Abstract
Background
Recently, several newer antiplatelet treatment strategies have been used in patients with coronary artery disease (CAD). Apart from the dual antiplatelet therapy (DAPT) consisting of aspirin and clopidogrel, double dose clopidogrel (DDC), triple antiplatelet therapy (TAPT) consisting of aspirin, clopidogrel and cilostazol and other newer antiplatelet agents have shown to be effective in different ways. In this analysis, we aimed to systematically compare the adverse clinical outcomes and the bleeding events which were observed when DDC was compared to the other antiplatelet regimens in patients with CAD.
Methods
English publications comparing DDC with other antiplatelet regimens were searched from MEDLARS/MEDLINE, EMBASE, www.ClinicalTrials.gov and Google Scholar. Adverse cardiovascular outcomes and bleeding events were the study endpoints. Statistical analysis was carried out by the RevMan 5.3 software whereby odds ratios (OR) with 95% confidence intervals (CIs) were calculated.
Results
A total number of 23,065 participants were included. Results of this analysis showed major adverse cardiac events (MACEs), all-cause mortality, cardiac death, stroke, stent thrombosis, revascularization and myocardial infarction (MI) to have been similarly manifested in patients who were treated with DDC versus the control group with OR: 0.98, 95% CI: 0.78–1.22; p = 0.83, OR: 0.95, 95% CI: 0.77–1.17; p = 0.62, OR: 0.97, 95% CI: 0.79–1.20; p = 0.81, OR: 0.98, 95% CI: 0.65–1.48; p = 0.94, OR: 0.84, 95% CI: 0.40–1.75; p = 0.64, OR: 0.88, 95% CI: 0.52–1.49; p = 0.63, and OR: 0.89, 95% CI: 0.65–1.21; p = 0.45 respectively. Any minor and major bleedings were also similarly manifested.
When DDC was compared to DAPT, no significant difference was observed in any bleeding event with OR: 1.58, 95% CI: 0.86–2.91; p = 0.14. Even when DDC was compared with either ticagrelor or prasugrel or TAPT, still no significant difference was observed in terms of bleeding outcomes.
Conclusions
In patients with CAD, adverse clinical outcomes were not significantly different when DDC was compared to the other antiplatelet regimens. In addition, bleeding events were also similarly manifested when DDC was compared to DAPT, TAPT or ticagrelor/prasugrel.
Similar content being viewed by others
Background
Coronary artery disease (CAD) is one among the most common non-communicable diseases affecting a large number of the elderly population around the globe [1]. As a measure of secondary prevention, several antiplatelet treatment strategies have been set up based upon the degree and type of intervention which was carried out. In patients with stable CAD where intervention was not required, a single antiplatelet agent was sufficient [2]. For those patients with acute coronary syndrome (ACS) or those patients undergoing percutaneous coronary intervention (PCI) with drug eluting stents (DES), dual antiplatelet therapy (DAPT) consisting of aspirin and clopidogrel has been the mainstay of treatment [3].
However, clopidogrel hyporesponsiveness [4] and platelet hyper-reactivity [5] have recently been observed in several subgroups of patients. Therefore, to overcome this problem, several new antiplatelet treatment strategies have been developed: Double dose clopidogrel (DDC) [6], triple antiplatelet therapy (TAPT) consisting of aspirin, clopidogrel and cilostazol [7] and other newer potential antiplatelet agents such as ticagrelor and prasugrel have been used [8].
Nevertheless, controversies have been observed with the use of DDC. Results of the CURRENT OASIS 7 Trial which was published in the New England Journal of Medicine showed no benefit of DDC in patients with ACS. However, a subgroup analysis of the same data (CURRENT OASIS 7) which was published in The Lancet indicated a beneficial effect of DDC in ACS patients following coronary stenting. However, DDC has never systematically been compared with the other antiplatelet agents.
In this analysis, we aimed to systematically compare the adverse clinical outcomes and the bleeding events which were observed with DDC versus the other antiplatelet regimens in patients with CAD.
Methods
Searched databases and searched strategies
MEDLARS or MEDLINE (Medical Literature Analysis and Retrieval System Online), EMBASE, www.ClinicalTrials.gov and Google Scholar were the online electronic databases which were searched for relevant English publications comparing DDC with other antiplatelet regimens in patients with CAD.
