Abstract
Background
Disruption of alternative splicing in apoptotic factors has been associated to chronic lymphocytic leukemia among other cancers and hematological malignancies. The proapoptotic proteins Caspase-9 and PP2Acα are functionally related in a direct interaction, which constitutes a promising target for cancer therapy. Both proteins present aberrant mRNA splicing variants that are antiapoptotic (Caspase-9b) and catalytically inactive (PP2Acα2), respectively.
Results
In this work we have analyzed the relative abundance of the aberrant spliced forms Caspase-9b and PP2Acα2 in several cell lines and chronic lymphocytic leukemia patients and correlated it with several parameters of the disease. Despite 40 % of the patients presented Caspase-9b dysregulation, there was no direct association between alterations in Caspase-9b relative abundance and the parameters analyzed in medical records. More importantly, PP2Acα2 dysregulation was observed in 88 % of CLL patients and was related with advanced stages of the malignancy.
Conclusions
Caspase-9b dysregulation seemed to be associated with the disease, although the differences between healthy donors and CLL patients were not statistically significant. However, PP2Acα2 dysregulation was significantly different between healthy donors and CLL patients and correlated with Binet B and C stages; therefore, we propose the use of PP2Acα2 dysregulation as a potential biomarker for advanced stages of chronic lymphocytic leukemia.
Similar content being viewed by others
Introduction
Chronic lymphocytic leukemia (CLL) is the most common B-cell malignancy in Caucasian aging adults, rarely younger than 50 years old [1]. Disruption of alternative splicing in many apoptotic factors is related to hematological malignancies and cancer, as CLL [2–6]. Abnormally expressed splicing factors in tumor cells induce the production of mRNA isoforms that are nonexistent or less abundant in normal cells, thus contributing to cancer development, tumor progression, different response to therapy and chemorefractoriness [7, 8].
Caspase-9 is a key point in the apoptotic signal transduction. The expression of its mRNA spliced variant Caspase-9b, lacking exons 3 to 6, inhibits apoptosis in a dominant-negative manner [9], which may establish a threshold to regulate Caspase-9 activation and prevent undesired apoptosis [10]. Caspase-9b is dysregulated in astrocytoma [11] and in several subtypes of non-small-cell lung cancer (NSCLC). Moreover, its overexpression was responsible of maintaining the tumorigenic capacity of NSCLC cells and made cells resistant to erlotinib [12]. Targeting the alternative splicing of Caspase-9 sensitized NSCLC cells to chemotherapies, increasing their efficiency and limiting their toxic side-effects [13].
The direct interaction between Caspase-9 and PP2Acα has been previously described [14]. PP2A is one of the major Ser/Thr phosphatases, whose dysregulation is associated with multiple cancers among other functions [15]. A catalytically inactive aberrant isoform of PP2A catalytic C subunit, PP2Acα2, has been reported, missing exon 5, which is close to the active site. PP2Acα2 has only been observed overexpressed in peripheral blood mononuclear cells (PBMC) under starvation conditions but the ratio PP2Acα:PP2Acα2 was reestablished once they were transferred to culture medium. When analyzed in cell lines, PP2Acα2 was present either as mRNA or as protein but at almost undetectable levels [16].
The aim of the present work was to study the expression ratios Caspase-9: Caspase-9b and PP2Acα:PP2Acα2 in cell lines and more importantly in healthy donors and CLL patients to evaluate their association with the disease.
Material and methods
Cell lines culture
HeLa cells were cultured in DMEM + 10 % FBS, Daudi and Jurkat cells in RPMI 1640 + 10 % FBS and SH-SY5Y in DMEM F12 + 10 % FBS, 1 % Glutamine and 1 % Hepes 1 M. All cell lines were grown at 37 °C and 5 % CO2 and underwent passage three times a week.
B cells isolation
Fresh blood from healthy donors was obtained from the Établissement français du sang. CLL samples were obtained from the Hematology Department of Saint Louis hospital (Paris). PBMC were isolated by Ficoll gradient centrifugation for 20 min at 2300 rpm, they were collected and washed twice with PBS. B cells were isolated using DynaI negative isolation kit (Invitrogen), reaching around 98 % purity.
Conventional PCR and Real Time PCR
Total RNA was extracted with TRIzol® (Life Technologies), cDNA was obtained performing RT-PCR using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) and conventional PCR was set up with Kapa HiFi DNA polymerase (Kapa Biosystems) at 95 °C-3’, 30 cycles of 98 °C-20”, 55 °C-20”, 72 °C-1’30” and a final extension of 72 °C-5’. The primers used were FWD: 5’-ATGGACGAAGCGGATCGG-3’ and REV: 5’-TTATGATGTTTTAAAGAAAAGTT-3’ for Caspase-9 and FWD 5’-GACGAGAAGGTGTTCACCAA-3’ and REV 5’-TTACAGGAAGTAGTCTGGGGTAC-3’ for PP2Acα.
