Abstract
Recently, the global incidence of gastric/gastroesophageal junction (G/GEJ) cancer has remained high. China is also a large country with a high gastric cancer (GC) incidence rate, where the cases of GC account for 40% of all cases worldwide. More than 90% of GEJ cancers are the adenocarcinoma pathological type. Patients with early-stage G/GEJ adenocarcinoma may have a better prognosis after surgery. In contrast, patients with advanced metastatic G/GEJ adenocarcinoma usually choose comprehensive treatment based on systemic pharmacotherapy, but the subsequent long-term survival is not optimistic. The discovery of various biomarkers, especially microsatellite instability (MSI), programmed cell death-ligand 1 (PD-L1), human epidermal growth factor receptor 2 (HER2), tumor mutational burden (TMB) and Epstein–Barr virus (EBV), has led to the identification of an increasing number of targeted populations and has greatly improved the clinical efficacy of treatments for G/GEJ adenocarcinoma. The ToGA trial added trastuzumab to standard chemotherapy, showed improved survival of patients with HER2-positive advanced G/GEJ adenocarcinoma and brought these patients into a new era of HER2-targeted therapy. Moreover, many HER2-targeted agents have been developed and studied in patients with advanced HER2-positive G/GEJ adenocarcinoma who have demonstrated excellent clinical outcomes. However, many patients experience disease progression with HER2-targeted therapy; hence, new anti-HER2 drugs keep being developed, significantly reducing HER2 resistance. This paper reviews HER2-targeted drugs for advanced metastatic G/GEJ adenocarcinoma, potential resistance mechanisms and future directions.
Similar content being viewed by others
Introduction
Gastric cancer (GC) is the fifth most commonly diagnosed cancer worldwide and is the fourth leading cause of cancer-related deaths, with approximately 1 million new cases and approximately 760,000 deaths due to GC worldwide in 2020 [1]. Most patients with nonmetastatic GC select endoscopic or surgical treatment [2], and for patients with advanced metastatic gastric/gastroesophageal junction (G/GEJ) adenocarcinoma, multidisciplinary treatment based on systemic pharmacotherapy is currently recommended. Standard chemotherapy regimens have usually consisted of fluorouracil and platinum with or without paclitaxel, and the resulting long-term survival has not been optimistic [3,4,5]. Therefore, it is necessary to develop new antitumor drugs and to search for additional therapeutic targets. Human epidermal growth factor receptor 2 (HER2) belongs to the epidermal growth factor receptor (EGFR) family, and its overexpression/amplification has been confirmed in various malignant neoplasms, such as breast cancer (BC), prostate cancer, and lung cancer. The application of HER2-targeted therapy has improved the survival prospects of patients with these malignancies [6,7,8]. Approximately 7.3–20.2% of patients with advanced G/GEJ adenocarcinoma have HER2 overexpression [9]. The completion of the ToGA trial and the approval of trastuzumab have positively affected the survival of patients with HER2-positive GC, as this drug has become the standard first-line treatment for patients with advanced metastatic HER2-positive GC, which has established the precedent for GC-targeted therapy [10]. The success of the CheckMate-649 trial has opened the door to immunotherapy for advanced GC, which has greatly improved the progression-free survival (PFS) and overall survival (OS) of patients with HER2-negative GC. Nevertheless, in addition to trastuzumab, the following HER2-targeted agents for advanced metastatic G/GEJ adenocarcinoma are still under investigation: monoclonal antibodies (mAbs) (e.g., pertuzumab, margetuximab, hersintuzumab), antibody–drug conjugates (ADCs) (e.g., T-DM1, DS-8201, Disitamab vedotin), bispecific antibodies (BsAbs) (e.g., ZW25, KN026), tyrosine kinase inhibitors (TKIs), and other novel therapeutic approaches (e.g., CAR-T, BVAC-B). In particular, Disitamab vedotin has been approved by the National Medical Products Administration (NMPA) for the second-line and above treatment of patients with HER2-overexpressing advanced metastatic G/GEJ adenocarcinoma. In this review, we summarize the clinical trials and potential resistance mechanisms of HER2-targeted therapy in advanced metastatic G/GEJ adenocarcinoma. We also discuss strategies to overcome resistance to HER2-targeted therapy and the development of new approaches.
Molecular mechanism of HER2-targeted therapy
HER2
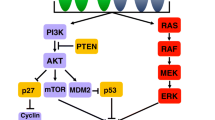
HER2, also known as ErbB2/Neu, belongs to the EGFR family and is located on human chromosome 17 (17q21); this gene encodes a 185 kDa transmembrane glycoprotein (p185). EGFR family members include HER1, HER2, HER3, and HER4, which are composed of three parts: an extracellular ligand-binding domain, a transmembrane domain, and an intracellular tyrosine kinase domain. HER2 lacks specific ligands and induces autophosphorylation of intracellular tyrosine residues by forming heterodimers with other members of the family, which activates downstream signaling (e.g., Ras-Raf-Mek-MAPK, PI3K-Akt-mTOR, JAK-STAT); this in turn promotes cell growth and proliferation and inhibits apoptosis [11] (Fig. 1).
Mechanism of action and potential resistance mechanisms of HER2-targeted therapy: A HER2-targeted ADCs enter cells through endocytosis and release toxins that act on microtubules, DNA, or other materials, thereby inhibiting cell growth, proliferation, survival and metastasis. B HER2-targeted antibodies inhibit downstream signaling by binding to the extracellular domain of HER2 and preventing the formation of dimers between HER2 and other EGFR family members, in addition to releasing perforins and granzymes to act on target cells through ADCC. C TKIs inhibit signal transduction by binding to the intracellular tyrosine kinase domain of HER2. D HER2 heterogeneity, loss of HER2-positivity, mutation/amplification, alterations in intracellular signaling, protein overexpression, miRNAs, and abnormalities in either component of the ADC process can all lead to the development of drug resistance, which prevents cell death
HER2 overexpression/amplification
HER2 overexpression is mainly due to HER2 amplification and leads to constitutive expression of the ERBB signaling network [11]. Overexpression of HER2 results in the formation of homodimers, which in turn results in ligand-independent signaling that leads to uncontrollable cell division, proliferation, differentiation, and apoptosis [11, 12]. HER2 expression is usually detected by immunohistochemistry (IHC) combined with fluorescence in situ hybridization (FISH) according to the following scale: IHC 3+ is HER2-positive and IHC 0/1+ is HER2-negative, whereas IHC 2+ requires FISH and is HER2-positive if FISH amplification is observed and is HER2-negative if FISH amplification is not observed [13]; thus, HER2 overexpression includes IHC 3+ and IHC 2+/FISH+. HER2 overexpression has been observed in BC, bladder cancer, lung cancer and other malignant tumors [6,7,8]. HER2 overexpression in GC was reported by K. Sakai et al. in 1986 [14]. HER2 positivity was more common in GEJ adenocarcinomas (32.2%) than in GC (21.4%) and was more common in intestinal tumors (31.8%) than in diffuse tumors (6.1%) [15]. In the most recent global report, HER2 overexpression in GC patients accounted for approximately 7.3–20.2% of all cases, and its expression rate varied according to country [9]. A retrospective study of 726 GC cases that underwent surgical resection at 4 clinical centers in China reported a HER2-positive rate of 13% [16], while another global multicenter study evaluated 734 patients with G/GEJ adenocarcinoma at 11 Chinese hospitals and found that 12% were HER2-positive [17].
Furthermore, the relationship between HER2 overexpression and the prognosis of GC patients remains controversial. A prospective study reported a poor prognosis in patients with HER2-overexpressing GC [18], and the results of a multicenter study in Japan that evaluated the relationship between HER2 status and prognosis in 1148 GC patients also showed that HER2 overexpression was associated with prognosis [19]. In contrast Sarah B Fisher et al. analyzed the HER2 status of 111 patients with G/GEJ adenocarcinoma and suggested that HER2 overexpression/amplification was not associated with poor prognosis [20]. Similarly, Shen et al. failed to find an association between HER2 expression levels and prognosis in 1562 GC patients in China [21]. Thus, the prognostic value of HER2 in patients with G/GEJ adenocarcinoma remains unclear and needs to be determined by further studies.
Low HER2 expression and mutations
Low HER2 expression includes HER2 IHC 1+ and IHC 2+/FISH+ [22]. The CheckMate-649 trial led to the addition of nivolumab to the first-line treatment of patients with HER2-low GC [23], but more response-persistent drugs are still needed. Recent clinical trials have shown that ADCs, such as DS-8201 and Disitamab vedotin, release intracellular toxins that can exert a killing effect on neighboring cells without target expression. This is termed the bystander effect and allows patients with HER2- low GC to benefit from HER2-targeted therapy [24, 25]. Therefore, ADCs may expand the population that can benefit from HER2-targeted therapy and are expected to be a novel option for patients whose tumors have low HER2 expression.
HER2 mutations have been demonstrated in a variety of malignancies, such as lung cancer, colorectal cancer (CRC), BC, and uroepithelial cancer (UC), and are associated with different outcomes in different cancers. Patients with HER2-mutated BC have a worse prognosis than those without mutations; in non-small cell lung cancer (NSCLC), patients with HER2 mutations are more sensitive to some HER2-targeted therapeutic agents. Patients with HER2-mutated CRC also have KRAS mutations, which suggests that concomitant HER2 and KRAS mutations may promote colorectal tumorigenesis, and these patients exhibit little response to EGFR-targeted therapy [26,27,28,29]. Several drugs are effective in patients with HER2-mutated NSCLC, such as poziotinib, DS-8201, and T-DM1 [30,31,32]. Therefore, the detection of HER2 mutations is essential for individualized treatment.
