertheless, One consistent result between experimental evidence and clinical evidence is that VGSCs can impact cancer migration.
Possible mechanisms underlying the impact of VGSCs on cancer
Persistent sodium current
Although membrane potentials in cancer cells can be dynamic and oscillating [92], the resting membrane potential of cancer cells has been reported to range from -5 to -52 mV, and that of highly proliferating non-cancer cells falls within the range of -5 to -25 mV [15]. By contrast, in non-cancer cells, the resting membrane potential is typically between -95 and -40 mV [15]. In the presence of Nav channels, this might result in a larger persistent sodium current, leading to downstream sodium gradient cascades and activating various signaling pathways and sodium-associated transmembrane mechanisms, such as Na + /H + exchangers [45] and sodium/calcium exchanger [93].
An exciting progression in the field is the identification of ranolazine, a specific blocker for sodium channel persistent current in cancer cells [94], as a clinically viable anti-metastatic drug that improves cancer survival [76, 78, 95]. Interestingly, in contrast to ranolazine, sodium channel blockers such as lidocaine, that inhibit peak current do not enhance cancer survival [82, 83]. These studies, highlighting the distinct roles between sodium peak current and sodium persistent current in cancer [82] should be given more prominence.
Sodium and hydrogen
The sodium–hydrogen antiporter 1 (sodium–proton exchanger proteins, NHE1) is co-expressed with VGSCs and can increase intracellular alkalization and extracellular acidity. The acidic microenvironment of cancer cells promotes the degradation of the extracellular matrix by cysteine cathepsins [96], thereby facilitating cancer migration and invasion [38, 97, 98]. An allosteric interaction between Nav1.5 and NHE1 has been suggested to explain a Nav1.5-dependent increase in H+ extrusion by NHE1 [39]. For the cardiac subtype VGSC, Nav1.5, another additional possibility is that Na+ influx through Nav1.5, rather than the Nav1.5 protein itself, increases H+ extrusion through NHE1 and other pH regulators, thereby resulting in extracellular acidification [39]. The extracellular acidification facilitate the invasion of cells [45]. Moreover, low pH can positively regulate Nav1.5 function in cardiomyocytes by increasing the persistent Nav current carried by Nav1.5 [99, 100], which might also occur in cancer cells.
Sodium and calcium
Although the whole-cell Ca2+ release-activated Ca2+ current was reported to be independent of extracellular and cytosolic Na+ [101], sodium/calcium exchanger, a unique calcium transport system that typically exports calcium ions out of the cell in exchange for sodium ions [102], might play a role in tumors [103, 104]. The sodium/calcium exchanger is another energetically unfavorable physiological processes that known to be driven by sodium gradients [93]. The sodium/calcium exchanger facilitates the movement of sodium down its concentration gradient and calcium in the opposite direction. Typically, sodium, which is at a higher concentration in the extracellular matrix (ECM), is transported into the cell, while calcium is moved out. However, sodium/calcium exchanger can also operate in reverse mode, bringing calcium into the cell. Calcium ions are crucial for numerous physiological processes, including vesicle transport and exocytosis [105], signal transduction as secondary messengers [105], muscle contraction [106], and as cofactors in various biological reactions. A rapid influx of sodium ions through voltage-gated sodium channels (VGSC) might affect sodium/calcium exchanger activity, subsequently altering calcium handling for processes such as vesicle exocytosis or signal transduction in invadopodia. Data also suggested that sodium ion influx can activate intracellular calcium signaling pathway [107]. This increases the uptake of calcium ions by mitochondria and further leads to their release of calcium ions into the cytosol [108]. Higher calcium concentrations in the cytosol promote the formation of invadopodia, facilitating cell movement [109, 110]. This hypothesis is largely based on the observation of VGSCs in macrophage and microglial podosomes [111] but might contribute to cancer cell migration.
β subunits
As immunoglobulin (Ig) family cell-adhesion molecules, VGSC β subunits are proposed to regulate cell adhesion, but some studies report that β subunit subtypes regulate cancer migration and invasion in a range of different ways. In breast cancer cells, β1 expression was negatively associated with cancer metastasis [112], while in prostate cancer, overexpression of β2 was associated with an increase in cancer migration and invasion [113]. The expression of β4 was reported to be downregulated in breast cancer cells compared to that in non-cancer epithelial cells. Reduced β4 expression was reported to promote migration and invasion while overexpressed β4 did the opposite [114]. β3 expression was absent in two breast cancer cell lines [112], but took place in other cancers such as prostate cancer [60] and liver cancer [115]. A recent study revealed that β3 can bind to tumor suppressor p53 and facilitate the degradation of p53 protein in liver cancer [115]. Although some effects of the β subunit on cancers have been reported, the underlying mechanisms remain largely unknown.
Growth factor
VGSCs have been suggested to be involved in growth factor regulation in cancers. Epidermal growth factor (EGF) was reported to promote the migration and invasion of prostate and non-small cell lung cancer cells by increasing Nav1.7 expression [59, 68, 116]. The regulatory role of nerve growth factor (NGF) in prostate cancer was also found to be associated with the up-regulation of Nav1.7 [117, 118]. Furthermore, some growth factors that are critical in cancers have been found to interact with VGSCs in non-cancer cells. For example, vascular endothelial growth factor (VEGF), a key regulator for cancer angiogenesis [119], has been found to increase VGSC expression in the DRG neurons [120]. However, another critical regulator in cancer, transforming growth factor-beta 1 (TGF-β1) has a paradoxical role in cancers [121] and was upregulated when Nav1.5 was inhibited in cardiac myocytes and fibroblasts [122]. These inferred that the VGSCs might not necessarily provide growth signals directly through growth factors, but are involved in more complex regulatory mechanisms.
Hormones
A number of studies have also shown that VGSCs are closely associated with the secretion of hormones, that are critical for some cancer types such as breast cancer and prostate cancer. In cardiomyocytes, insulin response elements in the SCN5A promoter region can affect the expression of Nav1.5 [123]. In adrenal chromaffin cells and breast cancer cells, insulin was also reported to regulate VGSC expression [124, 125]. Interestingly, the expression of functional VGSCs was found to be potentially associated with the expression of estrogen receptors (ERs) in breast cancer cells [32] and the expression of androgen receptors (ARs) in prostate cancer cells [126, 127].
VGSC-targeting drugs for cancers
In previous studies concerning VGSCs in the context of cancer, several VGSC-targeting drugs have been employed for research in this field. Table 5 provides a summary of VGSC-targeting drugs used in cancer studies as reported in the published papers. Not surprisingly, Tetrodotoxin, the most classic VGSC blocker, is used in many studies. Local anesthetics, which primarily target and inhibit VGSCs, have also been widely utilized in VGSC-cancer studies [49,50,51, 128]. Additionally, antibodies [48, 51], toxins [32, 37, 41, 45, 48, 50, 52, 53, 56, 57, 59, 67,68,69,70, 74, 76, 78, 79, 94, 127, 129, 130], chemical small molecules [45], and natural products [51, 67, 131] have been applied in VGSC-cancer studies. This summary of drug effects and doses provides a reference for future studies targeting VGSC in cancer and serves as a guide for locating relevant studies.
However, there is a limitation in the field due to the non-specific nature of many drugs targeting Nav channels. Although some of these drugs might preferentially inhibit certain subtypes of Nav channels (such as TTX – see Introduction), their application is generally not specific to a single subtype but has a broad effect on multiple Nav channel subtypes. This lack of specificity may be overlooked by many studies, as most do not suggest the role of a single Nav channel subtype in cancer, but rather focus on the general Na currents that all subtypes can mediate [32, 37, 41, 45, 48, 50, 52, 53, 57, 59, 67,68,69,70, 74, 79]. For example, sodium current-targeting nerve growth factor was identified for prostate cancer cell lines, without distinguishing among subtypes [117]. These pan-VGSC drugs could lead to non-specific effects on normal tissues, resulting in side effects that prevent these candidate drugs from progressing from in vitro studies to clinical application. Hence, it is essential to identify subtype-specific drugs to achieve cancer-specific treatment. An attempt to target neonatal Nav1.5 has generated a specific antibody against an epitope that is unique to neonatal Nav1.5, thus aiming to specifically target cancer [48]. Here, we urge the future development of more VGSC subtype-specific targeting strategies to achieve cancer-specific treatment.
