Abstract
Background
To tag large marine vertebrates, without the need to catch them, avoiding using barbs for tag retention, and precisely controlling tag location, the remote Tag Attachment Device on a pole (TADpole) was developed. This allows single-pin tags (Finmount, Wildlife Computers) to be attached to the dorsal fins of free-swimming large marine vertebrates.
Results
TADpole comprises a pole-mounted holster that carries a tag. It uses compressed air, and a micro-controller, to rapidly insert a stainless-steel pin through a corrodible metal retaining ring in the first tag attachment wing, the animal’s dorsal fin, and then a press fit Delrin retaining ring in the tag wing on the other side of the fin. Tagging only occurs when the trailing edge of the dorsal fin touches a trigger bar in the holster, ensuring optimal pin placement. It was developed using fins from cadavers, then trialed on briefly restrained coastal dolphins that could be followed in successive days and weeks, and then on free-swimming animals in the field. The latter showed very short touch/response intervals and highlighted the need for several iterative revisions of the pneumatic system. This resulted in reducing the total time from triggering to tag application to ~ 20 ms. Subsequent efforts expanded the TADpole’s applicability to sharks. One free-swimming Atlantic spotted dolphin, two white sharks, and one whale shark were then tagged using the TADpole.
Conclusions
Being able to tag free-swimming dolphins and sharks remotely and precisely with satellite-linked telemetry devices may contribute to solving conservation challenges. Sharks were easier to tag than dolphins. Dolphin touch-to-response times were 28 ms or less. Delphinid skin has unique polymodal axon bundles that project into the epidermis, perhaps a factor in their uniquely fast response, which is 10 × faster than humans. Their primary reaction to tagging is to abduct the flippers and roll the fin out of the TADpole holster. This device has the potential to deliver high-quality tag data from large vertebrates with dorsal fins without the stress and logistics associated with catch-and-release, and without the trauma of tags that use barbs for retention. It also collects a dorsal fin biopsy core.
Similar content being viewed by others
Background
Tracking movements of animals has long been a primary tool of wildlife biologists. The ability to deploy tracking tags remotely, without having to capture individuals for manual attachment, and avoiding barbed or intramuscular implants [1, 2], has many potential benefits, including reduced stress and tissue trauma during and after tag attachment, decreased risks to animals and people, and simplified logistics and costs.
In cetaceans, high-resolution short-duration archival tags such as the DTAG are typically attached with suction cups [3]. Satellite-linked radio transmitting (SLRT) tags allow for the collection of near-real-time geolocation data over broader time scales. To achieve longer attachment duration with this technology, barbed LIMPET (Wildlife Computers, Redmond, WA USA) tags attached via rifle, crossbow, or lance into the dorsal fin or base of the fin have been used. Attachments on small cetaceans are typically short. For SPLASH tags on rough-toothed dolphins (Steno bredanensis), mean duration was 13 days (maximum 17) [4]. Durations of up to three months were obtained for Cuvier’s beaked whales (Ziphius cavirostris) [5] and false killer whales (Pseudorca crassidens) [6]. Longer durations have been achieved with remotely attached transdermal intramuscular tags [1], but they have the potential for significant tissue trauma [7,8,9]. Dedicated tests and analyses of prior tagging data have demonstrated that a single-pin attachment of tags to the dolphin dorsal fin is minimally traumatic, without adverse impacts to health or behavior (e.g., FINMOUNT SPLASH tag,) [10,11,12,13]. However, use of this approach has been limited to attachment on small cetaceans caught briefly for tagging or for other interventions [11, 14,15,16,17,18,19,20,21]. The median tag durations of single-pin dorsal fin tags on bottlenose dolphins (Tursiops truncatus) for a recent study was 117–163 days [19], depending on the tag configuration. With a safe and effective tag now available, a means of safely attaching the tag to dorsal fins without the need for capture was desired.