Molecular mechanism of HER2 blockade
HER2 blockade improves the prognosis of patients with HER2-positive tumors by two primary mechanisms: (1) HER2 blockade prevents the binding of ligands to HER2 receptors and promotes the internalization and degradation of the receptors, which causes inhibition of their downstream signaling pathways, thereby regulating cell survival, proliferation, and invasion [33]; (2) HER2 blockade triggers antibody-dependent cell-mediated cytotoxicity (ADCC) by targeting HER2 receptors, which induces antitumor immunity [34]. Therefore, the development of targeted therapeutics that act on HER2 could be effective in prolonging the survival of patients.
HER2-targeted therapeutic agents
HER2-targeted antibodies
Monoclonal antibodies (mAbs)
Trastuzumab
Trastuzumab is a HER2-targeted mAb that binds to the extracellular domain of HER2 (domain IV) to inhibit downstream signaling and induce ADCC by blocking the activation of the intracellular tyrosine kinase domain [35]. The ToGA trial added trastuzumab to standard chemotherapy, which led to a significant survival benefit for patients with HER2-positive advanced G/GEJ adenocarcinoma (median OS, mOS 13.8 vs. 11.1 months; P = 0.046); consequently, trastuzumab in combination with chemotherapy is now the current first-line standard of care for patients with advanced metastatic HER2-positive GC [10]. Subsequent similar trials have yielded positive results and have demonstrated the feasibility of standard-dose trastuzumab in combination with chemotherapy as a first-line treatment regimen in dose-escalation studies [36,37,38,39,40,41].
Trastuzumab has also shown efficacy as a second-line therapy in untreated HER2-positive GC patients [42]. Moreover, trastuzumab is controversial as a cross-line therapy. A single-center trial in China found that continuation of trastuzumab was effective when progression occurred after first-line standard therapy in patients with advanced GC [43], while the phase II T-ACT trial failed to show improvements in PFS and OS in HER2-positive GC patients who progressed after first-line standard therapy. Notably, this trial did not assess HER2 status prior to continuation of trastuzumab, and in an exploratory analysis, loss of HER2-positive status occurred in 69% (11/16) of evaluable patients after first-line therapy [44].
Trastuzumab has also demonstrated promising utility as a perioperative therapy. Several trials using trastuzumab in combination with chemotherapy or radiotherapy for the perioperative treatment of HER2-positive resectable gastroesophageal adenocarcinoma (GEA) have reported considerable survival benefits [45,46,47]. The ongoing INNOVATION trial, which is testing trastuzumab or trastuzumab and pertuzumab as a perioperative treatment in patients with HER2-positive resectable G/GEJ adenocarcinoma, indicates that this therapy may be the standard of care in the perioperative period [48]. In contrast, a single-center retrospective study by Qifei He et al. found that trastuzumab added to perioperative therapy in patients with resectable HER2-positive GC resulted in tumor shrinkage and prolonged OS, but no meaningful improvement in OS was observed when trastuzumab was used as a neoadjuvant therapy (P = 0.126) [49]. As such, the value of trastuzumab as a neoadjuvant therapy in patients with HER2-positive G/GEJ adenocarcinoma requires further exploration.
Pertuzumab
Pertuzumab is a novel humanized anti-HER2 mAb that, unlike trastuzumab, inhibits downstream signaling by binding to the dimer-forming domain of HER2 (domain II) and preventing its dimerization with other EGFR family members [50]. The efficacy of pertuzumab in patients with HER2-positive advanced GC is unclear. In preclinical studies, although pertuzumab plus trastuzumab enhanced antitumor activity [51], the addition of pertuzumab to standard therapy failed to significantly improve the survival of patients with HER2-positive metastatic G/GEJ adenocarcinoma in the first-line placebo-controlled JACOB trial (mOS 17.5 vs. 14.2 months; P = 0.056) [52]. The PETRARCA trial evaluated the efficacy of trastuzumab and pertuzumab in combination with chemotherapy for HER2-positive resectable GEA during perioperative care, and although no significant survival benefit was observed, the pathological complete response rate (pCR) was higher in patients who received dual-target therapy [53].
Margetuximab
Margetuximab, an Fc-optimized mAb that targets HER2 with the same binding epitope as trastuzumab, affects the cell’s ability to kill tumor cells by increasing the affinity for activated Fc receptor (CD16A) and by decreasing the affinity for inhibitory Fc receptor (CD32B) and ADCC effects [54]. Phase I clinical trials that tested single-agent margetuximab reported favorable tolerability and activity [55]. The CPMGAH22–05 trial tested second-line therapy and beyond in patients with advanced HER2-positive GC, and chemo-free margetuximab plus pembrolizumab led to meaningful outcomes [56]. The ongoing phase II MAHOGANY trial will further explore the efficacy of margetuximab in combination with anti-PD-1 antibody for the first-line treatment of patients with G/GEJ adenocarcinoma [57].
Hersintuzumab
Hersintuzumab is a humanized mAb that targets HER2 extracellular domains I-II. In HER2-positive tumor xenograft models, the combination of hersintuzumab and trastuzumab inhibited tumor cell growth and showed the strongest antitumor activity in ovarian cancer and GC xenograft models [58]. This drug is currently being tested in preclinical studies.
Bispecific antibodies (BsAbs)
Zanidatamab (ZW25)
ZW25 is a novel enzymatic BsAb that targets both HER2 extracellular domains II and IV and activates ADCC. Reliable tolerability and monotherapeutic antitumor activity were demonstrated in a phase I clinical trial [59]. The ongoing phase II trial published data on ZW25 combined with chemotherapy in the first-line treatment of patients with HER2-positive GEA. The study reported a high disease control rate (DCR) of 100% in the ZW25 combined cohort and a reduction in tumor size in all but one patient, but 61% of patients were still receiving treatment at the time of data cutoff (DCR 89%; median PFS, mPFS 12 months) [60]. This finding supports the use of ZW25 in combination with chemotherapy as a potential new first-line standard of care for patients with HER2-positive GEA. Another ongoing trial (NCT04276493) is testing ZW25 in combination with chemotherapy and the anti-PD-1 antibody tislelizumab as a first-line treatment of patients with HER2-positive metastatic G/GEJ adenocarcinoma.
KN026
KN026 binds to HER2 domains II and IV to achieve the same effect as the combination of trastuzumab and pertuzumab. In a phase II clinical trial, KN026 showed favorable efficacy in patients with HER2-overexpressing G/GEJ adenocarcinoma with an objective response rate (ORR) of 55.6%; common adverse events included liver dysfunction and rash [61]. The ongoing KN026–001 phase II/III trial will further explore the survival benefit of KN026 in combination with chemotherapy as a second-line and beyond treatment in patients with HER2-positive advanced G/GEJ adenocarcinoma.
Other BsAbs
PRS-343 is a BsAb that targets HER2 and the costimulatory immunoreceptor 4-1BB on immune cells. In a phase I clinical trial, PRS-343 demonstrated antitumor activity as a single agent and in combination with the anti-PD-L1 antibody atezolizumab in previously treated patients with advanced HER2-positive solid tumors (including GC) [62]. The phase II trial is currently enrolling (NCT05190445) and will evaluate the efficacy of PRS-343 in combination with ramucirumab and paclitaxel in previously treated patients with treated HER2-overexpressing G/GEJ adenocarcinoma.
MM-111 is a BsAb that specifically binds to HER2 and HER3 and prevents the formation of HER2/HER3 heterodimers. MM-111 was discontinued in a trial of patients with advanced HER2-positive GEA due to worse PFS and OS, and all further clinical trials have now been withdrawn [63]. In addition, several drugs are currently being tested in preclinical studies [64] [65] (Tables 1, 2; Fig. 1B).
Antibody–drug conjugates (ADCs)
Trastuzumab emtansine (T-DM1)
T-DM1, an ADC of trastuzumab bound to the antitubulin molecule maytansine, delivers maytansine into tumor cells via receptor-mediated endocytosis, which leads to cell death [66]. T-DM1 inhibits the proliferation of both HER2-positive GC cell lines and trastuzumab-resistant GC cell lines in vivo and in vitro [67]. However, in the phase II/III GATSBY trial, the comparison of T-DM1 and paclitaxel for the second-line treatment of patients with HER2-positive advanced G/GEJ adenocarcinoma failed to show an advantage (mOS 7.9 vs. 8.6 months; P = 0.86) possibly because previously archived tumor tissue was selected for this study to assess HER2 status. Those patients had also received HER2-targeted therapy, and thus, HER2 negativity may have led to a lack of response to T-DM1 [68]. The subsequent GATHER3 trial compensated for this disappointing result by including a secondary biopsy in the assessment, and of the 13 HER2-positive GC patients treated with T-DM1, three were negative for HER2 during treatment, and therefore, no disease remission was observed; the ORR of the remaining patients was 44% [69]. This shows the importance of reassessing HER2 status before starting second-line HER2-targeted therapy. Potential drawbacks of T-DM1 have been reported, including a slow internalization rate, insufficient bystander effect, and a lack of intracellular transport and expression of the drug transporter protein MDR1 [70].
