Abstract
Background
Levels of toxic metal exposure in indigenous inhabitants are key bioindicators of the severity of environmental contamination. This study measured the seasonal variation of heavy metals and metallothionein (MT) contents in Asian swamp eels (Monopterus albus) from a paddy field situated in Tumpat, Kelantan, Malaysia, to identify prevalence, patterns and associations and togain insight on the suitability of MT as a biomarker for metal exposure.
Methods
Gill, muscle and liver tissues of M. albus (n = 50) sampled during the ploughing, seedling, growing and harvesting phases of rice growing were collected. The concentrations of copper (Cu), zinc (Zn), lead (Pb), nickel (Ni), and cadmium (Cd) in these tissues were determined by flame atomic absorption spectrometry. MT from each sample was isolated and purified, and subsequently quantitated using UV spectrophotometry. Associations between metal and MT concentrations, season and tissue type were evaluated using Pearson correlation and ANOVA with post-hoc Tukey HSD analysis.
Results
Zn was present in higher quantities in gill and liver tissues, while Cu levels were elevated solely in liver. Patterns of non-essential metal accumulation were varied: Cd was detected in low concentrations in all tissues, while Pb and Ni were abundant in gill tissues across all seasons. MT concentration in liver tissue was consistently higher than that found in muscle or gill tissue, except during the growing phase. Moreover, significant correlations (P < 0.05) were observed for Cd, Ni, and Zn when MT was employed as metal exposure biomarker. However, no significant association was found between high Pb and Ni levels and MT concentration in gill tissue.Variation of bioaccumulation rates of heavy metals among the different tissues was observed. Some of these metal concentration differences were found to be associated with MT concentration and, by extension, to its high metal-binding capacity.
Conclusions
Significant liver MT-Zn, MT-Cd, and MT-Ni correlations found in this study emphasised the role of metallothionein as a biomarker for exposure of zinc, cadmium and nickel metals in M. albus.
Similar content being viewed by others
Background
Monopterus albus, which is also known as Asian swamp eel, refers to a kind of fish that lives in freshwater, particularly in paddy fields. Paddy cultivation consists of several stages, namely, tilling the flooded soils or puddling, transplanting rice, and harvesting when the time is right [1, 2]. As for the Asian swamp eels that dwell in paddy fields, they have been exposed to various pollutants due to vast agrochemical usage of pesticides, fertilisers, herbicides, and polluted water.
The repetitive use of agrochemicals for paddy cultivation has escalated the amounts of pollutants in its soils. For instance, ploughing soils can cause pollutants from prior cycles of paddy to resurface, while dependency on chemical fertilisers for seedling and growing seasons adds to the amounts of pollutants. Fish uptakes important micronutrients, such as Zn, Cu, Pb, Cd, and Hg, for metabolism functions from its diet or surrounding (sediments and water), which will eventually accumulate in tissues [3,4,5]. This suggests that high accumulation of metals in tissues may turn into harmful toxic, for example, the high levels of Cu and Zn discovered by Pipe et al. [6] in fish tissues.
Metals accumulate in fish through several pathways, including exchange of metal ions via skin and gills, food consumption, and suspended particulate matter [7]. Additionally, Çoğun et al. [8] claimed that several factors dictate the bioaccumulation of metals, for instance, bioavailability of metals, as well as biotic and abiotic aspects including age, size, and feeding habits of species, and water temperature. Besides, liver has been reported to accumulate the highest levels of metals and thus, commonly examined to investigate bioaccumulation processes [9]. Miller et al. [10] asserted that liver is an exceptional indictor to determine inactivation and storage of metals accumulated.
Similar to other species of anguillid eel (A. marmorata), the Asian swamp eel has higher life longevity. At night, the fish migrate from one habitat to another for search of food, thus higher chances to get exposed to harmful pollutants. Hogstrand and Haux [11] reported that the levels of metallothionein (MT) or those similar to MT may increase in tissues due to excessive accumulation of heavy metals, such as Zn, Cu, Cd, and Hg.
According to Ureña et al. [12], MT has exhibited exceptional biochemical response towards exposure of metals. Some features of MT are listed as follows: low protein molecular weight that ranges from 6000 to 7000, rich in cysteine, resistant to heat, no disulfide bonds or aromatic amino acids, and selectively binds with heavy metals [13, 14]. In addition, MT stores and supplies important metals (Zn and Cu) for synthesis of protein, metabolism of nucleic acid [15], as well as metals detoxification. Although the analyses of MT in the species of Anguilla (eels) have begun receiving vast attention, such as that investigated by Langston et al. [16] and Ureña et al. [12]; the functions of MT in M. albus organs seem to be unpopular in the research arena.