Real Time PCR was performed using TaqMan PCR Mastermix in a 7500 Fast Real Time Applied Biosystems device. Caspase9 and PP2Acα probes corresponded to Hs00154261_m1 and Hs01003394_mH Life Technologies references. Caspase9b probe was previously described [17] and PP2Acα2 primers and probe were designed: 5’-CAAGAAGTTCCCCATGAGGGATATA-3’ (forward), 5’-CAACGATAACAATAGTTTGGAGCACT-3’ (reverse) and 5’-CGTTACTACATTCCGGTCATGGCACCA-3’ (probe). GAPDH was used as housekeeping reference gene (Hs99999905_m1, Life Technologies). Data analysis of relative expression was calculated following the 2-ΔΔC T method [18]. Differences between healthy donors and CLL patients were statistically evaluated with Student's t test and with a Two-way ANOVA with replication with a significance level of P < 0.005.
Results and discussion
Caspase-9b and PP2Acα2 expression in cell lines
Among the cell lines analyzed, derived from different oncologic malignancies, all of them showed a healthy ratio of Caspase-9b relative abundance in PCR and Real Time PCR assays (Fig. 1a and b). As established by Shultz et al. [19], the criteria used to classify Caspase-9b levels were: normal (Caspase-9: Caspase-9b mRNA ratio ≥3.3), moderately dysregulated (3.3 > X > 2.2) and highly dysregulated (≤2.2). Following the criteria that we established for CLL patients as described below, cell lines possessed PP2Acα2 mRNA levels corresponding to healthy state, obtaining very low values in Real Time PCR and a faint band in conventional PCR, when detected (Fig. 1a and b). These results suggest that Caspase-9b or PP2Acα2 dysregulation wouldn’t be characteristic of the analyzed cell lines, which prompted us to consider the approach of using samples from CLL patients instead of insisting with other established cell lines. Patients samples of these pathologies should be analyzed to confirm cell lines data; nevertheless in previous studies Caspase-9b dysregulation has been observed in the same extent in NSCLC cells and patients [12].
Analysis of Caspase-9b and PP2Acα2 in several cancer cell lines. a. Conventional PCR analysis of cancer cell lines showing Caspase-9 and PP2Acα full length and spliced variants expression. b. Real Time PCR of cancer cell lines. Caspase-9b and PP2Acα2 relative abundance is represented in the graph and normalized with respect to Caspase-9 and PP2Acα respectively, considered as 1
Caspase-9b and PP2Acα2 expression in healthy donors and CLL patients
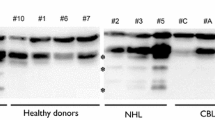
Conventional PCR showed that Caspase-9b was slightly expressed in healthy donors, while CLL patients showed a moderate overexpression. Healthy donors showed no detectable or very low expression of PP2Acα2, but the majority of CLL patients presented from moderate to high overexpression of the aberrant form (Fig. 2a).
Analysis of Caspase-9b and PP2Acα2 in healthy donors and CLL patients. a. Conventional PCR analysis of three healthy donors and three CLL patients for Caspase-9 and PP2Acα full length and spliced variants expression. b. Percentage of CLL patients that present normal, moderately dysregulated and highly dysregulated relative abundance of Caspase-9b and PP2Acα2. c. Real Time PCR analysis of healthy donors. Relative abundance values of spliced forms have been normalized with respect to full length forms, considered as 1. The limits for moderately dysregulated values are marked with a grey dotted line and the limits for highly dysregulated values with a black dotted line. d. Real Time PCR analysis of CLL patients. The same criteria as for Fig. 2c were applied for calculations and limits representation. Asterisk represents P < 0.005 (P = 0.0024), when compared with healthy donor
A cohort of 24 healthy donors and 25 CLL patients (15 men and 10 women) was analyzed by Real Time PCR. Figure 2b summarizes Caspase-9b and PP2Acα2 expression in CLL patients. 80 % of healthy donors showed Caspase-9b normal ratio and the rest were slightly over healthy values (Fig. 2c). Unlike in NSCLC [19], where 36 % of the patients were moderately dysregulated and 42 % were highly dysregulated, in CLL only 40 % of the patients had some alteration in Caspase-9b expression (Fig. 2d), although the highly dysregulated also overcame the moderate percentage (28 % versus 12 %).