Trastuzumab deruxtecan (T-Dxd, DS-8201)
DS-8201 is an ADC of trastuzumab coupled to a DNA topoisomerase I inhibitor, which causes cell cycle arrest and apoptosis of tumor cells by binding to topoisomerase I-DNA and inhibiting DNA replication. DS-8201 significantly reduced the risk of death in patients with HER2-positive BC compared with TDM-1 [71], and it was also approved by the U.S. Food and Drug Administration (FDA) in May 2022 for patients with HER2-positive metastatic BC who had previously been treated with anti-HER2 therapy and for those who were treated during or within 6 months of neoadjuvant or adjuvant therapy. In addition, DS-8201 also significantly prolonged PFS and OS in patients with HER2-low BC [22], possibly because the DS-8201 connexon is the enzyme unstable type that facilitates release of the payload after which the nonpolar payload crosses the cell membrane more easily, thus exerting a bystander effect [72]. Moreover, in an animal model study, it was shown that the bystander effect of DS-8201 is dependent on neighboring HER2-positive cells, affects only the tumor microenvironment and does not lead to systemic toxicity [73]. In GC, a phase I trial demonstrated acceptable safety and a favorable response rate [74]. The phase II DESTINY-Gastric01 trial evaluated the efficacy of DS-8201 plus chemotherapy in patients with HER2-positive G/GEJ adenocarcinoma who progressed after first-line treatment. Significant improvements in patient OS (12.5 vs. 8.4 months; P = 0.0097) and response rate (51.3% vs. 14.3%) compared with standard therapy were observed, and a survival benefit was also reported in patients with G/GEJ adenocarcinoma with low HER2 expression, with myelosuppression and interstitial lung disease being the major adverse events [24]. Therefore, DS-8201 may provide a new option for patients who progress on prior trastuzumab. In January 2021, the FDA approved DS-8201 for patients with HER2-positive G/GEJ adenocarcinoma who were treated with prior trastuzumab. Other trials are also in progress (NCT04014075, NCT04379596, NCT04704934) [75].
Disitamab vedotin
Disitamab vedotin is an ADC consisting of an anti-HER2 mAb and the antitubulin molecule monomethyl auristatin E. This agent has demonstrated safety and potent antitumor activity in a phase I trial in patients with advanced HER2-positive GC [76]. The phase II RC48-C008 trial, conducted in patients with advanced HER2-positive GC for third-line therapy and beyond, also showed a meaningful benefit (ORR 24.8%; mPFS 4.1 months; mOS 7.9 months); the main adverse effects were myelosuppression and malaise [77]. The ongoing phase III RC48-C007 (NCT04714190) trial will further compare the effectiveness of Disitamab vedotin with a standard treatment strategy as a second-line treatment and beyond in patients with advanced HER2-positive GC. In June 2021, Disitamab vedotin was approved in China as a second-line treatment for patients with HER2-overexpressing advanced or metastatic G/GEJ adenocarcinoma. Moreover, a recent preclinical study showed that Disitamab vedotin was more efficacious than trastuzumab in HER2-positive GC patient-derived xenografts (PDX) and demonstrated effectiveness in HER2-negative advanced GC [25].
ARX788
ARX788 is a novel ADC formed by the combination of the antitubulin molecule amberstatin 269 and an anti-HER2 mAb; this drug significantly inhibited the growth of tumor cells in a T-DM1-resistant HER2-positive GC model in a preclinical study and was superior to T-DM1 [78, 79]. The phase I ACE-Gastric-01 trial explored the tolerability and antitumor activity of ARX788 in patients with previously treated HER2-positive advanced G/GEJ adenocarcinoma [80]. An ongoing phase I trial will further validate its safety and efficacy (NCT03255070).
Other ADCs
With the more prevalent use of ADCs in patients with advanced HER2-positive GC, increasing numbers of drugs have been tested in phase I clinical trials. SYD985 and A166 (NCT03602079) were evaluated in patients with advanced HER2-positive GC and showed preliminary effects [81, 82]. MEDI4276 demonstrated stronger antitumor effects than T-DM1 in vitro, but an objective response was not observed in patients with HER2-positive GC who had previously received standard therapy [83, 84]. The phase 1 trial of ADCT-502 in HER2-positive advanced solid tumors, including GC (NCT03125200), was recently terminated due to safety concerns, and no additional trials are currently underway. PF-06804103 (NCT03284723), XMT-1522 (NCT02952729), GQ1001 (NCT04450732), MRG002 (NCT04492488), and ZW49 (NCT03821233) are also currently being tested in phase I clinical trials [85,86,87,88], while several other drugs are being tested in preclinical studies [89]. The development of these new drugs will bring more hope to patients (Tables 2, 3; Fig. 1A).
Tyrosine kinase inhibitors (TKIs)
Lapatinib
Lapatinib, a TKI that targets HER2 and EGFR, blocks autophosphorylation of HER2 intracellular tyrosine kinases, thereby inhibiting downstream signaling. The phase 3 TyTan trial that tested lapatinib as a second-line treatment in Asian patients with advanced GC showed a better survival benefit in the HER2 IHC 3+ subgroup (PFS 5.6 vs. 4.2 months; P = 0.0101) but failed to significantly improve OS in the total population (mOS 11.0 vs. 8.9 months; P = 0.104), possibly because the overall population of HER2 IHC 0/1+ patients (35%) was higher [90]. Moreover, the TRIO-013/LOGiC trial evaluated the efficacy of lapatinib in combination with standard chemotherapy in patients with HER2-positive advanced GC as a first-line treatment, but lapatinib did not improve patient OS compared with placebo (mOS 12.2 vs. 8.9 months; P = 0.349); however, OS was significantly prolonged in Asian and younger patients (< 60 years) in an exploratory analysis [91]. Although lapatinib was beneficial in HER2 IHC 3+ patients and in some Asian and younger patients, these trials did not meet the primary endpoint; thus, lapatinib is not recommended for patients with HER2-positive advanced GC, and its use may require a more rigorous screening strategy. In addition, lapatinib combined with chemotherapy has shown efficacy as a neoadjuvant therapy in patients with resectable HER2-positive GEA; in those patients, diarrhea was a common adverse event but did not interfere with surgical treatment [92].
Tucatinib
Tucatinib is a reversible HER2-targeted small-molecule TKI. Tucatinib plus trastuzumab has shown inhibition in HER2-positive GC xenograft models [93], and a phase Ib/II trial of tucatinib combined with trastuzumab and chemotherapy for patients with untreated advanced GC is ongoing (NCT04430738). As a second-line treatment of HER2-positive G/GEJ adenocarcinoma, trials of tucatinib in combination with several agents are currently recruiting patients (NCT04499924, NCT05190445), and the results of a phase I trial of tucatinib alone have just been published (NCT05382364) [94].
Pyrotinib
Pyrotinib is a novel oral TKI that targets EGFR, HER2, and HER4. A trial of pyrotinib combined with chemotherapy in patients with advanced HER2-overexpressing solid tumors included 9 patients with GC, whose outcomes were not promising (mPFS: 2.9 months, mOS: 5.9 months) and in whom diarrhea was the most common adverse event [95]. Another phase Ib trial combining pyrotinib with the CDK4/6 inhibitor SHR6390 showed a satisfactory benefit in patients with HER2-positive advanced GC who had received standard therapy, which suggests that this regimen may be a viable treatment strategy for patients with HER2-positive GC, but this requires confirmation in a larger sample [96].
Other TKIs
TKIs have been extensively studied in advanced/metastatic HER2-positive G/GEJ adenocarcinoma. The phase II DEBIOC trial of sapitinib in combination with chemotherapy demonstrated antitumor activity in the neoadjuvant treatment of patients with HER2-positive resectable GEA [97]. A phase II trial of dacomitinib monotherapy showed efficacy and safety in patients with HER2-positive GC who had received prior treatment [98]. After poziotinib showed reliable tolerability and toxicity in a phase I trial, a phase I/II trial was conducted in patients with HER2-positive advanced GC, and its combination with paclitaxel and trastuzumab exhibited promising antitumor activity and acceptable toxicity as a second-line therapy [99, 100]. Afatinib showed a favorable benefit in a phase II trial in patients with advanced refractory HER2-positive GEA, and phase II trials of afatinib in combination with paclitaxel as a second-line therapy for advanced/recurrent GEJ adenocarcinoma are ongoing (NCT02501603, NCT01522768) [101]. A phase I clinical trial of neratinib in patients with advanced HER2-positive GC is also ongoing (NCT05274048) (Tables 2, 4; Fig. 1C).
Combined immunotherapy
In recent years, the emergence of immune checkpoint inhibitors (ICIs) has opened new avenues for GC patients.
Pembrolizumab is a humanized mAb that inhibits PD-1 activity by binding to the PD-1 receptor on T cells. It has been shown that HER2-targeted therapy may increase PD-L1 expression on tumor cells, which would further enhance the potential synergy between these agents [102, 103]. Two clinical trials that tested first-line pembrolizumab in combination with trastuzumab and chemotherapy both showed good survival benefits [102, 104]. The ongoing phase 3 KEYNOTE-811 trial that compared pembrolizumab and placebo to chemotherapy combined with trastuzumab as a first-line treatment for patients with advanced G/GEJ adenocarcinoma has now reached the secondary endpoint with an impressive disease response (ORR 74.4% vs. 51.9%, DCR 96.2% vs. 89.3%) [105]. Based on this trial, the FDA granted accelerated approval of pembrolizumab plus trastuzumab combination chemotherapy in May 2021 for the first-line treatment of patients with locally advanced unresectable or metastatic HER2-positive G/GEJ adenocarcinoma. Moreover, a study of pembrolizumab plus margetuximab for second-line and beyond treatment in patients with advanced HER2-positive G/GEJ adenocarcinoma confirmed the synergistic antitumor effects of HER2-targeted agents and ICIs [56]. Another trial evaluating margetuximab plus the ICIs retifanlimab or tebotelimab with or without chemotherapy for the first-line treatment of patients with HER2-positive G/GEJ adenocarcinoma is underway [57].