VGSC and cancer drug resistance
As discussed, one of the most plausible mechanisms through which VGSCs impact cancer is by potential regulation of cancer cell migration. As the β subunit is less studied, this discussion focuses on the α subunits. Many studies have suggested that VGSCs may modulate migration by influencing the epithelial-to-mesenchymal transition (EMT) phenotype [46, 49, 50]. EMT has been linked to therapy resistance in many cancer types, such as lung cancer [134, 135], pancreatic cancer [136], and breast cancer [137, 138]. Therefore, the inhibition of VGSCs, hindering EMT, could represent a pathway through which VGSCs are involved in drug resistance.
In a prior study, a hypothesis was proposed suggesting that intervening with VGSCs could potentially overcome drug resistance in cancer [139]. According to this hypothesis, the inhibition of VGSCs has the potential to impede both EMT and angiogenesis through interactions with intracellular calcium activity and endothelial cells, respectively. Combining the blockage of VGSCs with other anticancer therapies may prove effective in both adjuvant and palliative settings. The inhibition of VGSCs might slow down the colonization at secondary sites by hindering angiogenesis, thereby providing temporary relief from symptoms associated with the tumor burden in patients with metastatic disease [139].
The VGSC inhibitors with the potential to inhibit EMT, could be particularly efficacious in the adjuvant setting. Disseminated and circulating tumor cells that have undergone EMT tend to be less proliferative, rendering them less responsive to chemotherapy. Inhibiting EMT may disrupt dormancy and enhance the chemosensitivity of cells, as observed with valproic acid in glioblastoma [140]. Cells in the disseminated and circulating tumor state exhibit mesenchymal characteristics due to EMT. Following the transition to a mesenchymal phenotype, cellular dependence on EGFR signaling diminishes, activating alternative growth factor pathways [141]. The reduction in EGFR expression during mesenchymal transition may explain the limited efficacy of incorporating anti-EGFR agents like cetuximab into chemotherapy in the adjuvant setting [142]. In addition, the epidermal growth factor was reported to increase Nav1.7 expression [59, 68, 116], which might be potentially involved in this regulatory pathway of drug resistance.
To date, only a limited number of experimental studies have delved into the role of VGSC in cancer drug resistance. Among these investigations, a study centered on leukemia has uncovered a direct association between VGSC and drug resistance in this context [143]. Specifically, this study has linked the augmentation of the voltage-gated sodium current to multidrug resistance in leukemia cells. Employing a patch clamp technique, the study measured the voltage-gated sodium current in a drug-sensitive human leukemia cell line, K562, and its multidrug-resistant counterpart (resistant to anthracycline antibiotics and Vinca alkaloids). The results indicated that a significant proportion of the multidrug-resistant cells exhibited voltage-gated sodium current, contrasting with the predominant absence of such current in the parental drug-sensitive cells. Unfortunately, doubts arose when tetrodotoxin failed to restore sensitivity to doxorubicin and vincristine, challenging the established link between drug resistance and VGSC [143].
Another study in the context of ovarian cancer reported that a VGSC-targeting drug, lidocaine, hinders the metastatic capabilities of ovarian cancer by impeding Nav1.5-mediated EMT and the focal adhesion kinase/Paxillin signaling pathway [144]. Elevated focal adhesion kinase levels were observed in advanced-stage ovarian cancers and correlated with advanced drug resistance to platinum- and taxane-based chemotherapy in ovarian cancer patients [145, 146]. In this study, when ovarian cancer cells were treated with 10 μM cisplatin combined with 5 mM lidocaine, cell viability decreased by 40% compared to cells treated with cisplatin alone. The combination of lidocaine and cisplatin enhanced the deactivation of the focal adhesion kinase/Paxillin signaling pathway and the induction of apoptosis compared to the effects observed with cisplatin alone [144]. In vivo experiments corroborated these findings, showing that the combined administration of lidocaine and cisplatin significantly decreased ovarian cancer loading. This combination exhibited superior inhibitory effects on cancer malignancy compared to individual drug treatments [144]. Besides the focal adhesion kinase/Paxillin signaling pathway, this study also attributed the effect of VGSC on drug resistance to the induction of apoptosis by the VGSC [144]. This finding aligns with another investigation indicating that Nav1.5 augments 5-Fluorouracil-stimulated apoptosis in colorectal cancer cells [46]. The study showed that stage II/III colorectal cancer patients with upregulated SCN5A expression demonstrated enhanced survival after 5-Fluorouracil-based adjuvant chemotherapy. In vitro experiments further suggested that SCN5A knockdown increased the IC50 for 5-Fluorouracil by elevating 5-Fluorouracil-induced apoptosis [46].
Future studies
The aim of this work was to identify potential new and unexplored scientific questions that could represent avenues for future research in this field. By doing so, we hope to expand our understanding of the subject and spur further exploration of its possibilities. To this end, we have identified gaps or areas that have yet to be explored based on the current published papers and our data reviewing. It is important to note that the majority of VGSC subtypes have never been studied in the context of cancer. This is due to the fact that some VGSCs are not expressed, or are expressed at extremely low levels, in some cancer types. Additionally, researchers often focus on one subtype of VGSCs and ignore the other subtypes in their studies. For example, many studies apply inhibitors that are not subtype-specific to VGSCs but attribute the effect to only one subtype.
A significant research area for future studies would be the investigation of the role of VGSCs in certain cancer types with potential clinical impact. Specifically, it is important to investigate VGSCs in cancer types where their gene expression is relatively high, in order to develop applicable biomarkers for clinical use. Furthermore, the expression or otherwise of the gene should have an impact on patient survival, as this would demonstrate that it is making a considerable difference in real patients. Additionally, it would be preferable (but not essential) to consider VGSCs that are aberrantly expressed in cancer tissue compared to normal tissue, as this could provide potential cancer-specific drug targets for treatment. To ensure reliability, such analysis should be conducted with a large sample size. To propose potentially significant research topics for future studies, the information on the top significant gene-cancer pairs from TCGA was displayed in Fig. 2 and S- Table 2. Among these gene-cancer pairs, SCN3A-LGG, SCN3B-LGG, SCN4A-KIRC, SCN1B-UVM, and SCN1B-PAAD have a relatively high gene expression level, and have a relatively large case number except for UVM (n = 79). No studies to date have investigated the potential connections between these gene-cancer pairs, therefore, SCN3A-LGG, SCN3B-LGG, SCN4A-KIRC, and SCN1B-PAAD would be potential clinically significant research topics for future study.
Another aspect of the potential study in this field in the future is to investigate the potential of VGSCs to be used for cancer therapies. Our bioinformatic analysis suggested a potential association between VGSC expression and the immune subtypes in certain cancer types (Table 4 column 10), hinting at the possibility of using VGSCs as a prediction biomarker or enhancer in cancer immunotherapy, such as Nav1.5 in colon, breast, and ovarian cancer and Nav1.7 in breast, prostate, lung, and gastric cancer. However, this analysis can provide some hints but not sufficient evidence to support the role of VGSCs in the cancer immune environment. In fact, a number of bioinformatic studies have used TCGA data for immune cell infiltration analysis and immune association studies of genes, but these bulk RNA sequencing analyses provide associations rather than causal effects of a gene on cancers [27,28,29,30, 147,148,149,150,151,152]. Emerging technologies, such as single-cell RNA sequencing and spatial transcriptomics, will hopefully offer more insights into the interactions between cancer cells and immune cells in the future, and reveal whether VGSCs play a role in this communication.