As such, this study has two objectives, which are: 1) to examine the content of metals (Zn, Cu, Cd, Ni, and Pb) in muscle, gill, and liver of M. albus obtained from a paddy field located at Tumpat, Kelantan during four varied seasons, and 2) to determine the presence of MT and some other metal-binding proteins in muscle, liver, and gills of M.albus.
Materials and methods
Study area and sampling location
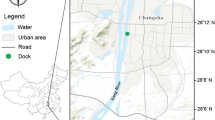
This study selected a paddy field located at Tumpat, Kelantan as the sampling area (see Fig. 1), where paddy cultivation has been carried out for a long time, along with several vegetables planted within the perimeter. Besides, only a handful of residents had been noted there with no heavy industries. Most paddy fields in Kelantan, including the study site, are managed by the Kemubu Agricultural Development Authority (KADA) and the Jal River flows near the study area to supply water during water shortage at dry season.
Sample collection
Before the sampling of eels, the permission for collecting the eels was acquired from the Kemubu Agricultural Development Authority (KADA) in Tumpat and from the owner of the paddy fields. In order to capture the eels, a tool called Tukil was used, which resembles a 36 × 2 in. semi-closed cylindrical tube comprised of a PVC pipe with a spiny entrance on one end and sealed at the opposite end. The eels were captured in year 2013 during each season; ploughing, seedling, growing, and harvesting. In capturing the eels, the tukil was placed at the paddy field for a whole day with some bait in it. If trapped, the eels were collected in the morning, placed in polyethylene plastic bags, sent to the laboratory, and kept in a freezer at − 20 °C until further analysis.
Analysis of heavy metals (Zn, cu, cd, Ni, and Pb)
A study determined the concentrations of Zn, Cu, Cd, Ni, and Pb in muscle, liver, and gill of Asian swamp eel through the use of an atomic absorption spectrophotometer, coupled with flame. In fact, the validity of the outcomes was verified by the National Research Council Canada (NRCC) by issuing certification of DORM-3 and PACS-2; reference materials for fish species. Table 1 tabulates the results of the analyses that displayed exceptional recovery percentage for each metal.
Determination of Metallothionein
As for analysis of MT, distilled water (DW) was used to rinse the eels a few times in order to discard slime on the skin. Next, the body sizes were recorded, which resulted as follows: the sample weights ranged between 29.0 and 271.0 g, while the total length had been in the range of 28.70 until 63.0 cm. After that, tissues from muscle, liver, and gill were extracted to determine the level of MT across the four paddy seasons. The samples were stored in a freezer at − 80 °C until further analyses. In total, fifty Asian swamp eels were examined in this study. MT was analysed in three types of tissues by adhering to the steps outlined by Viarengo et al. [17], as depicted by Amira et al. [18]. About 1 g of each tissue was homogenized in a buffer solution that consisted of 0.5 M sucrose, 20 mM Tris-HCl (pH 8.6), 0.006 mM leupeptin, 0.5 mM PMSF (phenylmethylsuphonylfuride), and 0.01% ß-mercaptoethanol. Next, centrifugation was carried out upon the samples at 30,000 g for 20 min at 0 °C.
Quantification of MT by spectrophotometer
The MT in cytosol was purified via precipitation using ethanol-chloroform to discard proteins sensitive to the solvent fractionation, as well as to obtain the MT concentration by adhering to Kimura et al. [19] and Dieter et al. [20]. Next, 1 ml of the resultant supernatant was purified using 80 μl chloroform and 1.05 ml of cold ethanol (− 20 °C). After that, these samples had been centrifuged at 6000 g for 10 min at 4 °C. Then, 6 ml of cold ethanol and 40 μl of 37% concentrated HCl had been added, while the protein was denatured at − 20 °C for an hour. Later, the mixtures had been centrifuged again at 6000 g for 10 min at 4 °C. The resultant, which was in the form of pellet had been kept. Next, after discarding the supernatant, the pellet was added the following: 1 ml of buffer solution, 6 ml of cold ethanol, and 80 μl of chloroform. This mixture was also centrifuged at 6000 g for 10 min at 4 °C. After discarding the supernatant, the pellet was dried using N2 gas. Then, the pellet was suspended in a solution made of 150 μl 0.25 M NaCl, 150 μl 1 N HCl, and 4 mM EDTA. The content of MT amongst the samples was examined by using 4.2 ml of a solution that consisted of 2 M NaCl and 0.43 mM DTNB (5,5′-Dithio-Bis(2-nitrobenzoic acid)) with pH adjusted to 8 using 0.2 M Na-phosphate (NaH2PO4) at ambient temperature. Again, the mixture was centrifuged for 5 min at 3000 g and then, measured at 412 nm using a UV-Visible Recording Spectrophotometer Shimadzu UV-160 A Model. The concentration of MT had been determined by using glutathione (GSH), which served as reference standard, as well as calibration curve [17].