Nivolumab is also a humanized mAb that inhibits PD-1 and that has demonstrated efficacy as a third-line therapy and beyond in patients with advanced G/GEJ adenocarcinoma who have been treated with trastuzumab [106]. A phase II trial of nivolumab and trastuzumab in combination with chemotherapy or other ICIs has been completed and is awaiting publication [107]. In addition, the PD-L1/CTLA-4 inhibitor BsAb KN046 in combination with the HER2-targeted BsAb KN026 also showed efficacy in HER-positive solid tumors [108].
Furthermore, the combination of ADCs and ICIs demonstrated antitumor activity in preclinical studies [109]. In the phase Ib DS8201-A-U105 trial, DS-8201 plus nivolumab did not demonstrate a better benefit than DS8201 monotherapy in patients with HER2-positive BC, but the results in the UC cohort were surprising (DCR 76.6%; mPFS 6.9 months; mOS 11.0 months) [110]. The RC48-C014 trial used Disitamab vedotin and toripalimab to treat patients with metastatic UC and showed a survival benefit regardless of whether the patients’ tumors expressed HER2 or PD-L1 [111]. No trials have tested the combination of ADCs and ICIs for advanced GC, and the combination regimen of ADCs and ICIs is inconclusive and requires further confirmation in additional clinical trials (Table 5).
Anti-HER2 chimeric antigen receptor (CAR) cellular therapies and other novel treatments
Anti-HER2 CAR cellular therapies include CAR-T-cell therapy, CAR-natural killer cell (NK) therapy, and CAR-macrophage (CAR-M) therapy. CAR-T-cell immunotherapy integrates the extracellular antigen-binding domain and transmembrane costimulatory domain of tumor-associated antigens (TAAs) with activated T cells, which are expanded in vitro and then infused back into the body to act on tumor cells, causing their death. CAR-T cells can persist in vivo for a long time and can produce durable tumor cell recognition and clearance effects [112]. Yanjing Song et al. found that CAR-T cells produced effective and durable antitumor effects in HER2-positive GC xenograft models [113]. This finding suggests that HER2-targeted CAR-T-cell therapy is a potential therapeutic strategy for patients with HER2-positive advanced GC, but this treatment approach still requires validation in future trials. Several phase I trials evaluating the safety, tolerability and antitumor activity of CAR-T cells in patients with relapsed or refractory HER2-positive solid tumors are currently recruiting patients (NCT04511871, NCT04650451). With the success of CAR-T-cell therapy, Xian Wu et al. introduced HER2-specific CAR (5.137.z) into NK-92 cells, which are termed NK-92/5. 137. z cells; these cells cleared tumor cells in HER2-positive GC xenograft models but did not effectively control larger tumors, which were improved by the addition of apatinib [114]. In addition, CAR-M therapy significantly reduced tumor load and prolonged OS in mouse xenograft models [115]. CT-0508 is a newly developed CAR-M therapy that has shown a survival benefit in a preclinical study of HER2-positive solid tumors and is currently being tested in a phase I clinical trial in patients with HER2-positive solid tumors (including GC) that have progressed after anti-HER2 therapy; the disclosure of the efficacy data is highly anticipated (NCT04660929). A phase I trial of MCY-M11, another CAR containing CAR-Ms, in patients with recurrent/refractory ovarian cancer and peritoneal mesothelioma was terminated due to a shift in focus by the sponsor (NCT03608618). The widespread use of HER2-targeted CAR cell therapy in solid tumors will lead to new drug candidates for patients with HER2-positive solid tumors.
Other novel therapeutic approaches include B-cell and monocyte-based immunotherapeutic vaccines (BVAC-B), ultrasound-mediated sonodynamic therapy, BAY2701439 and CAM-H2 targeted HER2 radiotherapy (NCT04147819, NCT04467515) [116, 117].
Potential resistance mechanisms of HER2-targeted therapy
The ToGA trial has brought GC into the era of HER2-targeted therapy, and increasing numbers of HER2-targeted therapeutics have been widely studied in GC, yet most patients with advanced GC still inevitably experience disease progression or death due to resistance. Primary or acquired resistance is a major challenge for GC patients, and multiple potential resistance mechanisms have been explored (Fig. 1D).
HER2 heterogeneity
HER2 heterogeneity includes changes in HER2 expression status and HER2 copy number. During HER2-targeted therapy, tumor cells with HER2 overexpression or HER2 amplification die, while residual drug-resistant cells proliferate and gradually dominate, which eventually leads to tumor recurrence. The heterogeneity of HER2 expression has been reported to be high (45–79%) in HER2-positive GC and is significantly correlated with patient survival; thus, the heterogeneity of HER2 expression was considered a factor in resistance to HER2-targeted therapy [118,119,120,121]. In addition, heterogeneity in HER2 expression is observed between primary and metastatic sites, which leads to an increased risk of false-positive HER2 failure of HER2-targeted therapy [122, 123].
Loss of HER2-positive status
In some clinical trials, 29–69% of GC patients may experience loss of HER2-positive status after trastuzumab progression, which is an important cause of resistance [44, 69, 118]. Therefore, the HER2 status of patients should be re-evaluated upon progression after HER2-targeted therapy to select the optimal treatment.
Mutation/amplification
Mutations are also a potential cause of resistance to HER2-targeted therapy. De novo mutations in HER2, which are located in the part of the protein involved in the regulation of kinase activity, have been identified in trastuzumab-treated HER2-overexpressing GC cell lines and lead to the maintenance of the active structure of HER2 by affecting its conformation [124]. Several studies have also reported that deletion of ErbB16 exons and comutation and/or amplification of MET, CCNE1, KRAS, EGFR, HER3, PI3K or PTEN can also lead to the development of resistance [101, 118, 125].
Alterations in intracellular signaling
HER2-targeted therapy inhibits the transduction of downstream signaling by blocking the binding of HER2 receptors and ligands, which inhibits the migration and proliferation of tumor cells and leads to apoptosis. Alterations in receptor tyrosine kinase-RAS-PI3K signaling have been reported to be associated with acquired resistance to trastuzumab [118]. Additionally, activation of the bypass pathway can lead to resistance. In their study, Aïda Sampera et al. found that the development of drug resistance in HER2-positive GC cell lines was associated with sustained activation of the MAPK-ERK and PI3K-mTOR pathways mediated by SRC [126]. In addition, NRF2 leads to the development of drug resistance by activating PI3K-mTOR [127].
Protein overexpression
Overexpression of some proteins leads to the development of resistance. High expression of PD-L1 was found in trastuzumab-resistant HER2-positive GC cells, and blockade of PD-L1 reversed trastuzumab resistance in trastuzumab-resistant cells. Preliminary results from the KEYNOTE-811 trial also confirmed the synergistic effect of trastuzumab combined with the anti-PD-L1 antibody pembrolizumab [105, 128]. Overexpression of HOTAIR, GSE1, CMIP, and IQGAP1 in GC cells also enhanced resistance to trastuzumab [124, 129,130,131].
MicroRNAs (miRNAs)
miRNAs are usually exosome-rich and can spread between cells and exert regulatory effects on drug resistance in various cancers. miR-301a-3p is induced by endoplasmic reticulum stress and mediates trastuzumab resistance by regulating the expression of multiple proteins in HER2-positive GC cells [132]. miR200c deletion induces trastuzumab resistance through TGF-β [133].
ADC-specific resistance mechanisms
After ADC enters the body and binds to the target antigen on the surface of tumor cells and is endocytosed by tumor cells, the ADC binds to the Fc receptor in the endosome and is then transported to the cell surface for release into the extracellular space. However, other ADC-antigen complexes enter the lysosome, where enzymes or the acidic environment can degrade the ADC, thus releasing cytotoxic chemicals that play a role in tumor cell death. Abnormalities in either component of this process can lead to the development of drug resistance. Preclinical studies have shown that altered internalization and transport pathways and abnormal metabolism are associated with T-DM1 resistance in HER2-positive GC cell lines, but these findings have not been confirmed in a clinical setting [134, 135].
Future prospects
The advent of HER2-targeted agents offers new hope for patients with advanced HER2-positive GC. The ToGA trial enabled the addition of trastuzumab to the first-line standard of care for patients with advanced HER2-positive GC and ushered in a new era of HER2-targeted therapy, while the benefits of trastuzumab as a neoadjuvant and adjuvant therapy have also been demonstrated. However, HER2-targeted agents other than trastuzumab, such as pertuzumab, T-DM1, and lapatinib, have failed to demonstrate better efficacy as a first-line therapy. Dual HER2-targeted therapy also did not significantly improve the prognosis of patients with advanced GC. Therefore, HER2-targeted therapy still needs to be improved. Several trials have revealed synergistic effects of HER2-targeted therapy combined with anti-PD-L1 antibody in HER2-positive advanced GC. With the approval of combination chemotherapy regimens consisting of pembrolizumab and trastuzumab, many researchers have also begun to experiment with other combinations of HER2-targeted agents and ICIs for advanced HER2-positive GC. This shows that the first-line treatment of advanced/metastatic G/GEJ adenocarcinoma has entered a new era of immunotherapy in combination with targeted therapy and chemotherapy. In addition, in G/GEJ adenocarcinoma with low HER2 expression (HER2 IHC 1+ and IHC 2+/FISH-), ADCs (e.g., Disitamab vedotin, DS-8201) use cleavable connexons that can release payloads before internalization, some of which are hydrophobic or nonpolar payloads that can easily cross cell membranes. They also demonstrate excellent ability through bystander effects but may also cause toxicity in nontumor tissues, and thus, this therapeutic approach is still not sufficiently mature; consequently, more targeted drugs with good safety and durable response profiles are urgently needed (Table 6).