According to Shultz et al.[19] criteria for Caspase-9b dysregulation, 80 % of the healthy donors that we analyzed fitted into normal values for Caspase-9b. However, PP2Acα2 was expressed in healthy donors in a PP2Acα:PP2Acα2 ratio from negligible values up to 1.27 in 80 % of the samples (Fig. 2c). Therefore, we formulated classification criteria that would fit a similar percentage of healthy donors in each category as the ones described by Schultz for Caspase-9b. We established a mRNA ratio PP2Acα:PP2Acα2 ≥ 1.33 for healthy expression, 0.67 < X < 1.33 for moderately dysregulated and ≤ 0.67 for highly dysregulated. According to this, 88 % of CLL patients analyzed had overexpression levels of PP2Acα2 (Figs. 2b and d). The efficacy of the use of PP2Acα2 as a CLL biomarker and the applicability of our criteria was validated with the calculation of several clinical parameters. The prevalence of the disease in the whole cohort was 51.02 % and the use of PP2Acα2 as a biomarker presented a sensitivity of 78.57 %, a specificity of 85.71 %, a positive predictive value of 88 % and a negative predictive value of 75 %. According to these results, the detection of a PP2Acα2 overexpression in a patient will allow to diagnose CLL in a 78.57 % of the cases. The specificity of this biomarker assures that 85.71 % of the patients without a dysregulation of PP2Acα2 don’t present the disease and only a 14.29 % would be diagnosed as false positives.
Gathering the data of relative abundance of spliced variants with the medical records available [20, 21] (Table 1), we couldn’t establish a direct correlation between the dysregulation in Caspase-9b and any of the disease parameters analyzed, but we don’t exclude that it may be related to other features of this malignancy. However, this dysregulation in 40 % of CLL patients makes us consider it as a manifestation of the disease and it could be a potential biomarker in some extent, although the differences between healthy donors and CLL patients were not statistically significant according to Student’s t test (P = 0.1878). Interestingly, all individuals classified in the medical records as B or C Binet score [20] presented highly dysregulated PP2Acα2, being the only patient with score C the one with the highest PP2Acα2 levels. Among A score patients, PP2Acα2 levels from normal to highly dysregulated were found. In this case, the differences between healthy donors and CLL patients for PP2Acα2 relative abundance were statistically significant (P < 0.005, P = 0.0024). A two-way ANOVA with replication was also performed between healthy donors and CLL patients 1–24 for Caspase-9b and PP2Acα2 relative abundance. The differences between healthy donors and CLL patients were significant (P < 0.0005) and also between Caspase-9b and PP2Acα2 (P < 0.00005). The interaction P-value (P < 0.005) suggested that the variables Caspase-9b and PP2Acα2 are different depending on the level of the other variable respectively. The distribution of Caspase-9b and PP2Acα2 relative abundance in the healthy donors and CLL patients populations was represented in Fig. 3. Although more extensive studies should be performed to confirm these data, we have promising evidences that suggest the importance of PP2Acα2 in the course of CLL. For the first time, the aberrant spliced variant PP2Acα2 has been related with a pathological state, suggesting its implication in the disease and a possible function for a protein that remains known as inactive.
Several markers as CD38, ZAP-70 and IgVH mutation status among others have been implemented as CLL progression and diagnosis predictors not without certain controversy [22–24]; therefore we propose also the use of PP2Acα2 high dysregulation as a potential CLL biomarker associated to severe stages of the disease.
Disruption of the PP2Acα:PP2Acα2 ratio in CLL patients may also alter the equilibrium of Caspase-9/PP2Acα interaction, giving rise to aberrant Caspase-9/PP2Acα2 complexes. Interestingly, we have previously reported a bifunctional peptide termed DPT-C9h, capable of dissociating Caspase-9/PP2Acα complex [14]. Given that Caspase-9 binding site is present in both PP2Acα and PP2Acα2, DPT-C9h may also interfere in the hypothetical Caspase-9/PP2Acα2 interaction, contributing to a possible modulation of the DPT-C9h mechanism of action and differential treatment response [14].
This bifunctional peptide induced apoptosis in CLL B cells without affecting healthy B cells nor the rest of peripheral blood mononuclear cells [25]. The bifunctional peptide DPT-C9h was also capable of inducing apoptosis in several cancer cell lines and in tumor xenograft models with treatment response in a different extent, which may be related to the relative abundance of PP2Acα2 in the cell lines and patients tested [14]. Further experiments will be performed to shed light on the role of PP2Acα2 in CLL and the hypothetical differential response to DPT-C9h depending on the level of PP2Acα2 dysregulation.