The following searched terms were used to retrieve publications:
-
Double dose clopidogrel and coronary artery disease;
-
Double dose clopidogrel and percutaneous coronary intervention;
-
Double dose clopidogrel and acute coronary syndrome;
-
Double dose clopidogrel and acute myocardial infarction;
-
Double dose clopidogrel and dual antiplatelet therapy;
-
Double dose clopidogrel and triple antiplatelet therapy;
-
Double dose clopidogrel and cilostazol;
-
Double dose clopidogrel and prasugrel;
-
Double dose clopidogrel and ticagrelor;
-
Double dose clopidogrel and antiplatelet agents.
The term ‘double dose’ was also replaced by the term ‘high dose’ in this search process.
Inclusion and exclusion criteria
Studies were included in this analysis if:
-
They compared double dose clopidogrel versus other antiplatelet agents in patients with CAD/PCI;
-
They reported adverse clinical outcomes and bleeding events as their endpoints.
Studies were excluded from this analysis if:
-
They were review articles, meta-analyses, case studies or letters to editors;
-
They did not compare DDC with other antiplatelet agents;
-
They did not report adverse clinical outcomes or bleeding events as their clinical endpoints; Instead, they only reported platelet activities;
-
They were duplicated studies.
Definitions, outcomes and follow-ups
DAPT: Dual antiplatelet therapy consisted of Aspirin and Clopidogrel;
TAPT: Triple antiplatelet therapy consisted of Aspirin, Clopidogrel and Cilostazol;
DDC: Double dose clopidogrel consisted twice the normal standard dose of clopidogrel given daily; that is, 150 mg clopidogrel.
The following outcomes were assessed:
-
Major adverse cardiac events (MACEs) consisting of mortality, myocardial infarction (MI), repeated revascularization, or stroke;
-
All-cause mortality;
-
Cardiac death;
-
MI;
-
Stroke;
-
Stent thrombosis;
-
Revascularization (target vessel revascularization or target lesion revascularization);
-
Any bleeding event consisting of any type of bleeding which was reported;
-
Any minor bleeding consisting of any minor type of bleeding or minimal bleeding;
-
Any major bleeding consisting of any type of major bleeding or serious bleeding.
The follow-up time period varied from study to study. The outcomes which were reported as well as the follow-up time periods have been listed in Table 1.
Data extraction, quality assessment and statistical analysis
Following the search of publications by the PRISMA guideline [9], and after selection of the most suitable articles which were relevant to this analysis, data extraction was carried out by five independent reviewers. The following data were extracted:
The type of study, the number of participants assigned to the DDC group and the control group respectively, the time period when the participants were enrolled, the type of participants, the baseline features, the cardiovascular and bleeding outcomes which were reported, the total number of events in each subgroups, the follow-up time periods and the methodological quality of the trials.
Any disagreement which followed was resolved by consensus.
The methodological quality of the trials was assessed in accordance to the Cochrane Collaboration [10].
Statistical analysis was carried out by the RevMan 5.3 software whereby odds ratios (OR) with 95% confidence intervals (CIs) were calculated.
Heterogeneity was assessed by the (1) Q statistic test whereby a p value less than 0.05 was considered statistically significant and (2) the I2 statistic test whereby a low heterogeneity was denoted by a low I2 value and a high heterogeneity was represented by an increased value of I2.
Concerning the statistical models which were used, a fixed effects model was used if I2 was less than 50% whereas a random effects model was used if I2 was greater than 50%.
Sensitivity analysis was carried out by a method of exclusion whereby each trial was excluded one by one and a new analysis was carried out each time to be compared with the main results for any significant difference.
Since this analysis did not include a large volume of studies, publication bias was best assessed through funnel plots which were obtained from the RevMan software.
Results
Searched outcomes
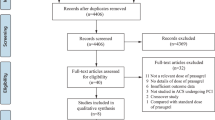
After a careful search, a total number of 1514 publications were obtained. Following an assessment of the titles and abstracts, 1398 articles were eliminated since they were not related to this research topic.