Statistical analysis
The findings are reported in mean ± standard deviation (SD) values. The Pearson Correlation was performed to determine the concentration of MT with Zn, Cu, Cd, Ni, and Pb in the sample muscle, liver, and gill of M.albus. In addition, a Post-Hoc HSD Tukey test had been carried out to identify the significantly differing values upon obtaining a significant ANOVA value. Further statistical analyses had been conducted with SPSS 21.0 with P < 0.05 and P < 0.01 as the significant levels.
Results
Zn, cu, cd, Ni and Pb (μg/g wet weight) accumulated in liver, gills, and muscle
The findings recorded regarding levels of Zn, Cu, Cd, Ni, and Pb found in Asian swamp eels are presented in Table 2. It was revealed that Zn concentrations ranged as follows: 15.82–20.24 μg/g wet weight in liver, 24.0–29.02 μg/g in gills, and 9.14–12.09 μg/g in muscle of M.albus. Besides, the levels of Zn seemed higher in both liver and muscle across the four seasons. Nevertheless, an insignificant variance was noted for Zn (p > 0.05) in the gills (see Table 2). Meanwhile, the Cu concentrations were: 0.53–2.45 μg/g in liver, 0.09–0.17 μg/g in gills, and an average of 0.04 μg/g in muscle across the four seasons. Both muscle and gills displayed insignificant variances at p > 0.05 throughout the seasons (see Table 2). However, Cu appeared higher in liver for seedling season perhaps due to excessive use of fertilisers to ascertain high paddy productivity. The ranges of Cd concentrations found in the samples of eels are as follows: 0.10–0.59 μg/g in liver, 0.09–0.66 μg/g in gills, and 0.08–0.16 μg/g in muscle. In fact, Table 2 presents that the Cd levels had been the lowest in samples, as compared to other metals in the eel tissues. Next, the levels of Ni in the tested tissues across the seasons are as recorded: 1.75 μg/g in liver, 9.21–15.92 μg/g in gills, and 1.40–2.57 μg/g in muscle (see Table 2). Besides, the Ni showed insignificant variance for gill tissues at p > 0.05. Lastly, Pb portrayed insignificants outcomes for all samples across all seasons (see Table 2).
Metallothionein concentration in liver, gills and muscle
Table 3 shows the levels of MT recorded for all samples of eels in the form of mean ± SD and expressed in μg/g MT. As a result, the levels of MT content refer to the following order: liver > gills > muscle. Nonetheless, liver seemed to disclose higher levels of MT, when compared to muscle and gills, except for the growing season. Meanwhile, an insignificant variance was discovered for MT in gill tissues at p > 0.05, but significant variances were revealed for MT in muscle and gills across all seasons at p < 0.05.
Metallothionein concentration with Zn, cu, cd, Ni and Pb in liver, gills and muscle
The results from Pearson correlation displayed significantly positive results (p < 0.05) for MT-Zn, MT-Cd, and MT-Ni in the liver samples, but exceptional for MT-Cu and MT-Pb. The muscle and gill tissues had correlations that were moderate and insignificant variances at p > 0.05 for MT-Zn, MT-Cu, MT-Cd, MT-Ni, and MT-Pb (see Table 4).
Discussions
This study found that the Asian swamp eels had the lowest metal concentrations for muscle tissues. For example, Mulk et al. [21] revealed the lowest heavy metals accumulation in fish muscle tissues that varied by types of fish [22]. Meanwhile, Cu and Zn were found in liver, whereas non-significant metals (Pb and Ni) were highly concentrated in gill tissues across the paddy seasons. As for Cd, its levels varied across the seasons with fluctuating recordings for all tissue samples.