Caspase-9b has been related to disease state in astrocytoma [11] and to play an important role in NSCLC treatment response [12]. Our results provide first evidences for the presence of aberrant PP2Acα/PP2Acα2 ratios in CLL advanced stage patients. Therefore, the dysregulation of the splicing variants of the association Caspase-9/PP2Acα is emerging as a valuable tool as biomarkers for prognosis in cancers that present aberrant expression of Caspase-9b or PP2Acα2 spliced variants. Further studies will need to be performed to evaluate Caspase-9/Caspase-9b and PP2Acα/PP2Acα2 ratios in other types of cancer and their possible application as biomarkers.
Ethics approval and consent to participate
The experiments with human samples included in this work were performed in accordance with the Declaration of Helsinki. No ethics committee approval was required for these experiments. Written informed consent was obtained from all patients.
Abbreviations
- CLL:
-
chronic lymphocytic leukemia
- DMEM:
-
Dulbecco’s modified Eagle medium
- DPT:
-
drug phosphatase technology
- FBS:
-
fetal bovine serum
- NSCLC:
-
non-small-cell lung cancer
- PBMC:
-
peripheral blood mononuclear cells
- RT-PCR:
-
reverse transcription polymerase chain reaction
References
Rozman C, Montserrat E. Chronic lymphocytic leukemia. N Engl J Med. 1995;333:1052–7.
Venables JP. Unbalanced alternative splicing and its significance in cancer. BioEssays. 2006;28:378–86.
Makishima H, Visconte V, Sakaguchi H, Jankowska AM, Kar SA, Jerez A, Przychodzen B, Bupathi M, Guinta K, Afable MG, Sekeres MA, Padgett RA, Tiu RV, Maciejewski JP: Mutations in the spliceosome machinery, a novel and ubiquitous pathway in leukemogenesis. Blood. 2012;119:3203–10.
Klein A, Miera O, Bauer O, Golfier S, Schriever F. Chemosensitivity of B cell chronic lymphocytic leukemia and correlated expression of proteins regulating apoptosis, cell cycle and DNA repair. Leukemia. 2000;14:40–6.
Ringshausen I, Schneller F, Bogner C, Hipp S, Duyster J, Peschel C, Decker T: Constitutively activated phosphatidylinositol-3 kinase (PI-3 K) is involved in the defect of apoptosis in B-CLL: Association with protein kinase Cδ. Blood. 2002;100:3741–8.
Packham G, Stevenson FK. Bodyguards and assassins: Bcl-2 family proteins and apoptosis control in chronic lymphocytic leukaemia. Immunology. 2005;114:441–9.
Sampath J, Long PR, Shepard RL, Xia X, Devanarayan V, Sandusky GE, Perry WL, Dantzig AH, Williamson M, Rolfe M, Moore RE: Human SPF45, a splicing factor, has limited expression in normal tissues, is overexpressed in many tumors, and can confer a multidrug-resistant phenotype to cells. Am J Pathol. 2003;163:1781–90.
Wang L, Lawrence MS, Wan Y, Stojanov P, Sougnez C, Stevenson K, Werner L, Sivachenko A, DeLuca DS, Zhang L, Zhang W, Vartanov AR, Fernandes SM, Goldstein NR, Folco EG, Cibulskis K, Tesar B, Sievers QL, Shefler E, Gabriel S, Hacohen N, Reed R, Meyerson M, Golub TR, Lander ES, Neuberg D, Brown JR, Getz G, Wu CJ. SF3B1 and other novel cancer genes in chronic lymphocytic leukemia. N Engl J Med. 2011;365:2497–506.
Seol DW, Billiar TR. A caspase-9 variant missing the catalytic site is an endogenous inhibitor of apoptosis. J Biol Chem. 1999;274:2072–6.
Srinivasula SM, Ahmad M, Guo Y, Zhan Y, Lazebnik Y, Fernandes-Alnemri T, Alnemri ES: Identification of an endogenous dominant-negative short isoform of caspase-9 that can regulate apoptosis. Cancer Res. 1999;59:999–1002.
Waltereit R, Weller M. The role of caspases 9 and 9-short (9S) in death ligand- and drug-induced apoptosis in human astrocytoma cells. Mol Brain Res. 2002;106:42–9.