In sharks, the use of satellite-linked tagging to examine and quantify distribution, habitat use, and movement ecology has increased dramatically over the last two decades [2]. The two most common technologies are pop-up satellite-linked archival transmitting (PSAT; e.g., MiniPAT, Wildlife Computers) and SLRT (e.g., SPOT and SPLASH tags) tags. The former are typically tethered to an intramuscular dart inserted into the base of the dorsal fin; PSAT tags can be programmed to detach (and transmit archived data) after a deployment duration of up to one year. In contrast, SLRT tags transmit near real-time data when the shark is at the surface, but they must be affixed to the apex of the shark’s dorsal fin. To do so, the shark is captured, restrained and/or lifted from the water, and the tag is usually attached by drilling four small holes through the fin and securing it with plastic bolts. These tags provide more accurate geolocations than PSAT tags and have been successfully deployed for up to seven years [22]. However, unlike air-breathing mammals, sharks are not obligated to spend time at the surface, and the amount of location data can vary within and between species. Moreover, sharks tagged using this method can be exposed to physiological stress and physical trauma associated with capture, handling, and tagging, which can impact post-release behavior [23] and cause permanent gross deformation of the fin [24]. As noted above for marine mammals, a single-pin tag that minimizes trauma to the fin and can be applied to free-swimming sharks would be of value.
Thus, if off-the-shelf single-pin tags could be routinely attached to free-swimming cetaceans and sharks that have suitable dorsal fins, without the need to catch them, there could be major improvement of medium-term tag attachment durations, reduced risk of injury to the animals and people, simplified logistics, reduced expense, and greatly increased deployment opportunities.
Methods
Overall approach
The design and development of a pole-based Tag Attachment Device (TADpole) evolved through iterative steps involving biologists, veterinarians, and engineers, working in the lab and in the field. While the TADpole was conceived in the early 2000s, and initial designs proposed in 2014, work to develop a prototype began in earnest when funding was first obtained in 2017. Field trials on dolphins spanned 2018–2023. In 2020, the device was tested off the bow of a small vessel off Massachusetts, USA tagging white sharks (Carcharodon carcharias). In 2022, the device was tested with an in-water approach to tagging whale sharks (Rhincodon typus) off Massachusetts, USA. In 2023 a bow-riding (Stenella frontalis) dolphin was tagged west of Sarasota, Florida, USA.
Specifications
The initial intent of the TADpole was for a pole-mounted apparatus to apply a single attachment pin SPLASH or SPOT tag onto free-swimming dolphins while riding the bow wave of a small boat. There were several design considerations. The system should collect a biopsy sample simultaneous with tagging to allow for genetic analyses to confirm species and sex of the animal and assist with genetic stock structure determinations. Similarly, the tag attachment system was also required to have a corrodible link for releasing the device from the animal following battery exhaustion. The tag pin location relative to the fin must enable the vee of the tag wings to be snug, but not compress the trailing edge of the dorsal fin, to minimize abrasion of the tag on the fin. The distance from the trailing edge of the fin to the tag attachment pin (38 mm) was established from examination of the relative success of previous tag attachments, and with the intent to reduce the potential of injury from tag pin migration to be no more serious than injuries dolphins and sharks inflict on one another [12, 25]. The design allowed for tagging a dorsal fin up to 26 mm wide at the pin location, being the maximum width of dorsal fin samples of common bottlenose dolphin dorsal fins measured at that position.
Design
Before embarking on a detailed design, a brief field test was conducted in April 2015 off Sarasota, FL, with a mock-up tool, a Y-shaped pole-end fitting, to ensure that it was indeed possible to place it around the caudal aspect of the dorsal fin of a bow-riding bottlenose dolphin. The initial tests were successful, and the design progressed into a conceptual design phase.
The initial, manual, catch-and-release approach for applying SPOT and SPLASH tags used a sharpened cork-borer to make a hole in the dorsal fin [10]. This was done by hand and estimated to require approximately 23 kg (50 lbs) of force. A cordless drill with a sharpened tube can also be used. Significantly more force would be required to rapidly tag a moving dolphin during the brief time it surfaces to breathe. Springs or compressed air were considered as a source of this force. With the requirement to recover a biopsy sample from the animal, a reciprocating linear motion was preferable.