Additionally, there is already in vitro data available that demonstrates the direct inhibition of VGSC blockers on cancer cells, suggesting that the development of VGSC blockers as chemotherapy or chemotherapy enhancers is promising. Our analysis also suggested that VGSC levels are associated with the molecular subtype of some cancer types (Table 4 column 11), implying that VGSC-targeting cancer drugs could be tailored to specific molecular subtypes. As summarized in this study, many VGSC-targeting drugs are readily accessible for research in this area, promising major future advances in this field. However, given the VGSC subtype similarity, specifically targeting VGSC subtypes has proven to be extremely difficult. Many current studies treat pan-VGSC as a single entity, which can lead to non-specific effects on normal tissues. Therefore, developing subtype-specific therapies is essential, as demonstrated by pioneering work on neonatal Nav1.5 [89]. We urge further research in this area to achieve more precise and effective treatments.
Almost all studies in this field so far focus on the downstream effects of VGSCs, demonstrating how VGSCs impact downstream functions in cancer. However, there is a lack of investigation into the upstream regulation of VGSC expression. A recent study proposed a hypothesis suggesting the presence of a feedback loop in Nav1.5-mediated cellular invasion that regulates the expression of Nav1.5 in cancer cells [153]. We emphasize the need for further research to validate these mechanisms. Given the importance of VGSC expression in cancer, additional research is essential to understand and validate the upstream pathways regulating VGSC expression. This study provides new insights and indicates upstream regulation of VGSC expression using open databases in aspects of copy number, methylation, and miRNAs. Hopefully, these pieces of information can inspire interest and highlight potential research candidates for future studies. This could also potentially lead to the identification of drug targets that mediate VGSC expression rather than merely blocking the channels and uncover the intrinsic relationships between VGSCs and other correlated oncogenes.
Availability of data and materials
No datasets were generated or analysed during the current study.
References
Liu, H.; Dilger, J.P.; Lin, J. A pan-cancer-bioinformatic-based literature review of TRPM7 in cancers. Pharmacol. Ther. 2022;108302. https://doi.org/10.1016/j.pharmthera.2022.108302.
Liu H, Dilger JP, Lin J. The role of transient receptor potential melastatin 7 (TRPM7) in cell viability: a potential target to suppress breast cancer cell cycle. Cancers. 2020;12:131. https://doi.org/10.3390/cancers12010131.
Liu H. Toxic medicine used in traditional Chinese medicine for cancer treatment: are ion channels involved? J Tradit Chin Med. 2022;42:1019–22. https://doi.org/10.19852/j.cnki.jtcm.20220815.005.
Liu H, Dilger JP, Lin J. Lidocaine suppresses viability and migration of human breast cancer cells: TRPM7 as a target for some breast cancer cell lines. Cancers. 2021;13:234. https://doi.org/10.3390/cancers13020234.
Huang X, Jan LY. Targeting potassium channels in cancer. J Cell Biol. 2014;206:151–62. https://doi.org/10.1083/jcb.201404136.
Wang H, Zou L, Ma K, Yu J, Wu H, Wei M, Xiao Q. Cell-specific mechanisms of TMEM16A Ca(2+)-activated chloride channel in cancer. Mol Cancer. 2017;16:152. https://doi.org/10.1186/s12943-017-0720-x.
Peretti M, Angelini M, Savalli N, Florio T, Yuspa SH, Mazzanti M. Chloride channels in cancer: focus on chloride intracellular channel 1 and 4 (CLIC1 and CLIC4) proteins in tumor development and as novel therapeutic targets. Biochim Biophys Acta. 2015;1848:2523–31. https://doi.org/10.1016/j.bbamem.2014.12.012.
Payne SL, Ram P, Srinivasan DH, Le TT, Levin M, Oudin MJ. Potassium channel-driven bioelectric signalling regulates metastasis in triple-negative breast cancer. EBioMedicine. 2022;75: 103767. https://doi.org/10.1016/j.ebiom.2021.103767.
Potier-Cartereau M, Raoul W, Weber G, Mahéo K, Rapetti-Mauss R, Gueguinou M, Buscaglia P, Goupille C, Le Goux N, Abdoul-Azize S, et al. Potassium and calcium channel complexes as novel targets for cancer research. Rev Physiol Biochem Pharmacol. 2022;183:157–76. https://doi.org/10.1007/112_2020_24.
Xu X, Li N, Wang Y, Yu J, Mi J. Calcium channel TRPV6 promotes breast cancer metastasis by NFATC2IP. Cancer Lett. 2021;519:150–60. https://doi.org/10.1016/j.canlet.2021.07.017.
Cheng S, Jiang D, Lan X, Liu K, Fan C. Voltage-gated potassium channel 1.3: A promising molecular target in multiple disease therapy. Biomed Pharmacother. 2024;175:116651. https://doi.org/10.1016/j.biopha.2024.116651.
Zhang L, Chen X, Yao S, Zheng L, Yang X, Wang Y, Li X, Wu E, Tuo B. Intracellular chloride channel 1 and tumor. Am J Cancer Res. 2023;13:3300–14.
Gao Y, Liao P. TRPM4 channel and cancer. Cancer Lett. 2019;454:66–9. https://doi.org/10.1016/j.canlet.2019.04.012.
O’Reilly D, Buchanan P. Calcium channels and cancer stem cells. Cell Calcium. 2019;81:21–8. https://doi.org/10.1016/j.ceca.2019.05.006.
Yang M, Brackenbury W. Membrane potential and cancer progression. Front Physiol. 2013;4:185. https://doi.org/10.3389/fphys.2013.00185.
Abdul Kadir, L.; Stacey, M.; Barrett-Jolley, R. Emerging roles of the membrane potential: action beyond the action potential. Front. Physiol. 2018, 9, https://doi.org/10.3389/fphys.2018.01661.
Orr CW, Yoshikawa-Fukada M, Ebert JD. Potassium: effect on DNA synthesis and multiplication of baby-hamster kidney cells: (cell cycle-membrane potential-synchronization-transformation). Proc Natl Acad Sci USA. 1972;69:243–7. https://doi.org/10.1073/pnas.69.1.243.
Binggeli R, Weinstein RC. Membrane potentials and sodium channels: hypotheses for growth regulation and cancer formation based on changes in sodium channels and gap junctions. J Theor Biol. 1986;123:377–401. https://doi.org/10.1016/s0022-5193(86)80209-0.
Smith NR, Sparks RL, Pool TB, Cameron IL. Differences in the intracellular concentration of elements in normal and cancerous liver cells as determined by X-ray microanalysis. Cancer Res. 1978;38:1952–9.
Cameron IL, Smith NK, Pool TB, Sparks RL. Intracellular concentration of sodium and other elements as related to mitogenesis and oncogenesis in vivo. Cancer Res. 1980;40:1493–500.
Sparks RL, Pool TB, Smith NK, Cameron IL. Effects of amiloride on tumor growth and intracellular element content of tumor cells in vivo. Cancer Res. 1983;43:73–7.
Namadurai S, Yereddi NR, Cusdin FS, Huang CL, Chirgadze DY, Jackson AP. A new look at sodium channel β subunits. Open Biol. 2015;5: 140192. https://doi.org/10.1098/rsob.140192.
Catterall WA. Forty years of sodium channels: Structure, function, pharmacology, and epilepsy. Neurochem Res. 2017;42:2495–504. https://doi.org/10.1007/s11064-017-2314-9.
de Lera Ruiz M, Kraus RL. Voltage-gated sodium channels: Structure, function, pharmacology, and clinical indications. J Med Chem. 2015;58:7093–118. https://doi.org/10.1021/jm501981g.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71.