Zhao et al. [23] reported that on the existence of metals discovered in liver is highly linked to metabolism functions. As for this current research, Cu and Zn were high in liver tissue due to MT; a binding protein [24] that stores metals so as to cater to the needs of enzymes and metabolic requirements [15, 25]. Zn was high in liver tissues across the four seasons due to essential biological needs [26] for fish samples taken from paddy fields in Tumpat [27]. Similarly, Zn was found in high concentration in gill tissues across the four seasons, mainly because this organ is the initial target of metals in water [28]. According to Reid and Mcdonald [29], the negatively-charged surface of the gills offers a viable interaction platform between metal and gill, especially for positively-charged metals. As for this study, the high concentrations of Zn discovered in gill and liver tissues signify that the Zn pathway has the dietary source, thus the spread to liver from gill. It is also important to highlight that paddy fields are inhabited by many other smaller freshwater invertebrates, which eases eels to seek for food. On top of that, the samples had Pb and Ni in gill tissues. This is in line with that discovered by Kargin [30] for Capoeta barroisi. The Pb and Ni bioaccumulation may have taken place in the gills due to the contaminated water at the study area [31]. In fact, Pb is a metal that is commonly found in the environment [32]. As for Cd, its levels appeared lower in gill tissue, as compared to that reported by El-Moselhy et al. [33]. The vast gill surface area promotes toxic metals diffusion in a rapid manner [34]. Hence, it has been hypothesised that the metals found in gill usually originate from water [33]. The metals in the gills, after that, are either transferred to other body parts, especially liver for detoxification or discarded into water [35].
Meanwhile, Velusamy et al. [36] asserted that the variance in uptake of elements among species depends on several fish biological traits, for instance, mobility, habitat, and trophic aspects. Hence, as Asian swamp eels live in paddy fields with varied seasons, their exposure to heavy metals is indeed high. Therefore, these eels project high levels of metal in their tissues samples. Furthermore, as these eels have increased longevity, these metals accumulate in their bodies as pollutants.
Apart from metabolic status, growth rate, ecological needs, and feeding habits; another factor that influences their exposure to metals is their patterns in life history [37]. Moreover, Suresh et al. [38] found that trace metals are seldom noted with uniform dispersion in the fish tissues, but accumulate in certain organs. This is in line with that reported by Hazrat and Ezzat [22] that vast factors dictate the accumulation of metalloids and heavy metals in freshwater aquatics, including fish traits and other external factors in their surroundings.
At recent times, the application of MT as a biomarker has begun to increase to evaluate biological impacts due to metals exposure [39]. As such, MT could be detected in several parts of the fish, such as intestine, liver, and gills [40]. Table 3 shows that MT is abundantly present in gill, muscle, and liver tissues. As for this research, the levels of MT are higher in liver, as compared to muscle and gill tissues, due to the role of liver in storing metals for detoxification purposes [41]. Similarly, Naji et al. [42] reported higher MT content in liver of Mozambique tilapia, than that in gills. Thus, it is a common scenario to detect high levels of MT in eel liver due to exposure to metals (Cd, Cu, and Zn) [43]. Nevertheless, compared to several other studies pertaining to discovery of MT in M.albus, this study displayed only slight variances for MT levels between gill and liver tissues. This could be due to the suitability of examining gills within the context of monitoring the environment [44]. Moreover, one vital outcome from this study is that although Cu, Cd, Ni, and Pb were detected in the tissues analysed, they did not necessarily display correlation with the level of MT in muscle and gill tissues. This indicates that the levels of MT depend on the type of tissue [31]. Amérand et al. [45] reported that high levels of MT found in muscle tissues could reflect the occurrence of intense oxidising metabolism activities that release free radicals into the system, thus inducing the synthesis of MT [46,47,48].
Furthermore, the positive relationship between Zn and MT in liver signifies the function of MT in homeostasis of Zn and the fact that Zn is a constituent element of MT [49]. In studying perch, Perca fluviatilis, Hogstrand et al. [50] revealed a positive relationship between Zn and MT (r = 0.75, P < 0.001), and a significantly lower and positive link between Cu and MT in the liver tissues, which was in line with the low Cu accumulation in liver (see Table 3). Hence, MT binds well with toxic metal, particularly Zn, but not as well as with Cu. Besides, the association of MT levels and Cd for this study in liver tissues is attributable to dietary uptake along the gastrointestinal tract of the Asian swamp eel. Additionally, McGeer et al. [51] asserted that Cd ions pass through intestinal walls with the aid of metal carriers, for instance, Ca, Cu, Zn, and Fe channels. Upon reaching the blood stream, Cd ions are carried by proteins - MT [52]. As liver seems to be the initial organ the metals are sent to, escalated accumulation of Cd is noted in liver, when compared to other tissue types. Next, MT and Ni also displayed a significantly positive relationship in liver since the MT refers to metal biomarker [53]. Cosson [54] mentioned that metal binding on MT platform does not only rely on the number of cells. As for Pb and MT, insignificant correlation was noted in the liver, perhaps due to either exceeding binding capacity of MT or incorporation of non-MT proteins with low molecular weight for sequestration of metal [4]. Meanwhile, the absence of link between metals and MT in muscle and gill tissues for this study is attributable to the presence of metal-protein complexes, instead of free ions [55, 56], or due to the low quantity of free ions to promote synthesis of MT.