One hundred and sixteen (116) full text articles were assessed for eligibility. Further elimination were carried out for the following reasons:
Meta-analysis (3), review of literature (5), case studies (3), letter to editors (4), did not compare DDC with other antiplatelet agents (32), did not report relevant adverse outcomes (25), and duplicated studies (30).
Finally only 14 studies [11,12,13,14,15,16,17,18,19,20,21,22,23,24] were considered relevant for this analysis as shown in Fig. 1.
General features of the studies
The general features have been listed in Table 2.
A total number of 23,065 participants were included in this analysis whereby 11,217 were assigned to the DDC group and 11,848 participants were assigned to the control group. In detail, the number of participants were assigned as followed: DDC (9019 participants) versus standard dual antiplatelet therapy (9172 participants), DDC (2355 participants) versus TAPT (2356 participants), and DDC (282 participants) versus ticagrelor/prasugrel (320 participants). Eleven studies were randomized trials whereas the other three studies were observational cohorts. The time period of patients’ enrollment varied from years 2006 to 2015 as shown in Table 2.
Patients with stable CAD, ACS, diabetes mellitus and those undergoing PCI were included in this analysis.
Baseline features of the studies
The baseline characteristics have been reported in Table 3. Majority of the participants were of male gender with a mean age ranging from 58.1 to 64.9 years. Hypertension, dyslipidemia and diabetes mellitus among the patients varied from 26.0 to 100% as shown in Table 3. According to the baseline features, no significant difference was observed between the two groups of participants.
Adverse cardiovascular outcomes associated with double dose clopidogrel versus the control group
When the adverse cardiovascular outcomes were compared, MACEs, all-cause mortality, cardiac death, stroke, stent thrombosis, revascularization and MI were similarly manifested in patients who were treated with DDC versus the control group with OR: 0.98, 95% CI: 0.78–1.22; p = 0.83, OR: 0.95, 95% CI: 0.77–1.17; p = 0.62, OR: 0.97, 95% CI: 0.79–1.20; p = 0.81, OR: 0.98, 95% CI: 0.65–1.48; p = 0.94, OR: 0.84, 95% CI: 0.40–1.75; p = 0.64, OR: 0.88, 95% CI: 0.52–1.49; p = 0.63, and OR: 0.89, 95% CI: 0.65–1.21; p = 0.45 respectively as shown in Fig. 2.
Bleeding associated with double dose clopidogrel versus the control group
The outcome ‘any bleeding event’ was not significantly different with DDC versus the control group with OR: 1.09, 95% CI: 0.84–1.42; p = 0.50. In addition, any minor bleeding and any major bleeding were also similarly manifested with OR: 0.62, 95% CI: 0.34–1.12; p = 0.11 and OR: 1.41, 95% CI: 0.96–2.07; p = 0.08 respectively as shown in Fig. 3.
Bleeding events were further analyzed whereby DDC was compared individually with different antiplatelet regimens.
Bleeding associated with double dose clopidogrel versus standard dual anti-platelet therapy
When DDC was compared with the standard dual antiplatelet therapy (Aspirin and clopidogrel), no significant difference was observed in any bleeding event with OR: 1.58, 95% CI: 0.86–2.91; p = 0.14 as shown in Fig. 4.
Bleeding associated with double dose clopidogrel versus ticagrelor or prasugrel
When DDC was compared with either ticagrelor or prasugrel, still no significant difference was observed in any bleeding events and any minor bleeding with OR: 0.67, 95% CI: 0.39–1.14; p = 0.14 and OR: 0.46, 95% CI: 0.20–1.08; p = 0.07 respectively as shown in Fig. 5.
Bleeding associated with double dose clopidogrel versus triple therapy (aspirin, clopidogrel and cilostazol)
When DDC was compared with TAPT, any bleeding event and any minor bleeding were not significantly different with OR: 0.87, 95% CI: 0.48–1.58; p = 0.65 and OR: 0.59, 95% CI: 0.25–1.38; p = 0.22 respectively as shown in Fig. 6.
Sensitivity analyzes showed consistent results accordingly. A low evidence of publication bias was observed which was visually assessed through funnel plots (Figs. 7 and 8) which were directly obtained through RevMan.