Liu H, Dilger JP, Lin J. Effects of local anesthetics on cancer cells. Pharmacol Ther. 2020;212:107558. https://doi.org/10.1016/j.pharmthera.2020.107558.
Leslie TK, Brackenbury WJ. Sodium channels and the ionic microenvironment of breast tumours. J Physiol. 2022;601:1543–53. https://doi.org/10.1113/jp282306.
Rajaratinam H, Mokhtar NF, Asma-Abdullah N, Fuad WEM. Discovering the triad between Nav1.5, breast cancer, and the immune system: A fundamental review and future perspectives. Biomolecules. 2022;12:310. https://doi.org/10.3390/biom12020310.
Luo Q, Wu T, Wu W, Chen G, Luo X, Jiang L, Tao H, Rong M, Kang S, Deng M. The functional role of voltage-gated sodium channel Nav1.5 in metastatic breast cancer. Front Pharmacol. 2020;11:1111. https://doi.org/10.3389/fphar.2020.01111.
Martin F, Ufodiama C, Watt I, Bland M, Brackenbury WJ. Therapeutic value of voltage-gated sodium channel inhibitors in breast, colorectal, and prostate cancer: A systematic review. Front Pharmacol. 2015;6:273. https://doi.org/10.3389/fphar.2015.00273.
Nelson M, Yang M, Dowle AA, Thomas JR, Brackenbury WJ. The sodium channel-blocking antiepileptic drug phenytoin inhibits breast tumour growth and metastasis. Mol Cancer. 2015;14:13. https://doi.org/10.1186/s12943-014-0277-x.
Fraser SP, Diss JK, Chioni AM, Mycielska ME, Pan H, Yamaci RF, Pani F, Siwy Z, Krasowska M, Grzywna Z, et al. Voltage-gated sodium channel expression and potentiation of human breast cancer metastasis. Clin Cancer Res. 2005;11:5381–9. https://doi.org/10.1158/1078-0432.Ccr-05-0327.
Yang M, Kozminski DJ, Wold LA, Modak R, Calhoun JD, Isom LL, Brackenbury WJ. Therapeutic potential for phenytoin: targeting Na(v)1.5 sodium channels to reduce migration and invasion in metastatic breast cancer. Breast Cancer Res Treat. 2012;134:603–15. https://doi.org/10.1007/s10549-012-2102-9.
Nelson M, Millican-Slater R, Forrest LC, Brackenbury WJ. The sodium channel β1 subunit mediates outgrowth of neurite-like processes on breast cancer cells and promotes tumour growth and metastasis. Int J Cancer. 2014;135:2338–51. https://doi.org/10.1002/ijc.28890.
Dulong C, Fang YJ, Gest C, Zhou MH, Patte-Mensah C, Mensah-Nyagan AG, Vannier JP, Lu H, Soria C, Cazin L, et al. The small GTPase RhoA regulates the expression and function of the sodium channel Nav1.5 in breast cancer cells. Int J Oncol. 2014;44:539–47. https://doi.org/10.3892/ijo.2013.2214.
Brisson L, Gillet L, Calaghan S, Besson P, Le Guennec JY, Roger S, Gore J. NaV1.5 enhances breast cancer cell invasiveness by increasing NHE1-dependent H(+) efflux in caveolae. Oncogene. 2011;30:2070–6. https://doi.org/10.1038/onc.2010.574.
Chioni AM, Shao D, Grose R, Djamgoz MB. Protein kinase A and regulation of neonatal Nav1.5 expression in human breast cancer cells: activity-dependent positive feedback and cellular migration. Int J Biochem Cell Biol. 2010;42:346–58. https://doi.org/10.1016/j.biocel.2009.11.021.
Gillet L, Roger S, Besson P, Lecaille F, Gore J, Bougnoux P, Lalmanach G, Le Guennec JY. Voltage-gated Sodium Channel Activity Promotes Cysteine Cathepsin-dependent Invasiveness and Colony Growth of Human Cancer Cells. J Biol Chem. 2009;284:8680–91. https://doi.org/10.1074/jbc.M806891200.
Brisson L, Driffort V, Benoist L, Poet M, Counillon L, Antelmi E, Rubino R, Besson P, Labbal F, Chevalier S, et al. NaV1.5 Na⁺ channels allosterically regulate the NHE-1 exchanger and promote the activity of breast cancer cell invadopodia. J Cell Sci. 2013;126:4835–42. https://doi.org/10.1242/jcs.123901.
Li X, Zhao L, Feng R, Du X, Guo Z, Meng Y, Zou Y, Liao W, Liu Q, Sheng Y, et al. Single molecule localizations of voltage-gated sodium channel Na(V)1.5 on the surfaces of normal and cancer breast cells. Anal Methods. 2023;15:1855–60. https://doi.org/10.1039/d3ay00208j.
Brackenbury WJ, Chioni AM, Diss JK, Djamgoz MB. The neonatal splice variant of Nav1.5 potentiates in vitro invasive behaviour of MDA-MB-231 human breast cancer cells. Breast Cancer Res Treat. 2007;101:149–60. https://doi.org/10.1007/s10549-006-9281-1.
Yamaci RF, Fraser SP, Battaloglu E, Kaya H, Erguler K, Foster CS, Djamgoz MBA. Neonatal Nav1.5 protein expression in normal adult human tissues and breast cancer. Pathol Res Pract. 2017;213:900–7. https://doi.org/10.1016/j.prp.2017.06.003.
Chioni AM, Fraser SP, Pani F, Foran P, Wilkin GP, Diss JK, Djamgoz MB. A novel polyclonal antibody specific for the Na(v)1.5 voltage-gated Na(+) channel “neonatal” splice form. J Neurosci Methods. 2005;147:88–98. https://doi.org/10.1016/j.jneumeth.2005.03.010.
Chatterjee SJ, McCaffrey L. Emerging role of cell polarity proteins in breast cancer progression and metastasis. Breast Cancer (Dove Med Press). 2014;6:15–27. https://doi.org/10.2147/bctt.S43764.
Lopez-Charcas O, Poisson L, Benouna O, Lemoine R, Chadet S, Pétereau A, Lahlou W, Guyétant S, Ouaissi M, Pukkanasut P, et al. Voltage-gated sodium channel Na(V)1.5 controls NHE-1-dependent invasive properties in colon cancer cells. Cancers. 2022;15(1):46. https://doi.org/10.3390/cancers15010046.
Sui Q, Peng J, Han K, Lin J, Zhang R, Ou Q, Qin J, Deng Y, Zhou W, Kong L, et al. Voltage-gated sodium channel Na(v)1.5 promotes tumor progression and enhances chemosensitivity to 5-fluorouracil in colorectal cancer. Cancer Lett. 2021;500:119–31. https://doi.org/10.1016/j.canlet.2020.12.017.
Poisson L, Lopez-Charcas O, Chadet S, Bon E, Lemoine R, Brisson L, Ouaissi M, Baron C, Besson P, Roger S, et al. Rock inhibition promotes Na(V)1.5 sodium channel-dependent SW620 colon cancer cell invasiveness. Sci Rep. 2020;10:13350. https://doi.org/10.1038/s41598-020-70378-3.
Guzel RM, Ogmen K, Ilieva KM, Fraser SP, Djamgoz MBA. Colorectal cancer invasiveness in vitro: Predominant contribution of neonatal Nav1.5 under normoxia and hypoxia. J Cell Physiol. 2019;234:6582–93. https://doi.org/10.1002/jcp.27399.
Baptista-Hon DT, Robertson FM, Robertson GB, Owen SJ, Rogers GW, Lydon EL, Lee NH, Hales TG. Potent inhibition by ropivacaine of metastatic colon cancer SW620 cell invasion and NaV1.5 channel function. Br J Anaesth. 2014;113(Suppl 1):i39–48. https://doi.org/10.1093/bja/aeu104.