Conclusions
As a conclusion, the outcomes of this study highlight the following: (1) the high presence of Zn was noted in gill and liver tissues, while Cu solely in liver; (2) accumulation of non-essential metal, such as Cd, had been low in all tissues, while Pb and Ni were abundant in gill tissues across all seasons; (3) the level of MT was higher in liver, when compared to muscle and gill tissues throughout the four paddy seasons; (4) MT-Zn, MT-Cd, and MT-Ni exhibited high correlations (p < 0.001), while significantly low correlation for MT-Cu in liver can be associated to the high metal-binding capacity exerted by MT with Zn, Cu, Cd, and Ni, hence emphasising the role of MT as a biomarker for exposure of metal in this particular eel species.
Abbreviations
- %:
-
Percentage
- °C:
-
Degree celcius
- AAS:
-
Atomic Absorption Spectrophotometer AAS
- Cd:
-
Cadmium
- Cm:
-
Centimetre
- CRM:
-
Certified reference material
- Cu:
-
Copper
- DTNB:
-
5,5-dithiobis-2-nitrobenzoic acid
- DW:
-
Distilled water
- G:
-
Gram
- M:
-
Molarity
- ml:
-
Millilitre
- mM:
-
Milimolar
- MT:
-
Metallothionein
- Ni:
-
Nickel
- Pb:
-
Lead
- PMSF:
-
Phenylmethylsuphonylfuride
- SD:
-
Standard deviation
- Zn:
-
Zinc
- μg/g:
-
Microgram per gram
- μl:
-
Microlitre
References
Zi-tong G. Pedogenesis of Paddy soil and its significance in soil classification. Soil Sci. 1983;135(1):5–10.
Sahrawat K. Fertility and organic matter in submerged Rice soils. Curr Sci. 2005:735–9.
Clearwater SJ, Farag AM, Meyer J. Bioavailability and toxicity of Dietborne Copper and zinc to fish. Comp Biochem Physiol C: Toxicol & Pharmacol. 2002;132(3):269–313.
Filipović V, Raspor B. Metallothionein and metal levels in cytosol of liver, kidney and brain in relation to growth parameters of Mullus surmuletus and Liza aurata from the eastern Adriatic Sea. Water Res. 2003;37(13):3253–62.
Neff JM. Bioaccumulation in marine organisms: effect of contaminants from oil well produced water. Elsevier; 2002.
Pipe R, Coles J, Carissan F, Ramanathan K. Copper induced immunomodulation in the Marine mussel, Mytilus edulis. Aquat Toxicol. 1999;46(1):43–54.
Nussey H. Bioaccumulation of chromium, manganese, nickel and Lead in the tissues of the Moggel, Labeo umbratus (Cyprinidae), from Witbank dam. Mpumalanga Water SA. 2000;26(2):269–84.
Çoğun HY, Yüzereroğlu TA, Firat Ö, Gök G, Kargin F. Metal concentrations in fish species from the Northeast Mediterranean Sea. Environ Monit Assess. 2006;121:431–8.
Akan JC, Mohmoud S, Yikala BS, Ogugbuaja VO. Bioaccumulation of some heavy metals in fish samples from river Benue in Vinikilang, Adamawa state, Nigeria. Am J Analyt Chem. 2012;3(11):727.
Miller P, Munkittrick K, Dixon D. Relationship between concentrations of Copper and zinc in water, sediment, benthic invertebrates, and tissues of white sucker (Catostomus commersoni) at metal-contaminated sites. Can J Fish Aquat Sci. 1992;49(5):978–84.
Hogstrand C, Lithner G, Haux C. The importance of Metallothionein for the accumulation of Copper, zinc and cadmium in environmentally exposed perch, Perca fluviatilis Basic. Cli PharmacolToxicol. 1991;68(6):492–501.
Ureña R, Peri S, Del Ramo J, Torreblanca A. Metal and Metallothionein content in tissues from wild and farmed Anguilla Anguilla at commercial size. Environ Int. 2007;33(4):532–9.
Kaegi JH, Schaeffer A. Biochemistry of Metallothionein. Biochemist. 1988;27(23):8509–15.
Roesijadi G, Robinson W. Metal regulation in aquatic animals: mechanisms of uptake, Accumulation and Release. Aquat Toxicol. 1994;102:125–33.