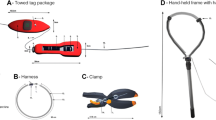
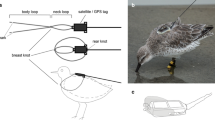
Figure 1 illustrates the overall design. Design drawings, specifications, dimensions, and operating instructions are archived at the Woods Hole Open Access Server [26]. Microcontroller code is archived on GitHub [27]. A tag is inserted into the Y-shaped TADpole holster, which is mounted on the base of a hollow, telescoping carbon fiber pole. The TADpole is deployed by an operator on the bow of a small vessel, ideally equipped with a bow platform or pulpit to provide a better view of bow-riding dolphins and allow a free range of movement of the TADpole system in front of the bow. The pole length is adjustable, 2–4 m long and 5 cm in diameter. The system weighs 3.6 kg (holster 2.1 kg, poles 1.5 kg). The operator maneuvers the holster behind the trailing edge of the dorsal fin of a bow-riding or surface-swimming animal (Fig. 2). The TADpole is configured as follows. The tag holster is attached to a valve housing at the base of the pole (Figs. 1 and 2). The tag is provided by the manufacturer with V-shaped wings that wrap around the fin from behind, with a mounting hole in each wing to secure the tag to a restrained animal’s dorsal fin. For the TADpole application, these holes are enlarged using a punch to enable retaining rings to press fit into each wing (Fig. 1 inset). The ring inserted in the right tag wing (looking down on the upper, antenna side of the tag) is corrodible (magnesium or aluminum), while the ring inserted in the left tag wing is Delrin (Fig. 1 inset) [26]. The rings in the tag wings are secured in U-shaped retaining clips on each side of the holster, with the holes in line with the axis of a pneumatic piston in an aluminum cylinder (Fig. 1) on the right side of the holster.
TADpole configuration used for dolphin and shark trials viewed from below. The aluminum holster has Delrin guides to position it around the dorsal fin (Fig. 2). The pole has a valve cylinder at its base, attached to the holster. A pneumatic cylinder is mounted to the holster with its piston in line with the Delrin and metal rings press fit into the tag wings. A pusher, sleeved by a dart, is threaded into the piston. When the trailing edge of the dorsal fin is surrounded by the tag wings, it triggers the piston to push a dart through the rings and the dorsal fin (insert). The dart is retained by a press fit into the Delrin ring. The piston retracts the pusher, leaving the animal free to swim off with the tag
Schematic drawing of the TADpole, positioned on a dorsal fin ready to apply a tag. The valve in the housing delivers compressed air to the cylinder to actuate the tagging cycle when triggered. The Delrin guides shown in Fig. 1 are not shown here
The tagging actuation cycle shown schematically in Fig. 3, involves a hollow pin, sleeved over a hollow pusher threaded into the piston (Figs. 4 and 5). The pusher passes the pin through the corrodible ring, with a loose fit. It then takes a biopsy core through the dorsal fin, and finally pushes the shouldered, beveled pin tip with a press fit through the Delrin ring (Figs. 4, 5 and 6). The base of the pin also has a shoulder that seats on the corrodible ring, and hence holds the right tag wing against the right side of the dorsal fin (Fig. 6). The press fit pin head in the Delrin ring holds it and the left tag wing against the left side of the fin. Once the pin, and hence tag and retaining rings, are attached to the dorsal fin, the piston rod and pusher retract (Fig. 7) with the biopsy retained by a small barb protruding into the hollow pusher. The swimming force of the animal pulls both retaining rings out of the clips on the holster, and the tagged animal swims free.
Flowchart for TADpole operation. Pneumatic cylinder, trigger and micro-switch are on holster (Figs. 1 and 2), valve in valve chamber at base of pole, to which holster is attached. Controller, batteries (2 × 12 V in series), and relays are in a control box on deck. Wiring from control box, and hose from dive cylinder pass inside pole to valve chamber and then holster
The actuation cycle is only triggered after turning on the controller power and pressurizing the solenoid valve, when the operator thrusts the holster forward immediately behind the trailing edge of the dorsal fin of a bow-riding animal, to the point that the dorsal fin depresses a horizontal metal trigger bar at the base of the holster vee (Fig. 2). This can only happen when the vee of the tag wings is snug against the trailing edge of the dorsal fin, a location previously shown to be optimal for single-pin dorsal fin tags [10, 13]. Trigger depression activates a micro-controller coded sequence of commands to the pneumatic valve to extend and retract the piston at defined intervals, to successfully attach the tag as described above. The actuation cycle is optimal at 20 ms (adjustable). The system’s working pressure is 1517 Pa (220 psi), using a regulated supply from a 20,685 kPa (3000 psi) capacity dive cylinder.