Brummelhuis IS, Fiascone SJ, Hasselblatt KT, Frendl G, Elias KM. Voltage-gated sodium channels as potential biomarkers and therapeutic targets for epithelial ovarian cancer. Cancers. 2021;13(21):5437. https://doi.org/10.3390/cancers13215437.
Liu J, Liu D, Liu JJ, Zhao C, Yao S, Hong L. Blocking the Nav1.5 channel using eicosapentaenoic acid reduces migration and proliferation of ovarian cancer cells. Int J Oncol. 2018;53:855–65. https://doi.org/10.3892/ijo.2018.4437.
Gao R, Shen Y, Cai J, Lei M, Wang Z. Expression of voltage-gated sodium channel alpha subunit in human ovarian cancer. Oncol Rep. 2010;23:1293–9. https://doi.org/10.3892/or_00000763.
Liu J, Tan H, Yang W, Yao S, Hong L. The voltage-gated sodium channel Na(v)1.7 associated with endometrial cancer. J Cancer. 2019;10:4954–60. https://doi.org/10.7150/jca.31544.
Wang J, Lu Z, Wu C, Li Y, Kong Y, Zhou R, Shi K, Guo J, Li N, Liu J, et al. Evaluation of the anticancer and anti-metastasis effects of novel synthetic sodium channel blockers in prostate cancer cells in vitro and in vivo. Prostate. 2019;79:62–72. https://doi.org/10.1002/pros.23711.
Shan B, Dong M, Tang H, Wang N, Zhang J, Yan C, Jiao X, Zhang H, Wang C. Voltage-gated sodium channels were differentially expressed in human normal prostate, benign prostatic hyperplasia and prostate cancer cells. Oncol Lett. 2014;8:345–50. https://doi.org/10.3892/ol.2014.2110.
Suy, S.; Hansen, T.P.; Auto, H.D.; Kallakury, B.V.; Dailey, V.; Danner, M.; Macarthur, L.; Zhang, Y.; Miessau, M.J.; Collins, S.P.; et al. Expression of voltage-gated sodium channel Na(v)18 in human prostate cancer is associated with high histological grade. J. Clin. Exp. Oncol. 2012, 1(2):https://doi.org/10.4172/2324-9110.1000102, https://doi.org/10.4172/2324-9110.1000102.
Gumushan Aktas H, Akgun T. Naringenin inhibits prostate cancer metastasis by blocking voltage-gated sodium channels. Biomed Pharmacother. 2018;106:770–5. https://doi.org/10.1016/j.biopha.2018.07.008.
Nakajima T, Kubota N, Tsutsumi T, Oguri A, Imuta H, Jo T, Oonuma H, Soma M, Meguro K, Takano H, et al. Eicosapentaenoic acid inhibits voltage-gated sodium channels and invasiveness in prostate cancer cells. Br J Pharmacol. 2009;156:420–31. https://doi.org/10.1111/j.1476-5381.2008.00059.x.
Uysal-Onganer P, Djamgoz MB. Epidermal growth factor potentiates in vitro metastatic behaviour of human prostate cancer PC-3M cells: involvement of voltage-gated sodium channel. Mol Cancer. 2007;6:76. https://doi.org/10.1186/1476-4598-6-76.
Diss JK, Fraser SP, Walker MM, Patel A, Latchman DS, Djamgoz MB. Beta-subunits of voltage-gated sodium channels in human prostate cancer: quantitative in vitro and in vivo analyses of mRNA expression. Prostate Cancer Prostatic Dis. 2008;11:325–33. https://doi.org/10.1038/sj.pcan.4501012.
Nelson M, Yang M, Millican-Slater R, Brackenbury WJ. Nav1.5 regulates breast tumor growth and metastatic dissemination in vivo. Oncotarget. 2015;6:32914–29. https://doi.org/10.18632/oncotarget.5441.
Chatterjee SJ, McCaffrey L. Emerging role of cell polarity proteins in breast cancer progression and metastasis. Breast Cancer (Dove Med Press). 2014;6:15–27. https://doi.org/10.2147/BCTT.S43764.
Smith P, Rhodes NP, Shortland AP, Fraser SP, Djamgoz MB, Ke Y, Foster CS. Sodium channel protein expression enhances the invasiveness of rat and human prostate cancer cells. FEBS Lett. 1998;423:19–24. https://doi.org/10.1016/s0014-5793(98)00050-7.
Xia J, Huang N, Huang H, Sun L, Dong S, Su J, Zhang J, Wang L, Lin L, Shi M, et al. Voltage-gated sodium channel Nav 1.7 promotes gastric cancer progression through MACC1-mediated upregulation of NHE1. Int J Cancer. 2016;139:2553–69. https://doi.org/10.1002/ijc.30381.
Sanchez-Sandoval AL, Gomora JC. Contribution of voltage-gated sodium channel β-subunits to cervical cancer cells metastatic behavior. Cancer Cell Int. 2019;19:35. https://doi.org/10.1186/s12935-019-0757-6.
Lopez-Charcas O, Espinosa AM, Alfaro A, Herrera-Carrillo Z, Ramirez-Cordero BE, Cortes-Reynosa P, Perez Salazar E, Berumen J, Gomora JC. The invasiveness of human cervical cancer associated to the function of Na(V)1.6 channels is mediated by MMP-2 activity. Sci. Rep. 2018;8:12995. https://doi.org/10.1038/s41598-018-31364-y.
Diaz D, Delgadillo DM, Hernández-Gallegos E, Ramírez-Domínguez ME, Hinojosa LM, Ortiz CS, Berumen J, Camacho J, Gomora JC. Functional expression of voltage-gated sodium channels in primary cultures of human cervical cancer. J Cell Physiol. 2007;210:469–78. https://doi.org/10.1002/jcp.20871.
Campbell TM, Main MJ, Fitzgerald EM. Functional expression of the voltage-gated Na⁺-channel Nav1.7 is necessary for EGF-mediated invasion in human non-small cell lung cancer cells. J Cell Sci. 2013;126:4939–49. https://doi.org/10.1242/jcs.130013.
Roger S, Rollin J, Barascu A, Besson P, Raynal PI, Iochmann S, Lei M, Bougnoux P, Gruel Y, Le Guennec JY. Voltage-gated sodium channels potentiate the invasive capacities of human non-small-cell lung cancer cell lines. Int J Biochem Cell Biol. 2007;39:774–86. https://doi.org/10.1016/j.biocel.2006.12.007.
Blandino JK, Viglione MP, Bradley WA, Oie HK, Kim YI. Voltage-dependent sodium channels in human small-cell lung cancer cells: role in action potentials and inhibition by Lambert-Eaton syndrome IgG. J Membr Biol. 1995;143:153–63. https://doi.org/10.1007/bf00234661.
Li H, Liu J, Fan N, Wang H, Thomas AM, Yan Q, Li S, Qin H. Nav1.6 promotes the progression of human follicular thyroid carcinoma cells via JAK-STAT signaling pathway. Pathol Res Pract. 2022;236:153984. https://doi.org/10.1016/j.prp.2022.153984.
Pukkanasut P, Whitt J, Guenter R, Lynch SE, Gallegos C, Rosendo-Pineda MJ, Gomora JC, Chen H, Lin D, Sorace A, et al. Voltage-gated sodium channel Na(V)1.7 inhibitors with potent anticancer activities in medullary thyroid cancer cells. Cancers. 2023;15(10):2806. https://doi.org/10.3390/cancers15102806.
Xu X, Dai Y, Feng L, Zhang H, Hu Y, Xu L, Zhu X, Jiang Y. Knockdown of Nav1.5 inhibits cell proliferation, migration and invasion via Wnt/β-catenin signaling pathway in oral squamous cell carcinoma. Acta Biochim Biophys Sin (Shanghai). 2020;52:527–35. https://doi.org/10.1093/abbs/gmaa021.