Roesijadi G. Metallothionein and its role in toxic metal regulation. Comp Biochem Physiol C: Pharmacol Toxicol Endocrinol. 1996;113(2):117–23.
Langston W, Chesman B, Burt G, Pope N, McEvoy J. Metallothionein in liver of eels Anguilla anguilla from the Thames estuary: an Indicator of environmental quality? Mar Environ Res. 2002;53(3):263–93.
Viarengo A, Ponzano E, Dondero F, Fabbri RA. Simple spectrophotometric method for Metallothionein evaluation in Marine Organisms: an application to Mediterranean and Antarctic Molluscs. Mar Environ Res. 1997;44(1):69–84.
Amira A, Merad L, Soltani N. Seasonal Variation in Biomarker Responses in Donax trunculus From Gulf of Annaba (Algeria):Implication of Marine Pollution. In: Elleuch B, editor. Proceedings of the 2nd ICIEN 2016, International Management For Sustainable Development (Sousse, Tunisa, 27–30 October 2016), vol. 1. Tunisia: Environmentally Sustainable Innovative Approaches & Methods Assessment; 2016. p. 232–44.
Kimura M, Otaki N, Imano M. Rabbit liver Metallothionein. Tentative amino acid sequence of Metallothionein-B. In Kagi JHR, Nordberg M. (editors). Metallothionein. Birkhauser, Basel. Experientia Suppl. 1979;24:163–8.
Dieter HH, Muller L, Abekl J, Summer KH. Metallothionein Determination in biological materials: Interlaboratory comparison of 5 current methods. In Kagi JHR, Kojima Y. (editors). Metallothionein II. Birkauser, Basel. Experientia Suppl. 1987;52:351–8.
Mulk S, Korai AL, Azizullah A, Shahi L, Khattak MNK. Marble industry effluents cause an increased Bioaccumulation of heavy metals in Mahaseer (tor Putitora) in Barandu River, district Buner. Pakistan Environ Sci Pollut Res. 2017;24(29):23039–56.
Ali H, Khan E. Bioaccumulationn of Nonpessential hazardous heavy metals and metalloids in freshwater fish. Risk to human health. Environ Chem Lett. 2018;16(3):903–17.
Zhao S, Feng C, Quan W, Chen X, Niu J, Shen Z. Role of living environments in the accumulation characteristics of heavy metals in fishes and crabs in the Yangtze River estuary. China Mar Pollut Bull. 2012;64(6):1163–71.
Görür FK, Keser R, Akcay N, Dizman S. Radioactivity and Heavy metal concentrations of some commercial fish species consumed in the Black Sea region of Turkey. Chemosphere. 2012;87(4):356–61.
Amiard JC, Amiard-Triquet C, Barka S, Pellerin J, Rainbow P. Metallothioneins in aquatic invertebrates: their role in metal detoxification and their use as biomarkers. Aquat Toxicol. 2006;76(2):160–202.
Ahdy Hoda HH, Tayel Fathy T. Assessment of Heavy Metals and Non-Essential Content of Some Edible and Soft Tissues. Egypt J Aquat Res. 2007;33:85–97.
Sow AY, Ahmad S, Syaizwan ZZ. An Assessment of Heavy Metals Bioaccumulation in Asian Swamp Eel, Monopterus albus During Plowing Stages of a Paddy Cycle. Bull Environ Cont Toxicol. 2013;91:6–12.
Saleh YS, Marie MAS. Assessment of metal contamination in water, sediment, and tissues of Arius thalassinus fish from the Red Sea coast of Yemen and the potential human risk assessment. Environ Sci Pollut Res. 2015;22(7):5481–90.
Reid SD, McDonald D. Metal Binding Activity of the Gills of Rainbow Trout (Oncorhynchus mykiss). Can J Fish Aquat Sci. 1991;48(6):1061–8.
Kargin F. Metal Concentrations in Tissues of the Freshwater Fish Capoeta barroisi from the Seyhan River (Turkey). Bull Environ Cont Toxicol. 1998;60(5):822–8.
Cretì P, Trinchella F, Scudiero R. Heavy Metal Bioaccumulation and Metallothionein Content in Tissues of the Sea Bream Sparus aurata from Three Different Fish Farming Systems. Environ Monit Asses J. 2010;165(1-4):321–9.
Goyer RA, Clarkson TW. Toxic Effects of Metals. Casarett & Doull’s Toxicology. In: Klaassen CD. (editor). The Basic Science of Poisons, Fifth Edition. McGraw-Hill; New York. Health Professions Division; 1996.