Trastuzumab resistance is also a major obstacle in the treatment of GC patients. Loss of HER2-positive status after disease progression with HER2-targeted therapy has been observed in several studies. Therefore, it is necessary to reassess HER2 status in patients who require later-line therapy.
In conclusion, HER2 is a promising therapeutic target, and although many HER2-targeted drugs are available, the treatment efficiency in patients with advanced GC is low, and the survival benefit is not satisfactory due to the high heterogeneity of GC and the development of resistance. Therefore, new HER2-targeted drugs should continue to be developed to improve the survival of GC patients.
Availability of data and materials
The material supporting the conclusion of this review has been included within the article.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Ono H, Yao K, Fujishiro M, Oda I, Uedo N, Nimura S, et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer (second edition). Dig Endosc. 2021;33(1):4–20.
Luo HY, Xu RH, Wang F, Qiu MZ, Li YH, Li FH, et al. Phase II trial of XELOX as first-line treatment for patients with advanced gastric cancer. Chemotherapy. 2010;56(2):94–100.
Xu R-h, Wang Z-Q, Shen L, Wang W, Lu J-W, Dai G, et al. S-1 plus oxaliplatin versus S-1 plus cisplatin as first-line treatment for advanced diffuse-type or mixed-type gastric/gastroesophageal junction adenocarcinoma: a randomized, phase 3 trial. J Clin Oncol. 2019;37(15_suppl):4017.
Lu Z, Zhang X, Liu W, Liu T, Hu B, Li W, et al. A multicenter, randomized trial comparing efficacy and safety of paclitaxel/capecitabine and cisplatin/capecitabine in advanced gastric cancer. Gastric Cancer. 2018;21(5):782–91.
Arteaga CL, Sliwkowski MX, Osborne CK, Perez EA, Puglisi F, Gianni L. Treatment of HER2-positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol. 2011;9(1):16–32.
Neto AS, Tobias-Machado M, Wroclawski ML, Fonseca FL, Teixeira GK, Amarante RD, et al. Her-2/neu expression in prostate adenocarcinoma: a systematic review and meta-analysis. J Urol. 2010;184(3):842–50.
Junker K, Stachetzki U, Rademacher D, Linder A, Macha HN, Heinecke A, et al. HER2/neu expression and amplification in non-small cell lung cancer prior to and after neoadjuvant therapy. Lung Cancer. 2005;48(1):59–67.
Abrahao-Machado LF, Scapulatempo-Neto C. HER2 testing in gastric cancer: an update. World J Gastroenterol. 2016;22(19):4619–25.
Bang Y-J, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–97.
Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127–37.
Worthylake R, Opresko LK, Wiley HS. ErbB-2 amplification inhibits down-regulation and induces constitutive activation of both ErbB-2 and epidermal growth factor receptors. J Biol Chem. 1999;274(13):8865–74.
Bartley AN, Washington MK, Colasacco C, Ventura CB, Ismaila N, Benson AB 3rd, et al. HER2 testing and clinical decision making in gastroesophageal adenocarcinoma: guideline from the College of American Pathologists, American Society for Clinical Pathology, and the American Society of Clinical Oncology. J Clin Oncol. 2017;35(4):446–64.
Sakai K, Mori S, Kawamoto T, Taniguchi S, Kobori O, Morioka Y, et al. Expression of epidermal growth factor receptors on normal human gastric epithelia and gastric carcinomas. J Natl Cancer Inst. 1986;77(5):1047–52.
Van Cutsem E, Bang YJ, Feng-Yi F, Xu JM, Lee KW, Jiao SC, et al. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. 2015;18(3):476–84.
Sheng WQ, Huang D, Ying JM, Lu N, Wu HM, Liu YH, et al. HER2 status in gastric cancers: a retrospective analysis from four Chinese representative clinical centers and assessment of its prognostic significance. Ann Oncol. 2013;24(9):2360–4.
Huang D, Lu N, Fan Q, Sheng W, Bu H, Jin X, et al. HER2 status in gastric and gastroesophageal junction cancer assessed by local and central laboratories: Chinese results of the HER-EAGLE study. PLoS One. 2013;8(11):e80290.
García I, Vizoso F, Martín A, Sanz L, Abdel-Lah O, Raigoso P, et al. Clinical significance of the epidermal growth factor receptor and HER2 receptor in resectable gastric cancer. Ann Surg Oncol. 2003;10(3):234–41.
Kurokawa Y, Matsuura N, Kimura Y, Adachi S, Fujita J, Imamura H, et al. Multicenter large-scale study of prognostic impact of HER2 expression in patients with resectable gastric cancer. Gastric Cancer. 2015;18(4):691–7.
Fisher SB, Fisher KE, Squires MH 3rd, Patel SH, Kooby DA, El-Rayes BF, et al. HER2 in resected gastric cancer: is there prognostic value? J Surg Oncol. 2014;109(2):61–6.
Shen GS, Zhao JD, Zhao JH, Ma XF, Du F, Kan J, et al. Association of HER2 status with prognosis in gastric cancer patients undergoing R0 resection: a large-scale multicenter study in China. World J Gastroenterol. 2016;22(23):5406–14.
Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387(1):9–20.
Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet (London, England). 2021;398(10294):27–40.
Shitara K, Bang YJ, Iwasa S, Sugimoto N, Ryu MH, Sakai D, et al. Trastuzumab Deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. 2020;382(25):2419–30.
Chen Z, Yuan J, Xu Y, Zhang C, Li Z, Gong J, et al. From AVATAR mice to patients: RC48-ADC exerted promising efficacy in advanced gastric cancer with HER2 expression. Front Pharmacol. 2021;12:757994.
Connell CM, Doherty GJ. Activating HER2 mutations as emerging targets in multiple solid cancers. ESMO open. 2017;2(5):e000279.
Petrelli F, Tomasello G, Barni S, Lonati V, Passalacqua R, Ghidini M. Clinical and pathological characterization of HER2 mutations in human breast cancer: a systematic review of the literature. Breast Cancer Res Treat. 2017;166(2):339–49.
Greulich H, Kaplan B, Mertins P, Chen TH, Tanaka KE, Yun CH, et al. Functional analysis of receptor tyrosine kinase mutations in lung cancer identifies oncogenic extracellular domain mutations of ERBB2. Proc Natl Acad Sci U S A. 2012;109(36):14476–81.
Kloth M, Ruesseler V, Engel C, Koenig K, Peifer M, Mariotti E, et al. Activating ERBB2/HER2 mutations indicate susceptibility to pan-HER inhibitors in lynch and lynch-like colorectal cancer. Gut. 2016;65(8):1296–305.
Li BT, Smit EF, Goto Y, Nakagawa K, Udagawa H, Mazières J, et al. Trastuzumab Deruxtecan in HER2-mutant non-small-cell lung cancer. N Engl J Med. 2022;386(3):241–51.
Elamin YY, Robichaux JP, Carter BW, Altan M, Gibbons DL, Fossella FV, et al. Poziotinib for patients with HER2 exon 20 mutant non-small-cell lung cancer: results from a phase II trial. J Clin Oncol. 2022;40(7):702–9.
Li BT, Shen R, Buonocore D, Olah ZT, Ni A, Ginsberg MS, et al. Ado-Trastuzumab Emtansine for patients with HER2-mutant lung cancers: results from a phase II basket. Trial. 2018;36(24):2532–7.
Hsu JL, Hung MC. The role of HER2, EGFR, and other receptor tyrosine kinases in breast cancer. Cancer Metastasis Rev. 2016;35(4):575–88.
Cooley S, Burns LJ, Repka T, Miller JS. Natural killer cell cytotoxicity of breast cancer targets is enhanced by two distinct mechanisms of antibody-dependent cellular cytotoxicity against LFA-3 and HER2/neu. Exp Hematol. 1999;27(10):1533–41.
Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med. 2007;357(1):39–51.
Kurokawa Y, Sugimoto N, Miwa H, Tsuda M, Nishina S, Okuda H, et al. Phase II study of trastuzumab in combination with S-1 plus cisplatin in HER2-positive gastric cancer (HERBIS-1). Br J Cancer. 2014;110(5):1163–8.
Miura Y, Sukawa Y, Hironaka S, Mori M, Nishikawa K, Tokunaga S, et al. Five-weekly S-1 plus cisplatin therapy combined with trastuzumab therapy in HER2-positive gastric cancer: a phase II trial and biomarker study (WJOG7212G). Gastric Cancer. 2018;21(1):84–95.
Gong J, Liu T, Fan Q, Bai L, Bi F, Qin S, et al. Optimal regimen of trastuzumab in combination with oxaliplatin/ capecitabine in first-line treatment of HER2-positive advanced gastric cancer (CGOG1001): a multicenter, phase II trial. BMC Cancer. 2016;16:68.