Zhang J, Mao W, Dai Y, Qian C, Dong Y, Chen Z, Meng L, Jiang Z, Huang T, Hu J, et al. Voltage-gated sodium channel Nav1.5 promotes proliferation, migration and invasion of oral squamous cell carcinoma. Acta Biochim Biophys Sin (Shanghai). 2019;51:562–70. https://doi.org/10.1093/abbs/gmz044.
Li S, Han J, Guo G, Sun Y, Zhang T, Zhao M, Xu Y, Cui Y, Liu Y, Zhang J. Voltage-gated sodium channels β3 subunit promotes tumorigenesis in hepatocellular carcinoma by facilitating p53 degradation. FEBS Lett. 2020;594:497–508. https://doi.org/10.1002/1873-3468.13641.
Driffort V, Gillet L, Bon E, Marionneau-Lambot S, Oullier T, Joulin V, Collin C, Pagès JC, Jourdan ML, Chevalier S, et al. Ranolazine inhibits NaV1.5-mediated breast cancer cell invasiveness and lung colonization. Mol Cancer. 2014;13:264. https://doi.org/10.1186/1476-4598-13-264.
Batcioglu K, Uyumlu AB, Satilmis B, Yildirim B, Yucel N, Demirtas H, Onkal R, Guzel RM, Djamgoz MB. Oxidative stress in the in vivo DMBA rat model of breast cancer: suppression by a voltage-gated sodium channel inhibitor (RS100642). Basic Clin Pharmacol Toxicol. 2012;111:137–41. https://doi.org/10.1111/j.1742-7843.2012.00880.x.
Bugan I, Kucuk S, Karagoz Z, Fraser SP, Kaya H, Dodson A, Foster CS, Altun S, Djamgoz MBA. Anti-metastatic effect of ranolazine in an in vivo rat model of prostate cancer, and expression of voltage-gated sodium channel protein in human prostate. Prostate Cancer Prostatic Dis. 2019;22:569–79. https://doi.org/10.1038/s41391-019-0128-3.
Yildirim S, Altun S, Gumushan H, Patel A, Djamgoz MBA. Voltage-gated sodium channel activity promotes prostate cancer metastasis in vivo. Cancer Lett. 2012;323:58–61. https://doi.org/10.1016/j.canlet.2012.03.036.
Davis GC, Kong Y, Paige M, Li Z, Merrick EC, Hansen T, Suy S, Wang K, Dakshanamurthy S, Cordova A, et al. Asymmetric synthesis and evaluation of a hydroxyphenylamide voltage-gated sodium channel blocker in human prostate cancer xenografts. Bioorg Med Chem. 2012;20:2180–8. https://doi.org/10.1016/j.bmc.2011.08.061.
Paul D, Maggi P, Piero FD, Scahill SD, Sherman KJ, Edenfield S, Gould HJ 3rd. Targeted osmotic lysis of highly invasive breast carcinomas using pulsed magnetic field stimulation of voltage-gated sodium channels and pharmacological blockade of sodium pumps. Cancers. 2020;12(6):1420. https://doi.org/10.3390/cancers12061420.
Fairhurst C, Martin F, Watt I, Bland M, Doran T, Brackenbury WJ. Sodium channel-inhibiting drugs and cancer-specific survival: a population-based study of electronic primary care data. BMJ Open. 2023;13: e064376. https://doi.org/10.1136/bmjopen-2022-064376.
Fairhurst C, Martin F, Watt I, Doran T, Bland M, Brackenbury WJ. Sodium channel-inhibiting drugs and cancer survival: protocol for a cohort study using the CPRD primary care database. BMJ Open. 2016;6: e011661. https://doi.org/10.1136/bmjopen-2016-011661.
Lastraioli E, Fraser SP, Guzel RM, Iorio J, Bencini L, Scarpi E, Messerini L, Villanacci V, Cerino G, Ghezzi N, et al. Neonatal nav1.5 protein expression in human colorectal cancer: immunohistochemical characterization and clinical evaluation. Cancers. 2021;13(15):3832. https://doi.org/10.3390/cancers13153832.
Lin S, Lv Y, Xu J, Mao X, Chen Z, Lu W. Over-expression of Nav1.6 channels is associated with lymph node metastases in colorectal cancer. World J Surg Oncol. 2019;17:175. https://doi.org/10.1186/s12957-019-1715-4.
Peng J, Ou Q, Wu X, Zhang R, Zhao Q, Jiang W, Lu Z, Wan D, Pan Z, Fang Y. Expression of voltage-gated sodium channel Nav1.5 in non-metastatic colon cancer and its associations with estrogen receptor (ER)-β expression and clinical outcomes. Chin J Cancer. 2017;36:89. https://doi.org/10.1186/s40880-017-0253-0.
Takada M, Fujimoto M, Motomura H, Hosomi K. Inverse association between sodium channel-blocking antiepileptic drug use and cancer: data mining of spontaneous reporting and claims databases. Int J Med Sci. 2016;13:48–59. https://doi.org/10.7150/ijms.13834.
Diss JK, Stewart D, Pani F, Foster CS, Walker MM, Patel A, Djamgoz MB. A potential novel marker for human prostate cancer: voltage-gated sodium channel expression in vivo. Prostate Cancer Prostatic Dis. 2005;8:266–73. https://doi.org/10.1038/sj.pcan.4500796.
Fraser SP, Onkal R, Theys M, Bosmans F, Djamgoz MBA. Neonatal Na(V) 1.5 channels: pharmacological distinctiveness of a cancer-related voltage-gated sodium channel splice variant. Br J Pharmacol. 2022;179:473–86. https://doi.org/10.1111/bph.15668.
Grzywna ZJ, Borys P. On the significance of sodium ionic channels in analysis of the breast cancer metastaticity. Biosystems. 2019;177:34–8. https://doi.org/10.1016/j.biosystems.2018.10.007.
Győrffy B. Survival analysis across the entire transcriptome identifies biomarkers with the highest prognostic power in breast cancer. Comput Struct Biotechnol J. 2021;19:4101–9. https://doi.org/10.1016/j.csbj.2021.07.014.
Quicke P, Sun Y, Arias-Garcia M, Beykou M, Acker CD, Djamgoz MBA, Bakal C, Foust AJ. Voltage imaging reveals the dynamic electrical signatures of human breast cancer cells. Commun Biol. 2022;5:1178. https://doi.org/10.1038/s42003-022-04077-2.
Khananshvili D. The SLC8 gene family of sodium-calcium exchangers (NCX) - structure, function, and regulation in health and disease. Mol Aspects Med. 2013;34:220–35. https://doi.org/10.1016/j.mam.2012.07.003.
Lee A, Fraser SP, Djamgoz MBA. Propranolol inhibits neonatal Nav1.5 activity and invasiveness of MDA-MB-231 breast cancer cells: Effects of combination with ranolazine. J Cell Physiol. 2019;234:23066–81. https://doi.org/10.1002/jcp.28868.
Djamgoz MBA. Ranolazine: a potential anti-metastatic drug targeting voltage-gated sodium channels. Br J Cancer. 2024;130:1415–9. https://doi.org/10.1038/s41416-024-02622-w.
Vidak E, Javoršek U, Vizovišek M, Turk B. Cysteine cathepsins and their extracellular roles: shaping the microenvironment. Cells. 2019;8:264. https://doi.org/10.3390/cells8030264.
Busco G, Cardone RA, Greco MR, Bellizzi A, Colella M, Antelmi E, Mancini MT, Dell’Aquila ME, Casavola V, Paradiso A. NHE1 promotes invadopodial ECM proteolysis through acidification of the peri-invadopodial space. FASEB J. 2010;24:3903–15. https://doi.org/10.1096/fj.09-152488.