El-Moselhy KM, Othman A, El-Azem HA, El-Metwally M. Bioaccumulation of Heavy Metals in some Tissues of Fish in the Red Sea, Egypt. Egypt J Basic Appl Sci. 2014;1(2):97–105.
Dhaneesh KV, Gopi M, Ganeshamurthy R, Kumar TTA, Balasubramanian T. Bio-Accumulation of Metals on Reef Associated Organisms of Lakshadweep Archipelago. Food Chem. 2012;131(3):985–91.
Kalay M, Canli M. Elimination of Essential (Cu, Zn) and Non-Essential (Cd, Pb) Metals from Tissues of a Freshwater Fish Tilapia Zilli. Turk J Zool. 2000;24(4):429–36.
Velusamy A, Kumar PS, Ram A, Chinnadurai S. Bioaccumulation of Heavy Metals in Commercially Important Marine Fishes from Mumbai Harbor, India. Mar Pollut Bull. 2014;81(1):218-24.
Yılmaz, AB, Sangün MK, Yağlıoğlu D, Turan, C. Metals (Major, Essential to Non-Essential) Composition of the Different Tissues of Three Demersal Fish Species From Iskenderun Bay, Turkey. Food Chem. 2010;123(2):410–5.
Suresh A, Sivaramakrishna B, Radhakrishnaiah K. Patterns of Cadmium Accumulation in the Organs of Fry and Fingerlings of Freshwater Fish Cyprinus carpio Following Cadmium Exposure. Chemosphere. 1993;26(5):945-53.
Hauser-Davis RA, Bastos FF, Tuton B, Rocha RC, Saint’Pierre T, Ziolli RL, Arruda MA. Bile and Liver Metallothionein Behavior in Copper-Exposed Fish. J Trace Elem Med Biol. 2014;28(1):70-4.
Mohanty d, Samantha L. Multivariate analysis of potential biomarkers of oxidative stress in Notopterus notopterus tissues from Mahanadi River as a function of concentration of heavy metals. Chemosphere. 2016;155:28-38.
Labarrère CR, Menezes BD, Melo MM. Avaliação Dos Teores De Zinco Em Brânquias, Carcaça, Fígado E Musculatura De Diferentes Espécies De Peixes Capturados No Rio São Francisco (MG, Brasil). Revista Geonomos. 2012;20(1).
Naji A, Ismail A, Kamrani E, Sohrabi T. Correlation of MT Levels in Livers and Gills ith Heavy Metals in Wild Tilapia (Oreochromis mossambicus) from the Klang River, Malaysia. Bull Environ Contam Toxicol. 2014;92(6):674-9.
Monikh FA, Peery S, Karami O, Hosseini M, Afshin AB, Amir FG. Distribution of metals in the tissues of benthic Euryglossa orientalis and Cynoglossus arel, and bentho-pelagic, Johnius belangerii, fish from three estuaries, Persian Gulf. Bull Environ Contam Toxicol. 2012;89(3):489-94.
Pacheco CSV, da Silva EGP, Hauser-Davis RA, Dias F, Amorim FAC, de Jesus RM, . . . Saint’Pierre TD. Determination and Evaluation of Metallothionein and Metals in Mugil cephalus (Mullet) from Pontal Bay, Brazil. Bull Environ Contam Toxicol. 2017;98(1):84-90.
Amérand A, Vettier A, Sébert P, Moisan C. A Comparative Study of Reactive Oxygen Species in Red Muscle: Pressure Effects. UNDERSEA HYPERBARIC M. 2006;33(3):161.
Ghoshal K, Jacob ST. Regulation Of Metallothionein Gene Expression. Biochem Phamarcol. 2000;59(1):95-104.
Chesman B, O’hara S, Burt G, Langston W. Hepatic Metallothionein and Total Oxyradical Scavenging Capacity in Atlantic Cod Gadus Morhua Caged in Open Sea Contamination Gradients. Aquat Toxicol. 2007;84(3):310-20.
Atif F, Kaur M, Ansari RA, Raisuddin S. Channa punctata Brain Metallothionein is A Potent Scavenger of Superoxide Radicals and Prevents Hydroxyl Radical‐Induced in Vitro DNA Damage. J Biochem Molecular Toxicol. 2008;22(3):202-8.
Hamza-Chaffai A, Cosson R, Amiard-Triquet C, El Abed A. Physico-chemical forms of storage of metals (Cd, Cu and Zn) and metallothionein-like proteins in gills and liver of marine fish from the Tunisian coast: ecotoxicological consequences. Comp.Biochem. Physiol., Part C: Phamrcol. Toxicol. Endocrinol. 1995;111(2):329–341.