Discussion
This is the first analysis to systematically compare DDC versus the other antiplatelet agents. The current results showed that adverse clinical outcomes were not significantly different with DDC versus the other antiplatelet regimens. In addition, bleeding events (including major and minor bleeding) were also similarly manifested.
Results of the Adjunctive Cilostazol Versus High Maintenance Dose Clopidogrel in Patients With AMI (ACCEL-AMI) Study showed that TAPT demonstrated greater platelet inhibition compared to DDC [12]. However, no major cardiovascular or bleeding outcomes were observed in any of the groups supporting the results of this current analysis. The Adjunctive Cilostazol versus double-dose ClopidogrEL in Diabetes Mellitus study (ACCEL-DM) also showed that when cilostazol was added to DAPT, the new regimen showed greater platelet inhibition in comparison to DDC [21]. However, no major bleeding was observed in any of the two groups.
Similarly, in the Gauging Responsiveness with A VerifyNow assay-Impact on Thrombosis And Safety (GRAVITAS) randomized study [25], whereby a total number of 2214 patients were assigned to DDC and standard clopidogrel dose, the former did not decrease the incidence of adverse cardiac outcomes in those patients who underwent PCI. Severe or moderate bleeding were also not significantly different.
Results of the Clopidogrel Response Evaluation and AnTi-platelet InterVEntion in High Thrombotic Risk PCI Patients (CREATIVE) Trial which compared DDC with that of the standard DAPT or cilostazol associated TAPT showed the latter to significantly improved outcomes [14]. However, no significant difference was observed when DDC was compared to the standard DAPT.
In contrast, in the CURRENT OASIS 7 randomized trial, where DDC (whereby 8560 patients were assigned to) was compared with the standard DAPT (where 8703 patients were assigned to) in patients with acute coronary syndrome, the former significantly improved cardiovascular outcomes and stent thrombosis [11]. In addition, major bleeding was significantly higher with DDC during a follow-up time period of 30 days. However, it should be noted that in the CURRENT OASIS 7, the patients were also exposed to a low versus a high dosage of aspirin in addition to the DDC. However, in this current analysis, most of the studies included patients who did not receive a high dosage of aspirin.
Nevertheless, other studies have shown an impact of the CYP2C19 variant to also have interacted with platelet reactivity. For example, the Accelerated Platelet Inhibition by a Double Dose of Clopidogrel According to Gene Polymorphism study showed that among post-PCI treated patients who received DDC, carriage of CYP2C19 variant was associated with a high platelet reactivity which might have shown that DDC was non-inferior to the standard DAPT or TAPT [26]. However, the RESET GENE Trial showed this high treatment platelet reactivity to be completely abolished by prasugrel [27].
In addition, the Atorvastatin and Clopidogrel High Dose in stable patients with residual high platelet activity (ACHIDO) study showed that a high dose of atorvastatin significantly improved the pharmacodynamics effect of DDC [28], which was ignored in this current study.
Novelty
This analysis is new because it is the first research paper to systematically compare DDC with the other antiplatelet regimens in patients with coronary artery disease. In addition, this is an important piece of information which might contribute to the literature of cardiovascular diseases. DDC was compared with the standard dual antiplatelet regimen, the triple antiplatelet regimen, and newer antiplatelet drugs such as prasugrel and ticagrelor which might represent a new feature. Moreover, a low level of heterogeneity was reported among almost all the subgroups, which might further contribute to the novelty of this analysis.
Limitations
Limitations were as followed: Several trials consisted of a very small number of participants which might have been affected by larger trials. However, the proportion of participants were adjusted in larger trials to compensate for the small number of participants in other studies in order to have a final fair result. Different studies had different follow up time periods, and this might have influenced the final outcomes following statistical analysis. In addition, a few of the original studies were pilot studies whereby crossing over of clopidogrel and the control group was reported. This could have influenced the results to a minor extent. Moreover, major and minor bleedings were not reported when a few antiplatelet agents were compared with DDC since data concerning major and minor bleeding were missing in the original papers or they were reported in only one study and a comparison would have not been possible. At last, patients with different stages of coronary disease or intervention were combined and assessed: following PCI, patients with stable coronary artery disease, patients with AMI and other ACS were all together systematically analyzed.