Brisson L, Gillet L, Calaghan S, Besson P, Le Guennec JY, Roger S, Gore J. NaV1.5 enhances breast cancer cell invasiveness by increasing NHE1-dependent H+ efflux in caveolae. Oncogene. 2011;30:2070–6. https://doi.org/10.1038/onc.2010.574.
Khan A, Kyle JW, Hanck DA, Lipkind GM, Fozzard HA. Isoform-dependent interaction of voltage-gated sodium channels with protons. J Physiol. 2006;576:493–501. https://doi.org/10.1113/jphysiol.2006.115659.
Jones DK, Peters CH, Tolhurst SA, Claydon TW, Ruben PC. Extracellular proton modulation of the cardiac voltage-gated sodium channel, Nav1.5. Biophys J. 2011;101:2147–56. https://doi.org/10.1016/j.bpj.2011.08.056.
Samanta K, Bakowski D, Amin N, Parekh AB. The whole-cell Ca(2+) release-activated Ca(2+) current, I(CRAC), is regulated by the mitochondrial Ca(2+) uniporter channel and is independent of extracellular and cytosolic Na. J Physiol. 2020;598:1753–73. https://doi.org/10.1113/jp276551.
Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiol Rev. 1999;79:763–854. https://doi.org/10.1152/physrev.1999.79.3.763.
Chovancova B, Liskova V, Babula P, Krizanova O. Role of sodium/calcium exchangers in tumors. Biomolecules. 2020;10(9):1257. https://doi.org/10.3390/biom10091257.
Rodrigues T, Estevez GNN, Tersariol I. Na(+)/Ca(2+) exchangers: Unexploited opportunities for cancer therapy? Biochem Pharmacol. 2019;163:357–61. https://doi.org/10.1016/j.bcp.2019.02.032.
McAinsh MR, Brownlee C, Hetherington AM. Calcium ions as second messengers in guard cell signal transduction. Physiol Plant. 1997;100:16–29.
Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–29. https://doi.org/10.1038/nrm1155.
Tajada S, Villalobos C. Calcium Permeable Channels in Cancer Hallmarks. Front Pharmacol. 2020;11:968. https://doi.org/10.3389/fphar.2020.00968.
Rizzuto R, Brini M, Murgia M, Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744–7. https://doi.org/10.1126/science.8235595.
Leverrier-Penna S, Destaing O, Penna A. Insights and perspectives on calcium channel functions in the cockpit of cancerous space invaders. Cell Calcium. 2020;90: 102251. https://doi.org/10.1016/j.ceca.2020.102251.
Xue Y, Li M, Hu J, Song Y, Guo W, Miao C, Ge D, Hou Y, Wang X, Huang X, et al. Ca(v)2.2-NFAT2-USP43 axis promotes invadopodia formation and breast cancer metastasis through cortactin stabilization. Cell Death Dis. 2022;13:812. https://doi.org/10.1038/s41419-022-05174-0.
Carrithers MD, Chatterjee G, Carrithers LM, Offoha R, Iheagwara U, Rahner C, Graham M, Waxman SG. Regulation of podosome formation in macrophages by a splice variant of the sodium channel SCN8A. J Biol Chem. 2009;284:8114–26. https://doi.org/10.1074/jbc.M808894200.
Chioni AM, Brackenbury WJ, Calhoun JD, Isom LL, Djamgoz MB. A novel adhesion molecule in human breast cancer cells: voltage-gated Na+ channel beta1 subunit. Int J Biochem Cell Biol. 2009;41:1216–27. https://doi.org/10.1016/j.biocel.2008.11.001.
Jansson KH, Lynch JE, Lepori-Bui N, Czymmek KJ, Duncan RL, Sikes RA. Overexpression of the VSSC-associated CAM, beta-2, enhances LNCaP cell metastasis associated behavior. Prostate. 2012;72:1080–92.
Bon E, Driffort V, Gradek F, Martinez-Caceres C, Anchelin M, Pelegrin P, Cayuela M-L, Marionneau-Lambot S, Oullier T, Guibon R, et al. SCN4B acts as a metastasis-suppressor gene preventing hyperactivation of cell migration in breast cancer. Nat Commun. 2016;7:12357. https://doi.org/10.1038/ncomms12357.
Li S, Han J, Guo G, Sun Y, Zhang T, Zhao M, Xu Y, Cui Y, Liu Y, Zhang J. Voltage-gated sodium channels beta3 subunit promotes tumorigenesis in hepatocellular carcinoma by facilitating p53 degradation. FEBS Lett. 2020;594:497–508. https://doi.org/10.1002/1873-3468.13641.
Ding Y, Brackenbury WJ, Onganer PU, Montano X, Porter LM, Bates LF, Djamgoz MB. Epidermal growth factor upregulates motility of Mat-LyLu rat prostate cancer cells partially via voltage-gated Na+ channel activity. J Cell Physiol. 2008;215:77–81. https://doi.org/10.1002/jcp.21289.
Brackenbury WJ, Djamgoz MB. Nerve growth factor enhances voltage-gated Na+ channel activity and Transwell migration in Mat-LyLu rat prostate cancer cell line. J Cell Physiol. 2007;210:602–8. https://doi.org/10.1002/jcp.20846.
Diss JK, Calissano M, Gascoyne D, Djamgoz MB, Latchman DS. Identification and characterization of the promoter region of the Nav1.7 voltage-gated sodium channel gene (SCN9A). Mol Cell Neurosci. 2008;37:537–47.
Goumans, M.J.; Ten Dijke, P. TGF-β signaling in control of cardiovascular function. Cold Spring Harb Perspect Biol 2018;10, https://doi.org/10.1101/cshperspect.a022210.
Malykhina AP, Lei Q, Erickson CS, Epstein ML, Saban MR, Davis CA, Saban R. VEGF induces sensory and motor peripheral plasticity, alters bladder function, and promotes visceral sensitivity. BMC Physiol. 2012;12:15. https://doi.org/10.1186/1472-6793-12-15.
Haque S, Morris JC. Transforming growth factor-β: A therapeutic target for cancer. Hum Vaccin Immunother. 2017;13:1741–50. https://doi.org/10.1080/21645515.2017.1327107.
Colak S, Ten Dijke P. Targeting TGF-β signaling in cancer. Trends Cancer. 2017;3:56–71. https://doi.org/10.1016/j.trecan.2016.11.008.
Mao W, You T, Ye B, Li X, Dong HH, Hill JA, Li F, Xu H. Reactive oxygen species suppress cardiac NaV1.5 expression through Foxo1. PLoS One. 2012;7:e32738. https://doi.org/10.1371/journal.pone.0032738.
Nemoto T, Yanagita T, Kanai T, Wada A. Drug development targeting the glycogen synthase kinase-3beta (GSK-3beta)-mediated signal transduction pathway: the role of GSK-3beta in the maintenance of steady-state levels of insulin receptor signaling molecules and Na(v)1.7 sodium channel in adrenal chromaffin cells. J Pharmacol Sci. 2009;109:157–61. https://doi.org/10.1254/jphs.08r20fm.
Pan H, Djamgoz MB. Biochemical constitution of extracellular medium is critical for control of human breast cancer MDA-MB-231 cell motility. J Membr Biol. 2008;223:27–36. https://doi.org/10.1007/s00232-008-9110-z.
Diss JK, Archer SN, Hirano J, Fraser SP, Djamgoz MB. Expression profiles of voltage-gated Na+ channel α-subunit genes in rat and human prostate cancer cell lines. Prostate. 2001;48:165–78.