Shultz JC, Goehe RW, Murudkar CS, Wijesinghe DS, Mayton EK, Massiello A, Hawkins AJ, Mukerjee P, Pinkerman RL, Park MA, Chalfant CE. SRSF1 regulates the alternative splicing of caspase 9 via a novel intronic splicing enhancer affecting the chemotherapeutic sensitivity of non-small cell lung cancer cells. Mol Cancer Res. 2011;9:889–900.
Shultz JC, Chalfant CE. Caspase 9b: a new target for therapy in non-small-cell lung cancer. Expert Rev Anticancer Ther. 2011;11:499–502.
Arrouss I, Nemati F, Roncal F, Wislez M, Dorgham K, Vallerand D, Rabbe N, Karboul N, Carlotti F, Bravo J, Mazier D, Decaudin D, Rebollo A: Specific targeting of Caspase-9/PP2A interaction as potential new anti-cancer therapy. PLoS One. 2013;8:e60816.
Lechward K, Awotunde OS, Swia̧Tek W, Muszyńska G. Protein phosphatase 2A: Variety of forms and diversity of functions. Acta Biochim Pol. 2001;48:921–33.
Migueleti DLS, Smetana JHC, Nunes HF, Kobarg J, Zanchin NIT. Identification and characterization of an alternatively spliced isoform of the human protein phosphatase 2A catalytic subunit. J Biol Chem. 2012;287:4853–62.
Vu NT, Park MA, Shultz JC, Goehe RW, Hoeferlin LA, Shultz MD, Smith SA, Lynch KW, Chalfant CE. HnRNP U enhances caspase-9 splicing and is modulated by AKT-dependent phosphorylation of hnRNP L. J Biol Chem. 2013;288:8575–84.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 − ΔΔCT method. Methods. 2001;25:402–8.
Shultz JC, Goehe RW, Wijesinghe DS, Murudkar C, Hawkins AJ, Shay JW, Minna JD, Chalfant CE:. Alternative splicing of caspase 9 is modulated by the phosphoinositide 3-kinase/Akt pathway via phosphorylation of SRp30a. Cancer Res. 2010;70:9185–96.
Binet JL, Auquier A, Dighiero G, Chastang C, Piguet H, Goasguen J, Vaugier G, Potron G, Colona P, Oberling F, Thomas M, Tchernia G, Jacquillat C, Boivin P, Lesty C, Duault MT, Monconduit M, Belabbes S, Gremy F. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer. 1981;48:198–206.
Matutes E, Owusu-Ankomah KA, Morilla RM, García Marco J, Houlihan A, Que TH, Catovsky D: The immunological profile of B-cell disorders and proposal of a scoring system for the diagnosis of CLL. Leukemia. 1994;8:1640–5.
Rassenti LZ, Huynh L, Toy TL, Chen L, Keating MJ, Gribben JG, Neuberg DS, Flinn IW, Rai KR, Byrd JC, Kay NE, Greaves A, Weiss A, Kipps TJ. ZAP-70 compared with immunoglobulin heavy-chain gene mutation status as a predictor of disease progression in chronic lymphocytic leukemia. N Engl J Med. 2004;351:893–901.
Inamdar KV, Bueso-Ramos CE. Pathology of chronic lymphocytic leukemia: an update. Ann Diagn Pathol. 2007;11:363–89.
Matutes E, Wotherspoon A, Catovsky D. Differential diagnosis in chronic lymphocytic leukaemia. Best Pract Res Clin Haematol. 2007;20:367–84.
Arrouss I, Decaudin D, Choquet S, Azar N, Parizot C, Zini JM, Nemati F, Rebollo A: Cell penetrating peptides as a therapeutic strategy in chronic lymphocytic leukemia. Protein Pept Lett. 2015;22:539–46.
Funding
This work was supported by grants from the Ministerio de Economía y Competitividad (Spain) SAF2012-31405, SAF2015-67077-R and the Generalitat Valenciana (Spain) (PROMETEO/2012/061) for sample preparation, experiment performance, data collection and analysis and manuscript writing and INSERM (France) for sample extraction, preparation and transport. We acknowledge support of the publication fee by the CSIC Open Access Publication Support Initiative through its Unit of Information Resources for Research (URICI).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
LD-B designed experiments and wrote the paper; JMZ and AR provided samples and medical records; LD-B and XZ performed experiments; LD-B, JF, AR and JB analyzed results and revised the manuscript. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Domínguez-Berrocal, L., Zhang, X., Zini, J.M. et al. Evaluation of Caspase-9b and PP2Acα2 as potential biomarkers for chronic lymphocytic leukemia. Biomark Res 4, 9 (2016). https://doi.org/10.1186/s40364-016-0063-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40364-016-0063-6