Yuki S, Shinozaki K, Kashiwada T, Kusumoto T, Iwatsuki M, Satake H, et al. Multicenter phase II study of SOX plus trastuzumab for patients with HER2(+) metastatic or recurrent gastric cancer: KSCC/HGCSG/CCOG/PerSeUS 1501B. Cancer Chemother Pharmacol. 2020;85(1):217–23.
Rivera F, Romero C, Jimenez-Fonseca P, Izquierdo-Manuel M, Salud A, Martinez E, et al. Phase II study to evaluate the efficacy of Trastuzumab in combination with Capecitabine and Oxaliplatin in first-line treatment of HER2-positive advanced gastric cancer: HERXO trial. Cancer Chemother Pharmacol. 2019;83(6):1175–81.
Shah MA, Xu RH, Bang YJ, Hoff PM, Liu T, Herráez-Baranda LA, et al. HELOISE: phase IIIb randomized multicenter study comparing standard-of-care and higher-dose Trastuzumab regimens combined with chemotherapy as first-line therapy in patients with human epidermal growth factor receptor 2-positive metastatic gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol. 2017;35(22):2558–67.
Nishikawa K, Takahashi T, Takaishi H, Miki A, Noshiro H, Yoshikawa T, et al. Phase II study of the effectiveness and safety of trastuzumab and paclitaxel for taxane- and trastuzumab-naive patients with HER2-positive, previously treated, advanced, or recurrent gastric cancer (JFMC45-1102). Int J Cancer. 2017;140(1):188–96.
Li Q, Jiang H, Li H, Xu R, Shen L, Yu Y, et al. Efficacy of trastuzumab beyond progression in HER2 positive advanced gastric cancer: a multicenter prospective observational cohort study. Oncotarget. 2016;7(31):50656–65.
Makiyama A, Sukawa Y, Kashiwada T, Kawada J, Hosokawa A, Horie Y, et al. Randomized, phase II study of Trastuzumab beyond progression in patients with HER2-positive advanced gastric or gastroesophageal junction cancer: WJOG7112G (T-ACT study). J Clin Oncol. 2020;38(17):1919–27.
Rivera F, Izquierdo-Manuel M, Garcia-Alfonso P, Martinez de Castro E, Gallego J, Limon ML, et al. Perioperative trastuzumab, capecitabine and oxaliplatin in patients with HER2-positive resectable gastric or gastro-oesophageal junction adenocarcinoma: NEOHX phase II trial. Eur J Cancer. 2021;145:158–67.
Hofheinz RD, Hegewisch-Becker S, Kunzmann V, Thuss-Patience P, Fuchs M, Homann N, et al. Trastuzumab in combination with 5-fluorouracil, leucovorin, oxaliplatin and docetaxel as perioperative treatment for patients with human epidermal growth factor receptor 2-positive locally advanced esophagogastric adenocarcinoma: a phase II trial of the Arbeitsgemeinschaft Internistische Onkologie Gastric Cancer Study Group. Int J Cancer. 2021;149(6):1322–31.
Safran HP, Winter K, Ilson DH, Wigle D, DiPetrillo T, Haddock MG, et al. Trastuzumab with trimodality treatment for oesophageal adenocarcinoma with HER2 overexpression (NRG Oncology/RTOG 1010): a multicentre, randomised, phase 3 trial. Lancet Oncol. 2022;23(2):259–69.
Wagner AD, Grabsch HI, Mauer M, Marreaud S, Caballero C, Thuss-Patience P, et al. EORTC-1203-GITCG - the “INNOVATION”-trial: effect of chemotherapy alone versus chemotherapy plus trastuzumab, versus chemotherapy plus trastuzumab plus pertuzumab, in the perioperative treatment of HER2 positive, gastric and gastroesophageal junction adenocarcinoma on pathologic response rate: a randomized phase II-intergroup trial of the EORTC-Gastrointestinal Tract Cancer Group, Korean Cancer Study Group and Dutch Upper GI-Cancer group. BMC Cancer. 2019;19(1):494.
He Q, Chen J, Zhou K, Jin C, Wang A, Ji K, et al. Effect of additional Trastuzumab in neoadjuvant and adjuvant treatment for patients with resectable HER2-positive gastric cancer. Ann Surg Oncol. 2021;28(8):4413–22.
Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5(4):317–28.
Yamashita-Kashima Y, Iijima S, Yorozu K, Furugaki K, Kurasawa M, Ohta M, et al. Pertuzumab in combination with trastuzumab shows significantly enhanced antitumor activity in HER2-positive human gastric cancer xenograft models. Clin Cancer Res. 2011;17(15):5060–70.
Tabernero J, Hoff PM, Shen L, Ohtsu A, Shah MA, Cheng K, et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2018;19(10):1372–84.
Al-Batran SE, Haag GM, Ettrich TJ, Borchert K, Kretzschmar A, Teschendorf C, et al. 1421MO final results and subgroup analysis of the PETRARCA randomized phase II AIO trial: perioperative trastuzumab and pertuzumab in combination with FLOT versus FLOT alone for HER2 positive resectable esophagogastric adenocarcinoma. Ann Oncol. 2020;31(suppl_4): S841–73.
Nordstrom JL, Gorlatov S, Zhang W, Yang Y, Huang L, Burke S, et al. Anti-tumor activity and toxicokinetics analysis of MGAH22, an anti-HER2 monoclonal antibody with enhanced Fcγ receptor binding properties. Breast Cancer Res. 2011;13(6):R123.
Bang YJ, Giaccone G, Im SA, Oh DY, Bauer TM, Nordstrom JL, et al. First-in-human phase 1 study of margetuximab (MGAH22), an fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors. Ann Oncol. 2017;28(4):855–61.
Catenacci DVT, Kang Y-K, Park H, Uronis HE, Lee K-W, Ng MCH, et al. Margetuximab plus pembrolizumab in patients with previously treated, HER2-positive gastro-oesophageal adenocarcinoma (CP-MGAH22–05): a single-arm, phase 1b–2 trial. Lancet Oncol. 2020;21(8):1066–76.
Catenacci DV, Rosales M, Chung HC, Yoon HH, Shen L, Moehler M, et al. MAHOGANY: margetuximab combination in HER2+ unresectable/metastatic gastric/gastroesophageal junction adenocarcinoma. Fut Oncol (London, England). 2021;17(10):1155–64.
Shiravi F, Mohammadi M, Golsaz-Shirazi F, Bahadori T, Judaki MA, Fatemi F, et al. Potent synergistic anti-tumor activity of a novel humanized anti-HER2 antibody hersintuzumab in combination with trastuzumab in xenograft models. Invest New Drugs. 2021;39(3):697–704.
Meric-Bernstam F, Beeram M, Mayordomo JI, Hanna DL, Ajani JA, Murphy MAB, et al. Single agent activity of ZW25, a HER2-targeted bispecific antibody, in heavily pretreated HER2-expressing cancers. J Clin Oncol. 2018;36(15_suppl):2500.
Ku G, Elimova E, Denlinger CS, Mehta R, Lee KW, Iqbal S, et al. 1380P phase (Ph) II study of zanidatamab + chemotherapy (chemo) in first-line (1L) HER2 expressing gastroesophageal adenocarcinoma (GEA). Ann Oncol. 2021;32:S1044–S5.
Xu J, Zhang Y, Wu J, Xu N, Ying J, Xiang X, et al. The preliminary efficacy of KN026 (anti-HER2 BsAb) in advanced gastric and gastroesophageal junction cancer patients with HER2 expression. J Clin Oncol. 2021;39(15_suppl):e16005–e.
Piha-Paul S, Bendell J, Tolcher A, Hurvitz S, Patnaik A, Shroff R, et al. O82 A phase 1 dose escalation study of PRS-343, a HER2/4–1BB bispecific molecule, in patients with HER2-positive malignancies. J Immunother Cancer. 2020;8(Suppl 1):A1–2.
Denlinger CS, Maqueda MA, Watkins DJ, Sym SJ, Bendell JC, Park SH, et al. Randomized phase 2 study of paclitaxel (PTX), trastuzumab (T) with or without MM-111 in HER2 expressing gastroesophageal cancers (GEC). J Clin Oncol. 2016;34(15_suppl):4043.
Mohammadi M, Jeddi-Tehrani M, Golsaz-Shirazi F, Arjmand M, Bahadori T, Judaki MA, et al. A novel anti-HER2 bispecific antibody with potent tumor inhibitory effects in vitro and in vivo. Front Immunol. 2020;11:600883.
Rau A, Kocher K, Rommel M, Kuhl L, Albrecht M, Gotthard H, et al. A bivalent, bispecific Dab-Fc antibody molecule for dual targeting of HER2 and HER3. MAbs. 2021;13(1):1902034.
Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68(22):9280–90.
Barok M, Tanner M, Koninki K, Isola J. Trastuzumab-DM1 is highly effective in preclinical models of HER2-positive gastric cancer. Cancer Lett. 2011;306(2):171–9.
Thuss-Patience PC, Shah MA, Ohtsu A, Van Cutsem E, Ajani JA, Castro H, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 2017;18(5):640–53.
Seo S, Ryu MH, Park YS, Ahn JY, Park Y, Park SR, et al. Loss of HER2 positivity after anti-HER2 chemotherapy in HER2-positive gastric cancer patients: results of the GASTric cancer HER2 reassessment study 3 (GASTHER3). Gastric Cancer. 2019;22(3):527–35.