Anderson JD, Hansen TP, Lenkowski PW, Walls AM, Choudhury IM, Schenck HA, Friehling M, Höll GM, Patel MK, Sikes RA, et al. Voltage-gated sodium channel blockers as cytostatic inhibitors of the androgen-independent prostate cancer cell line PC-3. Mol Cancer Ther. 2003;2:1149–54.
Li R, Xiao C, Liu H, Huang Y, Dilger JP, Lin J. Effects of local anesthetics on breast cancer cell viability and migration. BMC Cancer. 2018;18:666. https://doi.org/10.1186/s12885-018-4571-6.
BenAissa R, Othman H, Villard C, Peigneur S, Mlayah-Bellalouna S, Abdelkafi-Koubaa Z, Marrakchi N, Essafi-Benkhadir K, Tytgat J, Luis J, et al. AaHIV a sodium channel scorpion toxin inhibits the proliferation of DU145 prostate cancer cells. Biochem Biophys Res Commun. 2020;521:340–6. https://doi.org/10.1016/j.bbrc.2019.10.115.
Chen B, Zhang C, Wang Z, Chen Y, Xie H, Li S, Liu X, Liu Z, Chen P. Mechanistic insights into nav17-dependent regulation of rat prostate cancer cell invasiveness revealed by toxin probes and proteomic analysis. FEBS J. 2019;286:2549–61. https://doi.org/10.1111/febs.14823.
Fang Q, Xin W, Chen L, Fu Y, Qi Y, Ding H, Fang L. Caffeic acid phenethyl ester suppresses metastasis of breast cancer cells by inactivating FGFR1 via MD2. PLoS ONE. 2023;18:e0289031. https://doi.org/10.1371/journal.pone.0289031.
Fuchs E, Messerer DAC, Karpel-Massler G, Fauler M, Zimmer T, Jungwirth B, Föhr KJ. Block of voltage-gated sodium channels as a potential novel anti-cancer mechanism of TIC10. Front Pharmacol. 2021;12: 737637. https://doi.org/10.3389/fphar.2021.737637.
Aktas HG, Ayan H. Oleuropein: a potential inhibitor for prostate cancer cell motility by blocking voltage-gated sodium channels. Nutr Cancer. 2021;73:1758–67. https://doi.org/10.1080/01635581.2020.1807575.
Thomson S, Petti F, Sujka-Kwok I, Epstein D, Haley JD. Kinase switching in mesenchymal-like non-small cell lung cancer lines contributes to EGFR inhibitor resistance through pathway redundancy. Clin Exp Metastasis. 2008;25:843–54.
Shintani Y, Okimura A, Sato K, Nakagiri T, Kadota Y, Inoue M, Sawabata N, Minami M, Ikeda N, Kawahara K. Epithelial to mesenchymal transition is a determinant of sensitivity to chemoradiotherapy in non-small cell lung cancer. Ann Thorac Surg. 2011;92:1794–804.
Arumugam T, Ramachandran V, Fournier KF, Wang H, Marquis L, Abbruzzese JL, Gallick GE, Logsdon CD, McConkey DJ, Choi W. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res. 2009;69:5820–8.
Zhang W, Feng M, Zheng G, Chen Y, Wang X, Pen B, Yin J, Yu Y, He Z. Chemoresistance to 5-fluorouracil induces epithelial–mesenchymal transition via up-regulation of snail in mcf7 human breast cancer cells. Biochem Biophys Res Commun. 2012;417:679–85.
Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, Rimm DL, Wong H, Rodriguez A, Herschkowitz JI, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A. 2009;106:13820–5.
Eren OO, Ozturk MA, Sonmez OU, Oyan B. Voltage-gated sodium channel blockers can augment the efficacy of chemotherapeutics by their inhibitory effect on epithelial-mesenchymal transition. Med Hypotheses. 2015;84:11–3. https://doi.org/10.1016/j.mehy.2014.11.006.
Kerkhof M, Dielemans JC, van Breemen MS, Zwinkels H, Walchenbach R, Taphoorn MJ, Vecht CJ. Effect of valproic acid on seizure control and on survival in patients with glioblastoma multiforme. Neuro Oncol. 2013;15:961–7.
Zeisberg M, Shah AA, Kalluri R. Bone morphogenic protein-7 induces mesenchymal to epithelial transition in adult renal fibroblasts and facilitates regeneration of injured kidney. J Biol Chem. 2005;280:8094–100.
Oyan B. Why do targeted agents not work in the adjuvant setting in colon cancer? Expert Rev Anticancer Ther. 2012;12:1337–45.
Yamashita N, Hamada H, Tsuruo T, Ogata E. Enhancement of voltage-gated Na+ channel current associated with multidrug resistance in human leukemia cells. Cancer Res. 1987;47:3736–41.
Liu C, Yu M, Li Y, Wang H, Xu C, Zhang X, Li M, Guo H, Ma D, Guo X. Lidocaine inhibits the metastatic potential of ovarian cancer by blocking Na(V) 1.5-mediated EMT and FAK/Paxillin signaling pathway. Cancer Med. 2021;10:337–49. https://doi.org/10.1002/cam4.3621.
Levy A, Alhazzani K, Dondapati P, Alaseem A, Cheema K, Thallapureddy K, Kaur P, Alobid S, Rathinavelu A. Focal adhesion kinase in ovarian cancer: A potential therapeutic target for platinum and taxane-resistant tumors. Curr Cancer Drug Targets. 2019;19:179–88. https://doi.org/10.2174/1568009618666180706165222.
Sood AK, Coffin JE, Schneider GB, Fletcher MS, DeYoung BR, Gruman LM, Gershenson DM, Schaller MD, Hendrix MJ. Biological significance of focal adhesion kinase in ovarian cancer: role in migration and invasion. Am J Pathol. 2004;165:1087–95. https://doi.org/10.1016/s0002-9440(10)63370-6.
Liu H, Tang T. MAPK signaling pathway-based glioma subtypes, machine-learning risk model, and key hub proteins identification. Sci Rep. 2023;13:19055. https://doi.org/10.1038/s41598-023-45774-0.
Liu H, Tang T. Pan-cancer genetic analysis of disulfidptosis-related gene set. Cancer Genet. 2023;278–279:91–103. https://doi.org/10.1016/j.cancergen.2023.10.001.
Liu H, Tang T. A bioinformatic study of IGFBPs in glioma regarding their diagnostic, prognostic, and therapeutic prediction value. Am J Transl Res. 2023;15:2140–55.
Liu H, Tang T. Pan-cancer genetic analysis of cuproptosis and copper metabolism-related gene set. Front Oncol. 2022;12: 952290. https://doi.org/10.3389/fonc.2022.952290.
Gillet L, Roger S, Bougnoux P, Le Guennec JY, Besson P. Beneficial effects of omega-3 long-chain fatty acids in breast cancer and cardiovascular diseases: Voltage-gated sodium channels as a common feature? Biochimie. 2011;93:4–6. https://doi.org/10.1016/j.biochi.2010.02.005.
Onkal R, Djamgoz MB. Molecular pharmacology of voltage-gated sodium channel expression in metastatic disease: Clinical potential of neonatal Nav1.5 in breast cancer. Eur J Pharmacol. 2009;625:206–19. https://doi.org/10.1016/j.ejphar.2009.08.040.
Leslie TK, Tripp A, James AD, Fraser SP, Nelson M, Toss M, Fadhil W. A novel Nav1.5-dependent feedback mechanism driving glycolytic acidification in breast cancer metastasis. bioRxiv. 2023.06. 16.545273.
Acknowledgements
The author thanks the comments from Professor Laura M. Machesky and the support of Weifen Chen, Zongxiong Liu, Yaqi Yang, and Bryan Liu.
Funding
This study received no funding.
Author information
Authors and Affiliations
Contributions
HL conducted the analysis and literature review and drafted the manuscript. JW provided clinical insight and comments on the paper. CH and APJ edited the manuscript and supervised the project.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.