Conclusions
In patients with CAD, adverse clinical outcomes were not significantly different when DDC was compared to the other antiplatelet regimens. In addition, bleeding events were also similarly manifested when DDC was compared to DAPT, TAPT or ticagrelor/prasugrel. Larger upcoming trials should be able to confirm this hypothesis.
Abbreviations
- CAD:
-
Coronary artery disease
- DAPT:
-
Dual antiplatelet therapy
- DDC:
-
Double dose clopidogrel
- PCI:
-
Percutaneous coronary intervention
- TAPT:
-
Triple antiplatelet therapy
References
Mahmood SS, Levy D, Vasan RS, Wang TJ. The Framingham heart study and the epidemiology of cardiovascular disease: a historical perspective. Lancet. 2014;383(9921):999–1008.
Knight CJ. Antiplatelet treatment in stable coronary artery disease. Heart. 2003;89(10):1273–8.
Degrauwe S, Pilgrim T, Aminian A, Noble S, Meier P, Iglesias JF. Dual antiplatelet therapy for secondary prevention of coronary artery disease. Open Heart. 2017;4(2):e000651.
Lee GY, Hahn JY, Lee SY, Kim HJ, Kim JH, Lee SY, Song YB, Choi SH, Choi JH, Gwon HC. Adjunctive cilostazol versus high maintenance dose of clopidogrel in patients with hyporesponsiveness to chronic clopidogrel therapy. Yonsei Med J. 2013;54(1):34–40.
Montalescot G, Rangé G, Silvain J, Bonnet JL, Boueri Z, Barthélémy O, Cayla G, Belle L, Van Belle E, Cuisset T, Elhadad S, Pouillot C, Henry P, Motreff P, Carrié D, Rousseau H, Aubry P, Monségu J, Sabouret P, O'Connor SA, Abtan J, Kerneis M, Saint-Etienne C, Beygui F, Vicaut E, Collet JP, ARCTIC Investigators. High on-treatment platelet reactivity as a risk factor for secondary prevention after coronary stent revascularization: A landmark analysis of the ARCTIC study. Circulation. 2014;129(21):2136–43.
Chen S, Zhang Y, Wang L, Geng Y, Gu J, Hao Q, Wang H, Qi P. Effects of dual-dose Clopidogrel, Clopidogrel combined with Tongxinluo capsule, and Ticagrelor on patients with coronary heart disease and CYP2C19*2 Gene mutation after percutaneous coronary interventions (PCI). Med Sci Monit. 2017;23:3824–30.
Bundhun PK, Qin T, Chen MH. Comparing the effectiveness and safety between triple antiplatelet therapy and dual antiplatelet therapy in type 2 diabetes mellitus patients after coronary stents implantation: a systematic review and meta-analysis of randomized controlled trials. BMC Cardiovasc Disord. 2015;15:118.
Schulz S, Angiolillo DJ, Antoniucci D, Bernlochner I, Hamm C, Jaitner J, Laugwitz KL, Mayer K, von Merzljak B, Morath T, Neumann FJ, Richardt G, Ruf J, Schömig G, Schühlen H, Schunkert H, Kastrati A; Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment (ISAR-REACT) 5 Trial Investigators. Randomized comparison of ticagrelor versus prasugrel in patients with acute coronary syndrome and planned invasive strategy--design and rationale of the iNtracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment (ISAR-REACT) 5 trial. J Cardiovasc Transl Res. 2014;7(1):91–100.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcareinterventions: explanation and elaboration. BMJ. 2009;339:b2700.
Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Mehta SR, Tanguay JF, Eikelboom JW, Jolly SS, Joyner CD, Granger CB, Faxon DP, Rupprecht HJ, Budaj A, Avezum A, Widimsky P, Steg PG, Bassand JP, Montalescot G, Macaya C, Di Pasquale G, Niemela K, Ajani AE, White HD, Chrolavicius S, Gao P, Fox KA, Yusuf S, CURRENT-OASIS 7 trial investigators. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet. 2010;376(9748):1233–43.