The tag remains attached to the fin until the corrodible ring has lost sufficient material that its inside diameter exceeds the outside diameter of the shoulder at the base of the pin. At that point the right tag wing will detach from the fin, and the ensuing asymmetric drag on the tag will work the pin back out of the tag attachment hole and release the tag, leaving no foreign body in the fin, allowing repair/healing to ensue.
The micro-controller and batteries to power the controller and valve are in a customized, waterproof Pelican case secured to the deck of the vessel. The cable connecting the holster micro-switch and valve to the micro-controller, and the air hose from the compressed air tank to the valve pass down the hollow pole. To control the cylinder’s actuation with millisecond time resolution, an Arduino Uno micro-controller (https://store-usa.arduino.cc/products/arduino-uno-rev3), a waterproof MIL spec micro-switch (https://www.mcmaster.com/7517K33/), and a 5-way solenoid valve were selected (https://www.automationdirect.com/adc/shopping/catalog/pneumatic_components/directional_control_solenoid_valves/solenoid_valves/avs-5121-24d). For low-cost compressed air, a portable air compressor capable of 1138 kPa (165 psi) max was used at first. Subsequent iterations of the design used a standard SCUBA compressed air tank with a single stage regulator. The exhaust air from the valve chamber is discharged via a 1 m air hose extending up the pole to avoid sea water ingress into the valve assembly. Corrodible rings for tag release were made of magnesium for short-term initial testing, with a plan for using aluminum for longer deployments. The current design is illustrated and detailed in Figs. 1, 2, 3, 4, 5 and 6. The holster is loosely tethered to the vessel by a 6 mm diameter Dyneema (https://www.applied-fiber.com/) braided line, to retain the holster in the event of a failure of the pusher to retract during the actuation cycle, or a fracture of the pole/holster connection.
Laboratory testing
Best practice guidelines for cetacean tagging [1] encourage refinement of tags and attachment techniques, with suitable testing on carcass tissue, to achieve effective and consistent operation before use on live animals. Frozen cadaver bottlenose dolphin dorsal fin samples from beach stranding mortalities were thawed, and the TADpole system iteratively tested to ensure complete tagging cycles occurred, with minimum actuation cycle time. Tests in the lab and in the field were documented through video recordings, allowing frame-by-frame analysis. Success with lab tests led to field tests on dolphins during 2018–2023, and tests with white sharks in November 2020, and whale sharks in September 2022 with each field test leading to further refinements and lab testing.
Iterative modifications
During laboratory cadaver sample tests, to minimize the delay in the pneumatic system, the valve that was initially located in the deck control box was moved to the valve chamber at the base of the pole (Fig. 2). Higher flow and pressure valves were tested, pressure was increased from 896 kPa (130 psi) with a battery powered air compressor, to a SCUBA tank, with pressure options up to 20,685 kPa (3000 psi). Different cylinder sizes and pressure capacities were tested. The specifications for the current system components are available [26]. Step-by-step detailed instructions for use of the TADpole, and description of the hardware and software are provided in the TADpole Operations Manual. The operational pressure for the system is 1517 kPa (220 psi), being the upper functional limit for the solenoid valve.
Pin sterilization and handling
Pins were autoclaved prior to field trials and then handled using sterile gloves. A small amount of electrical insulating compound #4 (Dow Corning) was applied to the base of the pin before inserting it on to the pusher to avoid it sliding down the pusher shaft while the holster is being maneuvered to the dorsal fin.