Hogstrand C, Lithner G, Haux C. The importance of metallothionein for the accumulation of copper, zinc and cadmium in environmentally exposed perch, Perca fluviatilis. Basic Clin. Pharmacol. Toxicol. 1991;68(6):492–501.
McGeer JC, Niyogi S, Scott Smith D. 3-cadmium. In: Wood, CM. APF, CJB, editors. Fish Physiology, Homeostasis and Toxicology of Non-essential Metals. Academic Press; 2011. p.125–184.
Le Croizier G, Lacroix C, Artigaud S, Le Floch S, Raffray J, Penicaud V, Le Bayon N. Significance of metallothioneins in differential cadmium accumulation kinetics between two marine fish species. Environ.Pollut. 2018;236:462–476.
Roch M, Noonan P, McCarter J. Determination of No Effect Levels of Heavy Metals for Rainbow Trout Using Hepatic Metallothionein. Water Res. 1986;20(6):771-4.
Cosson RP. Heavy Metal Intracellular Balance and Relationship With Metallothionein Induction in the Liver of Carp after Contamination by Silver, Cadmium and Mercury Following or not Pretreatment by Zinc. Biometals. 1994;7(1):9-19.
Moltedo O, Verde C, Capasso A, Parisi E, Remondelli P, Bonatti S, Leone A. Zinc Transport and Metallothionein Secretion in the Intestinal Human Cell Line Caco-2. J Biol Chem. 2000;275(41):31819-25.
Trinchella F, Riggio M, Filosa S, Volpe MG, Parisi E, Scudiero R. Cadmium Distribution and Metallothionein Expression in Lizard Tissues following Acute and Chronic Cadmium Intoxication. Comp Biochem Physiol C: Toxicol Pharmacol. 2006;144(3):272-8.
Acknowledgements
Not applicable.
Funding
This study was jointly supported by the Research University Grant Scheme, RUGS (Project No: 03–01-11-1155RU) with the grant provided by Universiti Putra Malaysia as well as the Fundamental Research Grant scheme (FRGS) (Project No: FRGS/1/11/ST/UPM/02/12) awarded by the Malaysia Ministry of Higher Education (MOHE).
Availability of data and materials
The datasets used and/or analysed during the current study are available from corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
AYS performed the analysis of heavy metals in Asian eel and writing the manuscript. AI substantially contributed to conception or design on how to sampling the Asian eel and drafted the manuscript and gave final approval. SZZ performed analysis or interpretation of the data and was a major contributor in writing the manuscript. MNA critically revised the manuscript and drafted the manuscript. KH contributed for the ideas, design, and revised the manuscript and drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Authors’ information
Ai Yin Sow currently is a post-doctoral fellowship in Universiti Malaysia Kelantan (UMK) who worked out in breeding and culturing of clams. She obtained her Ph.D in Ecotoxicology from Universiti Putra Malaysia (UPM) and had written manuscripts regarding on bioaccumulation of heavy metals in Asian swamp eels on four different paddy seasons. Ahmad Ismail is a retired professor in Wildlife Ecology and Ecotoxicology and was the head of Department of Biology, Faculty of Science, UPM. His academic background, active in teaching and research, continuous involvement with NGOs and government agencies have contributed a lot in the development of interest in science and nature among young generations. Syaizwan Zahmir Zulkifli is currently working as Professor in the Department of Biology from Universiti Putra Malaysia. He showed highly passion and interested in research on ecotoxicology, coastal ecochemistry and isotopes ecology. Mohammad Noor Amal being selected as Associate Professor in Department of Biology from Universiti Putra Malaysia. He’s also one of the top researchers in Epidemiology and aquaculture on fishes. Kamarul Hambali is a researcher in wildlife ecology from Universiti Malaysia Kelantan. His passion in educating young generations towards ecology awareness and had written manuscripts on wildlife and ecology, eligible him as one of recognized researcher in Malaysia.
Ethics approval and consent to participate
The eels were sampled, handled and sacrified according to methods approved by Institutional Animal Care and Use Committee, Universiti Putra Malaysia.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Sow, A.Y., Ismail, A., Zulkifli, S.Z. et al. Seasonal variation of heavy metals and metallothionein contents in Asian swamp eels, Monopterus albus (Zuiew, 1793) from Tumpat, Kelantan, Malaysia. BMC Pharmacol Toxicol 20, 8 (2019). https://doi.org/10.1186/s40360-019-0286-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40360-019-0286-x