Jeong YH, Hwang JY, Kim IS, Park Y, Hwang SJ, Lee SW, Kwak CH, Park SW. Adding cilostazol to dual antiplatelet therapy achieves greater platelet inhibition than highmaintenance dose clopidogrel in patients with acute myocardial infarction: results of the adjunctive cilostazol versus high maintenance dose clopidogrel in patients with AMI (ACCEL-AMI) study. Circ Cardiovasc Interv. 2010;3(1):17–26.
Angiolillo DJ, Shoemaker SB, Desai B, Yuan H, Charlton RK, Bernardo E, Zenni MM, Guzman LA, Bass TA, Costa MA. Randomized comparison of a high clopidogrel maintenance dose in patients with diabetes mellitus and coronary artery disease: results of the optimizing antiplatelet therapy in diabetes mellitus (OPTIMUS) study. Circulation. 2007;115(6):708–16.
Tang YD, Wang W, Yang M, Zhang K, Chen J, Qiao S, Yan H, Wu Y, Huang X, Xu B, Gao R, Yang Y, CREATIVE Investigators. Randomized comparisons of double-dose Clopidogrel or adjunctive Cilostazol versus StandardDual antiplatelet in patients with high posttreatment platelet reactivity: results of the CREATIVE trial. Circulation. 2018;137(21):2231–45.
Chen S, Zhang Y, Wang L, Geng Y, Gu J, Hao Q, Wang H, Qi P. Effects of dual-dose Clopidogrel, Clopidogrel combined with Tongxinluo capsule, and Ticagreloron patients with coronary heart disease and CYP2C19*2 Gene mutation after PercutaneousCoronary interventions (PCI). Med Sci Monit. 2017;23:3824–30.
Khatri S, Pierce T. Comparing prasugrel to twice daily clopidogrel post percutaneous coronary intervention in a veterans affairs population. Am J Health Syst Pharm. 2015;72(17 Suppl 2):S98–S103.
Angiolillo DJ, Badimon JJ, Saucedo JF, Frelinger AL, Michelson AD, Jakubowski JA, Zhu B, Ojeh CK, Baker BA, Effron MB. A pharmacodynamic comparison of prasugrel vs. high-dose clopidogrel in patients with type 2diabetes mellitus and coronary artery disease: results of the optimizing anti-platelet therapy in diabetes MellitUS (OPTIMUS)-3 trial. Eur Heart J. 2011;32(7):838–46.
Wiviott SD, Trenk D, Frelinger AL, O'Donoghue M, Neumann FJ, Michelson AD, Angiolillo DJ, Hod H, Montalescot G, Miller DL, Jakubowski JA, Cairns R, Murphy SA, McCabe CH, Antman EM, Braunwald E, PRINCIPLE-TIMI 44 Investigators. Prasugrel compared with high loading- and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 trial. Circulation. 2007;116(25):2923–32.
Dridi NP, Johansson PI, Clemmensen P, Stissing T, Radu MD, Qayyum A, Pedersen F, Helqvist S, Saunamäki K, Kelbæk H, Jørgensen E, Engstrøm T, Holmvang L. Prasugrel or double-dose clopidogrel to overcome clopidogrel low-response--the TAILOR(thrombocytes and IndividuaLization of ORal antiplatelet therapy in percutaneous coronaryintervention) randomized trial. Platelets. 2014;25(7):506–12.
Wu X, Liu G, Lu J, Zheng XX, Cui JG, Zhao XY, Huang XH. Administration of Ticagrelor and Double-Dose Clopidogrel Based on platelet ReactivityDetermined by VerifyNow-P2Y12 for Chinese subjects after elective PCI. Int Heart J. 2017;58(2):167–73.
Jeong YH, Tantry US, Park Y, Kwon TJ, Park JR, Hwang SJ, Bliden KP, Koh EH, Kwak CH, Hwang JY, Kim S, Gurbel PA. Pharmacodynamic effect of cilostazol plus standard clopidogrel versus double-dose clopidogrelin patients with type 2 diabetes undergoing percutaneous coronary intervention. Diabetes Care. 2012;35(11):2194–7.