Field testing
Once consistent results were obtained in the laboratory, dolphin field trials were undertaken off Sarasota, Florida, U.S.A., during April–June 2018, June 2019, May 2021, October 2022, May 2023, and August 2023, and off the island of Hawai‘i, U.S.A., during November 2018. The vessel was operated near dolphins. If they chose to bow ride, a tagging attempt was made if feasible. Trials with white sharks were undertaken east of Chatham, Massachusetts, U.S.A., in November 2020. Trials with the whale shark were undertaken south of Martha’s Vineyard, Massachusetts, USA, in September 2022. All these tests except the whale shark used the system on a handheld pole as described above. The whale shark trial vessel was incompatible with the pole approach, so the control system (in a waterproof enclosure), and the air tank were mounted on a small raft pushed by a swimmer following behind the tagging swimmer with hoses and control cable between them. The whale shark’s dorsal fin was too compliant for triggering the tag on its trailing edge, so the tag was manually placed and then triggered by the swimmer’s finger on the trigger.
Video analysis
Videos of dolphin trials in the field and laboratory were acquired using a GoPro Hero6 Black camera (https://gopro.com/). Then, using Final Cut Pro X 10.4.1 (https://apps.apple.com/) to scroll through frames to identify significant events—the numbers of frames between events were converted to elapsed time using frame per second recorded. Events included: first touch, behavioral changes such as bubble streams, roll, flipper movement, pump tail, and accelerate.
Results
Laboratory testing
As the hardware and software evolved, iterative lab tests were critical to ensure that the system was reliably tagging cadaver fins. However, the absence of the dynamics of sea state and animal behavior made successive field trials essential and informative.
Dolphin tagging efficiency in field
Days spent in the field to test the TADpole varied widely in terms of sea state and availability of potential tagging candidates. Table 1 summarizes the field sites, and species involved in tagging attempts at each site, including bottlenose, pantropical spotted (Stenella attenuata), Atlantic spotted, and rough-toothed dolphins (Steno bredanensis), and melon-headed whales (Peponocephala electra). Thirty-one individual dorsal fins were contacted over 22 days at sea. On 17 occasions, the device triggered, but the pin did not fully penetrate through the fin, and therefore failed to press fit into the second retaining ring to complete the tag attachment. On one occasion a tag was successfully attached and is described in detail below (Fig. 8). On four occasions, a partial biopsy sample was obtained from the pin dragging across the dorsal fin as the animal rolled out of the holster before the actuation cycle could be completed.
Assessment of restrained, tagged Sarasota Bay bottlenose dolphins
As part of Sarasota Dolphin Research Program bottlenose dolphin health assessments, during a brief restraint onboard a vessel, two dolphins were tagged in 2022 using the TADpole device, using magnesium corrodible rings. On May 18th, 2022, dolphin F293 was tagged. The tag continued to transmit through June 22nd, 2022. On July 6th, 2022, the animal was observed without the tag, with a small healing hole at the tag site. The tag was attached for 35–49 days. A second dolphin, F322 was tagged May 19th, 2022. The tag continued to transmit through June 10th, 2022, and then was observed on June 14th, 2022, with the tag off with no evidence of tag trauma except the small hole made by the pin (Fig. 9). The tag was attached for 22–26 days. Both dolphins were observed after tag loss, and the tag holes have fully healed without complications (Figs. 10 and 11).
Bottlenose dolphin tag F322. a Tag being applied using the TADpole during temporary restraint, May 19th, 2022. b Tag attached to F322, May 19th, 2022. c Individual sighted on June 8th, 2022. This individual was sighted again June 14th, with the tag gone, and the tag area looked ‘clean’, but no adequate photographs were available
In May 2023, two restrained dolphins were tagged by the TADpole during Sarasota Dolphin Research Program bottlenose dolphin health assessments, using aluminum corrodible links to test their durability. On May 11th, 2023, dolphin F292 was tagged (Fig. 12). The animal was first seen after tagging, without the tag, on May 23rd, 2023. Transmissions ceased on May 15th, 2023, so the dolphin lost the tag within 4–12 days of tagging. A small hole remained at the tag attachment site on May 23rd. On May 12th, 2023, dolphin F326 was tagged. The animal was last reported with the tag on September 5th, 2023, after transmissions had ceased, and was first seen without the tag on September 12th, 2023, indicating a tag attachment duration of 115–123 days, as desired. Figure 13 shows the tag site on September 12th, November 7th, 2023, and January 15th, 2024. In both cases, the tags came off the fin as designed leaving a small hole, and by August and November 2023, respectively, both fins were well-healed (Figs. 12 and 13). The reason for the short tag attachment duration for F292 is not known, but it was too brief to have been the result of corrosion. The attachment duration for F326 suggests that aluminum is a reasonable choice for the corrodible retaining ring.