Collins DM, Bossenmaier B, Kollmorgen G, Niederfellner G. Acquired resistance to antibody-drug conjugates. Cancers. 2019;11(3):394.
Cortés J, Kim SB, Chung WP, Im SA, Park YH, Hegg R, et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for breast cancer. N Engl J Med. 2022;386(12):1143–54.
Nakada T, Sugihara K, Jikoh T, Abe Y, Agatsuma T. The latest Research and Development into the antibody-drug conjugate, [fam-] Trastuzumab Deruxtecan (DS-8201a), for HER2 cancer therapy. Chem Pharm Bull. 2019;67(3):173–85.
Ogitani Y, Hagihara K, Oitate M, Naito H, Agatsuma T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016;107(7):1039–46.
Shitara K, Iwata H, Takahashi S, Tamura K, Park H, Modi S, et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive gastric cancer: a dose-expansion, phase 1 study. Lancet Oncol. 2019;20(6):827–36.
Janjigian YY, Viglianti N, Liu F, Mendoza-Naranjo A, Puvvada S. 1500TiP a phase Ib/II, multicenter, open-label, dose-escalation and dose-expansion study evaluating trastuzumab deruxtecan (T-DXd; DS-8201) monotherapy and combinations in patients with HER2-overexpressing gastric cancer (DESTINY-Gastric03). Ann Oncol. 2020;31:S930–S1.
Xu Y, Wang Y, Gong J, Zhang X, Peng Z, Sheng X, et al. Phase I study of the recombinant humanized anti-HER2 monoclonal antibody-MMAE conjugate RC48-ADC in patients with HER2-positive advanced solid tumors. Gastric Cancer. 2021;24(4):913–25.
Peng Z, Liu T, Wei J, Wang A, He Y, Yang L, et al. Efficacy and safety of a novel anti-HER2 therapeutic antibody RC48 in patients with HER2-overexpressing, locally advanced or metastatic gastric or gastroesophageal junction cancer: a single-arm phase II study. Cancer Commun (London, England). 2021;41(11):1173–82.
Barok M, Le Joncour V, Martins A, Isola J, Salmikangas M, Laakkonen P, et al. ARX788, a novel anti-HER2 antibody-drug conjugate, shows anti-tumor effects in preclinical models of trastuzumab emtansine-resistant HER2-positive breast cancer and gastric cancer. Cancer Lett. 2020;473:156–63.
Skidmore L, Sakamuri S, Knudsen NA, Hewet AG, Milutinovic S, Barkho W, et al. ARX788, a site-specific anti-HER2 antibody-drug conjugate, demonstrates potent and selective activity in HER2-low and T-DM1-resistant breast and gastric cancers. Mol Cancer Ther. 2020;19(9):1833–43.
Zhang Y, Qiu M, Wang J, Zhang Y, Yuan X, Zhang T, et al. A phase 1 multicenter, dose expansion study of ARX788 as monotherapy in patients with HER2-positive advanced gastric and gastroesophageal junction adenocarcinoma (ACE-Gastric-01). J Clin Oncol. 2021;39(15_suppl):e16059–e.
Banerji U, van Herpen CML, Saura C, Thistlethwaite F, Lord S, Moreno V, et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019;20(8):1124–35.
Liu Y, Lian W, Zhao X, Qi W, Xu J, Xiao L, et al. A first in-human study of A166 in patients with locally advanced/metastatic solid tumors which are HER2-positive or HER2-amplified who did not respond or stopped responding to approved therapies. J Clin Oncol. 2020;38(15_suppl):1049.
Li JY, Perry SR, Muniz-Medina V, Wang X, Wetzel LK, Rebelatto MC, et al. A Biparatopic HER2-targeting antibody-drug conjugate induces tumor regression in primary models refractory to or ineligible for HER2-targeted therapy. Cancer Cell. 2016;29(1):117–29.
Pegram MD, Hamilton EP, Tan AR, Storniolo AM, Balic K, Rosenbaum AI, et al. First-in-human, phase 1 dose-escalation study of Biparatopic anti-HER2 antibody-drug conjugate MEDI4276 in patients with HER2-positive advanced breast or gastric cancer. Mol Cancer Ther. 2021;20(8):1442–53.
Graziani EI, Sung M, Ma D, Narayanan B, Marquette K, Puthenveetil S, et al. PF-06804103, a site-specific anti-HER2 antibody-drug conjugate for the treatment of HER2-expressing breast, gastric, and lung cancers. Mol Cancer Ther. 2020;19(10):2068–78.
Le Joncour V, Martins A, Puhka M, Isola J, Salmikangas M, Laakkonen P, et al. A novel anti-HER2 antibody-drug conjugate XMT-1522 for HER2-positive breast and gastric cancers resistant to Trastuzumab Emtansine. Mol Cancer Ther. 2019;18(10):1721–30.
Li J, Guo Y, Xue J, Peng W, Ge X, Zhao W, et al. First-in-human phase I study of anti-HER2 ADC MRG002 in patients with relapsed/refractory solid tumors. J Clin Oncol. 2020;38(15_suppl):TPS1101–TPS.
Hamblett K, Barnscher S, Davies R, Hammond P, Hernandez A, Wickman G, et al. Abstract P6-17-13: ZW49, a HER2 targeted biparatopic antibody drug conjugate for the treatment of HER2 expressing cancers. Cancer Res. 2019;79(4_Supplement):P6-17-3–P6--3.
Shin SH, Park YH, Park SS, Ju EJ, Park J, Ko EJ, et al. An elaborate new linker system significantly enhances the efficacy of an HER2-antibody-drug conjugate against refractory HER2-positive cancers. Adv Sci. 2021;8(23):e2102414.
Satoh T, Xu RH, Chung HC, Sun GP, Doi T, Xu JM, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN--a randomized, phase III study. J Clin Oncol. 2014;32(19):2039–49.
Hecht JR, Bang YJ, Qin SK, Chung HC, Xu JM, Park JO, et al. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC--A randomized phase III trial. J Clin Oncol. 2016;34(5):443–51.
Smyth EC, Rowley S, Cafferty FH, Allum W, Grabsch HI, Stenning S, et al. Safety and efficacy of the addition of lapatinib to perioperative chemotherapy for resectable HER2-positive gastroesophageal adenocarcinoma: a randomized phase 2 clinical trial. JAMA Oncol. 2019;5(8):1181–7.
Kulukian A, Lee P, Taylor J, Rosler R, de Vries P, Watson D, et al. Preclinical activity of HER2-selective tyrosine kinase inhibitor tucatinib as a single agent or in combination with trastuzumab or docetaxel in solid tumor models. Mol Cancer Ther. 2020;19(4):976–87.
Strickler JH, Nakamura Y, Yoshino T, Catenacci DVT, Janjigian YY, Barzi A, et al. MOUNTAINEER-02: phase II/III study of tucatinib, trastuzumab, ramucirumab, and paclitaxel in previously treated HER2+ gastric or gastroesophageal junction adenocarcinoma—trial in progress. J Clin Oncol. 2021;39(3_suppl):TPS252–TPS.
Yin Y, Yang H, Liu Z, Tan J, Zhu C, Chen M, et al. Studies on the safety and efficacy of Pyrotinib in the treatment of HER2- positive advanced solid tumors excluding breast cancer. Cancer Manag Res. 2020;12:13479–87.
Chen Z, Xu Y, Gong J, Kou F, Zhang M, Tian T, et al. Pyrotinib combined with CDK4/6 inhibitor in HER2-positive metastatic gastric cancer: a promising strategy from AVATAR mouse to patients. Clin Transl Med. 2020;10(4):e148.
Thomas A, Virdee PS, Eatock M, Lord SR, Falk S, Anthoney DA, et al. Dual Erb B inhibition in Oesophago-gastric Cancer (DEBIOC): a phase I dose escalating safety study and randomised dose expansion of AZD8931 in combination with oxaliplatin and capecitabine chemotherapy in patients with oesophagogastric adenocarcinoma. Eur J Cancer. 2020;124:131–41.
Oh DY, Lee KW, Cho JY, Kang WK, Im SA, Kim JW, et al. Phase II trial of dacomitinib in patients with HER2-positive gastric cancer. Gastric Cancer. 2016;19(4):1095–103.
Kim TM, Lee KW, Oh DY, Lee JS, Im SA, Kim DW, et al. Phase 1 studies of Poziotinib, an irreversible Pan-HER tyrosine kinase inhibitor in patients with advanced solid tumors. Cancer Res Treat. 2018;50(3):835–42.
Kim TY, Han HS, Lee KW, Zang DY, Rha SY, Park YI, et al. A phase I/II study of poziotinib combined with paclitaxel and trastuzumab in patients with HER2-positive advanced gastric cancer. Gastric Cancer. 2019;22(6):1206–14.
Sanchez-Vega F, Hechtman JF, Castel P, Ku GY, Tuvy Y, Won H, et al. EGFR and MET amplifications determine response to HER2 inhibition in ERBB2-amplified Esophagogastric cancer. Cancer Discov. 2019;9(2):199–209.
Janjigian YY, Maron SB, Chatila WK, Millang B, Chavan SS, Alterman C, et al. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21(6):821–31.
Yamashita K, Iwatsuki M, Yasuda-Yoshihara N, Morinaga T, Nakao Y, Harada K, et al. Trastuzumab upregulates programmed death ligand-1 expression through interaction with NK cells in gastric cancer. Br J Cancer. 2021;124(3):595–603.