Ha SJ, Kim SJ, Hwang SJ, Woo JS, Kim W, Kim WS, Kim KS, Kim MK. Effect of cilostazol addition or clopidogrel doubling on platelet function profiles in diabeticpatients undergoing a percutaneous coronary intervention. Coron Artery Dis. 2013;24(8):690–7.
Park KW, Kang SH, Park JJ, Yang HM, Kang HJ, Koo BK, Park BE, Cha KS, Rhew JY, Jeon HK, Shin ES, Oh JH, Jeong MH, Kim S, Hwang KK, Yoon JH, Lee SY, Park TH, Moon KW, Kwon HM, Chae IH, Kim HS. Adjunctive cilostazol versus double-dose clopidogrel after drug-eluting stent implantation: the HOST-ASSURE randomized trial (Harmonizing Optimal Strategy for Treatment of Coronary Artery Stenosis-Safety & Effectiveness of Drug-Eluting Stents & Anti-platelet Regimen). JACC Cardiovasc Interv. 2013;6(9):932–42.
Jeong YH, Lee SW, Choi BR, Kim IS, Seo MK, Kwak CH, Hwang JY, Park SW. Randomized comparison of adjunctive cilostazol versus high maintenance dose clopidogrel in patients with high post-treatment platelet reactivity: results of the ACCEL-RESISTANCE (adjunctive Cilostazol versus high maintenance dose Clopidogrel in patients with ClopidogrelResistance) randomized study. J Am Coll Cardiol. 2009;53(13):1101–9.
Price MJ, Berger PB, Teirstein PS, Tanguay JF, Angiolillo DJ, Spriggs D, Puri S, Robbins M, Garratt KN, Bertrand OF, Stillabower ME, Aragon JR, Kandzari DE, Stinis CT, Lee MS, Manoukian SV, Cannon CP, Schork NJ, Topol EJ, GRAVITAS Investigators. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA. 2011;305(11):1097–105.
Jeong YH, Kim IS, Park Y, Kang MK, Koh JS, Hwang SJ, Kwak CH, Hwang JY. Carriage of cytochrome 2C19 polymorphism is associated with risk of high post-treatmentplatelet reactivity on high maintenance-dose clopidogrel of 150 mg/day: results of the ACCEL-DOUBLE (accelerated platelet inhibition by a double dose of Clopidogrel according to Gene polymorphism) study. JACC Cardiovasc Interv. 2010;3(7):731–41.
Sardella G, Calcagno S, Mancone M, Palmirotta R, Lucisano L, Canali E, Stio RE, Pennacchi M, Di Roma A, Benedetti G, Guadagni F, Biondi-Zoccai G, Fedele F. Pharmacodynamic effect of switching therapy in patients with high on-treatment plateletreactivity and genotype variation with high clopidogrel dose versus prasugrel: the RESET GENE trial. Circ Cardiovasc Interv. 2012;5(5):698–704.
Leoncini M, Toso A, Maioli M, Angiolillo DJ, Giusti B, Marcucci R, Abbate R, Bellandi F. High-dose atorvastatin on the pharmacodynamic effects of double-dose clopidogrel in patientsundergoing percutaneous coronary interventions: the ACHIDO (atorvastatin and Clopidogrel HIgh DOse in stable patients with residual high platelet activity) study. JACC Cardiovasc Interv. 2013;6(2):169–79.
Funding
There was no external funding for this research.
Availability of data and materials
All data and materials used in this research are freely available. References have been provided.
Author information
Authors and Affiliations
Contributions
XZ, BZ, SO, PN and MX were responsible for the conception and design, acquisition of data, analysis and interpretation of data, drafting the initial manuscript and revising it critically for important intellectual content. XZ and BZ wrote the final manuscript. All authors have read and approved the manuscript as it is.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was not applicable for this systematic review and meta-analysis.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhuo, X., Zhuo, B., Ouyang, S. et al. Adverse clinical outcomes associated with double dose clopidogrel compared to the other antiplatelet regimens in patients with coronary artery disease: a systematic review and meta-analysis. BMC Pharmacol Toxicol 19, 54 (2018). https://doi.org/10.1186/s40360-018-0247-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40360-018-0247-9