Behavioral responses by dolphins
For each restrained animal, a single startle/jerk reaction was observed as the pin passed through the fin. For bow-riding animals, the commonest response of a dolphin to the tool touching the dorsal fin was to quickly abduct the flippers and roll laterally, often to as much as 90 degrees. In some cases, forward acceleration or dropping in elevation was observed. When the initial designs of the device triggered, the animals usually reacted faster than the device could complete its task. This led to various adjustments to the hardware and software. These included minimizing the length of air hose between the valve and the cylinder, maximizing the working air pressure, reducing the wall thickness of the pin from 1.82 mm to 1.02 mm, and ensuring its cutting tip was freshly sharpened.
Video analysis of touch-to-response times
We used the video data from the 2021 season to evaluate the device. Table 2 shows that the touch-to-response time ranged from 3 to 28 ms. In one case the video also showed the movement of the trigger, and the pusher/pin assembly. The biggest delay was from the first touch to when the trigger began to move. To establish the deployment timing of the TADpole device in the laboratory, videos were taken of the device triggering without any dorsal fin in the holster. For three consecutive trials, the time from triggering to extension ranged from 9 to 15 ms, and from triggering to retraction was 20–26 ms.
Free-swimming dolphin tagging
A bow-riding presumed adult female Atlantic spotted dolphin was tagged on August 15th, 2023, 85 km offshore of Sarasota, Florida (Figs. 8 and 14). The tag was deployed from a custom bow pulpit on Mote Marine Laboratory’s 14-m R/V Eugenie Clark with the tag operator’s feet 0.5 m above the sea surface. The standard Wildlife Computers SPLASH10 Finmount tag was attached. To minimize the actuation cycle duration there was no biopsy retaining barb in the pusher. Despite this, a full fin core sample was retained. The corrodible ring was made of aluminum. Conditions were nearly ideal—calm seas and slow-moving, persistently bow-riding dolphins. The angle of the holster relative to the pole was altered to be more acute, in response to dolphins riding close to the bow of the vessel, facilitating trigger contact by the fin when the tool was in appropriate position. Two previous contacts on other dolphins earlier in the day with a more obtuse angle as would be required for dolphins farther ahead of the bow had resulted in premature triggering, before the tag was in place on the fin. The dolphin leaped repeatedly immediately after tagging, but returned to the bow multiple times, affording good views of the attached tag (Fig. 8). The tag transmitted for 31 days, as the animal ranged through waters frequented by Atlantic spotted dolphins (Fig. 15). Transmissions ceased due to battery exhaustion.
Shark tagging
Two white sharks, approximately 3.7 and 4.4 m in total length, were tagged with SPOT tags from the 3.4 m-long bow pulpit of a 7.3 m vessel off Chatham, MA on 7 November 2020. Both sharks were free-swimming < 1 m below the surface when tagged and reacted by moving slowly away from the tagging vessel. An overhead view of shark tagging is shown in Fig. 16 and an image of a successfully tagged shark is shown in Fig. 17. No locations were reported from either of these tags. However, one of the tags reported intermittently over the next five months until 17 April 2021; unfortunately, tag communication with the satellite was not long enough to calculate a position. When this shark was resighted after 2 years (28 November 2022), the tag was gone, and the fin was well-healed. One whale shark was successfully tagged by a swimmer as described above (Fig. 18), a second tag attempt was triggered, but the pin did not fully press into the Delrin ring, so the tag swimmer removed the tag manually. This resulted from inadequate air pressure in the small volume scuba cylinder that was used to power the system. The resulting tag data are shown in Fig. 19.
Discussion
The TADpole has potential for conservation research with dolphins, and white and whale sharks. The fundamental method appears to have value, but to enhance efficiency, especially for using it with small cetaceans, there are some matters to consider.