Rha SY, Lee C-K, Kim HS, Kang B, Jung M, Bae WK, et al. Targeting HER2 in combination with anti-PD-1 and chemotherapy confers a significant tumor shrinkage of gastric cancer: A multi-institutional phase Ib/II trial of first-line triplet regimen (pembrolizumab, trastuzumab, chemotherapy) for HER2-positive advanced gastric cancer (AGC). J Clin Oncol. 2020;38(15_suppl):3081-.
Janjigian YY, Kawazoe A, Yañez P, Li N, Lonardi S, Kolesnik O, et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. 2021;600(7890):727–30.
Satoh T, Kang YK, Chao Y, Ryu MH, Kato K, Cheol Chung H, et al. Exploratory subgroup analysis of patients with prior trastuzumab use in the ATTRACTION-2 trial: a randomized phase III clinical trial investigating the efficacy and safety of nivolumab in patients with advanced gastric/gastroesophageal junction cancer. Gastric Cancer. 2020;23(1):143–53.
Tintelnot J, Goekkurt E, Binder M, Thuss-Patience P, Lorenzen S, Knorrenschild JR, et al. Ipilimumab or FOLFOX with Nivolumab and Trastuzumab in previously untreated HER2-positive locally advanced or metastatic EsophagoGastric adenocarcinoma - the randomized phase 2 INTEGA trial (AIO STO 0217). BMC Cancer. 2020;20(1):503.
ALPHAMAB ONCOLOGY. https://www.alphamabonc.com/html/news/2492.html Accessed 11 Jul 2022.
Iwata TN, Ishii C, Ishida S, Ogitani Y, Wada T, Agatsuma T. A HER2-targeting antibody-drug conjugate, Trastuzumab Deruxtecan (DS-8201a), enhances antitumor immunity in a mouse model. Mol Cancer Ther. 2018;17(7):1494–503.
Galsky MD, Conte GD, Foti S, Yu EY, Machiels J-PH, Doger B, et al. Primary analysis from DS8201-A-U105: A phase 1b, two-part, open-label study of trastuzumab deruxtecan (T-DXd) with nivolumab (nivo) in patients (pts) with HER2-expressing urothelial carcinoma (UC). J Clin Oncol. 2022;40(6_suppl):438.
Zhou L, Xu H, Li S, Yan X, Li J, Wu X, et al. Study RC48-C014: preliminary results of RC48-ADC combined with toripalimab in patients with locally advanced or metastatic urothelial carcinoma. J Clin Oncol. 2022;40(6_suppl):515.
Knochelmann HM, Smith AS, Dwyer CJ, Wyatt MM, Mehrotra S, Paulos CM. CAR T cells in solid tumors: blueprints for building effective therapies. Front Immunol. 2018;9:1740.
Song Y, Tong C, Wang Y, Gao Y, Dai H, Guo Y, et al. Effective and persistent antitumor activity of HER2-directed CAR-T cells against gastric cancer cells in vitro and xenotransplanted tumors in vivo. Protein Cell. 2018;9(10):867–78.
Wu X, Huang S. HER2-specific chimeric antigen receptor-engineered natural killer cells combined with apatinib for the treatment of gastric cancer. Bull Cancer. 2019;106(11):946–58.
Klichinsky M, Ruella M, Shestova O, Lu XM, Best A, Zeeman M, et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat Biotechnol. 2020;38(8):947–53.
Lee JB, Kwon WS, Kim HS, Jung M, Kim S, Park M, et al. First-in-human phase I study of BVAC-B cell therapy in HER2-positive advanced gastric cancer. J Clin Oncol. 2020;38(15_suppl):4534.
Sun L, Zhang J, Xu M, Zhang L, Tang Q, Chen J, et al. Ultrasound microbubbles mediated sonosensitizer and antibody co-delivery for highly efficient synergistic therapy on HER2-positive gastric cancer. ACS Appl Mater Interfaces. 2022;14(1):452–63.
Janjigian YY, Sanchez-Vega F, Jonsson P, Chatila WK, Hechtman JF, Ku GY, et al. Genetic predictors of response to systemic therapy in Esophagogastric cancer. Cancer Discov. 2018;8(1):49–58.
Yagi S, Wakatsuki T, Yamamoto N, Chin K, Takahari D, Ogura M, et al. Clinical significance of intratumoral HER2 heterogeneity on trastuzumab efficacy using endoscopic biopsy specimens in patients with advanced HER2 positive gastric cancer. Gastric Cancer. 2019;22(3):518–25.
Kim KC, Koh YW, Chang HM, Kim TH, Yook JH, Kim BS, et al. Evaluation of HER2 protein expression in gastric carcinomas: comparative analysis of 1,414 cases of whole-tissue sections and 595 cases of tissue microarrays. Ann Surg Oncol. 2011;18(10):2833–40.
Haffner I, Schierle K, Raimúndez E, Geier B, Maier D, Hasenauer J, et al. HER2 expression, test deviations, and their impact on survival in metastatic gastric cancer: results from the prospective multicenter VARIANZ study. J Clin Oncol. 2021;39(13):1468–78.
Palle J, Rochand A, Pernot S, Gallois C, Taieb J, Zaanan A. Human epidermal growth factor receptor 2 (HER2) in advanced gastric cancer: current knowledge and future perspectives. Drugs. 2020;80(4):401–15.
Park SR, Park YS, Ryu MH, Ryoo BY, Woo CG, Jung HY, et al. Extra-gain of HER2-positive cases through HER2 reassessment in primary and metastatic sites in advanced gastric cancer with initially HER2-negative primary tumours: results of GASTric cancer HER2 reassessment study 1 (GASTHER1). Eur J Cancer. 2016;53:42–50.
Arienti C, Zanoni M, Pignatta S, Del Rio A, Carloni S, Tebaldi M, et al. Preclinical evidence of multiple mechanisms underlying trastuzumab resistance in gastric cancer. Oncotarget. 2016;7(14):18424–39.
Kim ST, Banks KC, Pectasides E, Kim SY, Kim K, Lanman RB, et al. Impact of genomic alterations on lapatinib treatment outcome and cell-free genomic landscape during HER2 therapy in HER2+ gastric cancer patients. Ann Oncol. 2018;29(4):1037–48.
Sampera A, Sanchez-Martin FJ, Arpi O, Visa L, Iglesias M, Menendez S, et al. HER-family ligands promote acquired resistance to Trastuzumab in gastric cancer. Mol Cancer Ther. 2019;18(11):2135–45.
Gambardella V, Gimeno-Valiente F, Tarazona N, Martinez-Ciarpaglini C, Roda D, Fleitas T, et al. NRF2 through RPS6 activation is related to anti-HER2 drug resistance in HER2-amplified gastric cancer. Clin Cancer Res. 2019;25(5):1639–49.
Jin MH, Nam AR, Bang JH, Oh KS, Seo HR, Kim JM, et al. WEE1 inhibition reverses trastuzumab resistance in HER2-positive cancers. Gastric Cancer. 2021;24(5):1003–20.
Bie L, Luo S, Li D, Wei Y, Lu XJCCDT. HOTAIR competitively binds MiRNA330 as a molecular sponge to increase the resistance of gastric cancer to Trastuzumab; 2020. p. 20.
Wang W, Wang S, Xu AM, Yuan X, Huang L, Li J. Overexpression of GSE1 related to Trastuzumab resistance in gastric cancer cells. Biomed Res Int. 2021;2021:8834923.
Xiang R, Han X, Ding K, Wu Z. CMIP promotes Herceptin resistance of HER2 positive gastric cancer cells. Pathol Res Pract. 2020;216(2):152776.
Guo J, Zhong X, Tan Q, Yang S, Liao J, Zhuge J, et al. miR-301a-3p induced by endoplasmic reticulum stress mediates the occurrence and transmission of trastuzumab resistance in HER2-positive gastric cancer. Cell Death Dis. 2021;12(7):696.
Zhou X, Men X, Zhao R, Han J, Fan Z, Wang Y, et al. Correction: miR-200c inhibits TGF-beta-induced-EMT to restore trastuzumab sensitivity by targeting ZEB1 and ZEB2 in gastric cancer. Cancer Gene Ther. 2020;27(12):976–8.
Sung M, Tan X, Lu B, Golas J, Hosselet C, Wang F, et al. Caveolae-mediated endocytosis as a novel mechanism of resistance to Trastuzumab Emtansine (T-DM1). Mol Cancer Ther. 2018;17(1):243–53.
Wang H, Wang W, Xu Y, Yang Y, Chen X, Quan H, et al. Aberrant intracellular metabolism of T-DM1 confers T-DM1 resistance in human epidermal growth factor receptor 2-positive gastric cancer cells. Cancer Sci. 2017;108(7):1458–68.
Acknowledgements
We appreciate Dr. Qiao Bin’s critical reading of the article.
Funding
This study was funded by Health Commission of Shanxi Province, China (2020129) for Jun Zhao.
Author information
Authors and Affiliations
Contributions
1.Design: JZ, W-LL, X-LZ and Y-YD. 2. Manuscript writing: W-LL and JZ. 3. Manuscript revision: JZ, W-LL, X-LZ, Y-YD, YZ, JL and W-QH. 4. Final approval of the manuscript: JZ and W-QH. All authors contributed to the final manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, W., Zhang, X., Du, Y. et al. HER2-targeted advanced metastatic gastric/gastroesophageal junction adenocarcinoma: treatment landscape and future perspectives. Biomark Res 10, 71 (2022). https://doi.org/10.1186/s40364-022-00416-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40364-022-00416-x