Dolphin evasive behavior
During the dolphin field trials, the biggest challenge was to complete the actuation cycle before the animal evaded the attempt by rolling, as described above. The dolphins were acutely sensitive to touch by the TADpole, and adept at avoiding it once sensed. Earlier studies of human reaction times (mean ± SEM milliseconds) to painful stimuli to the hand were 387 ± 20 ms whereas reaction to tactile stimulation was 361 ± 25 ms [28]. From a review by Caldwell et al. [29]: “Human tactile, perceptual mean reaction times from one study in untrained, healthy volunteers have been found to vary between 210 and 400 ms [30], but can range down to 140–150 ms with practice for certain individuals [31]. Reaction times for individuals tend to stay relatively constant between ages 25 and 60”. Thus, from the data in Table 2, dolphins seem to react to touch an order of magnitude faster than humans, although we cannot say for sure that they were not pre-alerted by visual or acoustic stimuli before the tool first touched the dolphin, or that the very act of bow riding puts them on high alert. But tactile stimulus reaction times in the teens for dolphins vs. hundreds of milliseconds in humans is striking.
In the skin of bottlenose dolphins, Palmer et al. [32] described richer, more elaborate and specialized neural structures than in humans. These tunneled into the rete peg base terminating in unique papillary wall complexes, some of which penetrated the epidermis up to three cell layers from the surface. Eldridge et al. [33] described how humpback whale (Megaptera novaeangliae) skin has been shaped by the aquatic environment to sense flow, turbulence, and boundary layers as well as touch and noxious stimuli. Visualizing afferent neural structures immunochemically, they described unique threadlike heterogenous axon bundles in humpback whale skin, that divided into smaller bundles without structural endings at the dermal/epidermal junction, with ‘an exceptionally dense low threshold mechanosensory system innervation most likely adapted for sensing hydrodynamic stimuli’. If proven relevant to all cetaceans, these observations may be the basis for the remarkably rapid dolphin touch reflex observed in our study. One might speculate that the dolphins’ rapid rolling reaction evolved at least in part in response to predation attempts by sharks, as evidenced by frequent observations of shark bite wounds exhibiting scraping marks from teeth in one jaw as the dolphin presumably rolled out of its mouth (Fig. 20).
Tagging efficiency
Success of the tagging effort was predicated on the ability to place the tool where it would trigger an event, the duration of the consequent actuation cycle, and the ability of the pin to penetrate the dorsal fin efficiently and press fit through the Delrin ring before the animal was able to react and evade the attempt. Data in Table 2 showing the collection of biopsies from the pin tip as dolphins dropped and rolled out of the TADpole holster reflect events where the tool fell short of completing its task. This resulted in changes being made to the pneumatic system such as minimizing the length of the air hoses between the valve and the cylinder, optimizing the size of the cylinder, maximizing the cutting efficiency of the pin, minimizing any mechanical latency in the trigger mechanics, optimizing the ergonomics of the angle of the tool relative to the pole, adjusting the angle relative to the distance of the dolphin from the bow of the boat, and positioning of the tagger relative to where the dolphin swam.
Metal retaining rings
We initially considered magnesium, zinc, and aluminum. We decided to do the initial attachment trials with magnesium to generate maximum corrosion, and thence, short attachment times. The two tags remained attached for about a month from the Sarasota Bay follow-up observations. We did this in case there were any unforeseen health impacts from the tag attachment. We observed none. Longer attachments could be attained with a metal lower in the galvanic series, such as aluminum or brass. Subsequent tests in Sarasota Bay used aluminum, and this was also used for the offshore tagging of the free-ranging dolphin. No adverse health effects were noted for the Sarasota Bay dolphins. One tag with an aluminum ring came off the dorsal fin of an inshore dolphin within 4–12 days of tagging for unknown reasons probably unrelated to the metal of the ring. The tag on the offshore dolphin ceased transmitting after 31 days due to battery failure, so the full duration of attachment could not be documented, and the other inshore dolphin retained the tag for 115–123 days, a reasonable amount of time for tracking SPLASH tags.
Biopsy
During the trials to minimize the TADpole actuation cycle, we elected to remove the biopsy retaining barb in the pusher as we were concerned it might slow down the extension phase. Once we have more experience with successful dolphin tagging events, using that barb should be a consideration. Future shark tagging efforts should use barbed pushers.
Pin length
When single-pin tags are attached to restrained animals, the width of the fin can be measured, and the pin length cut to match the fin width. Obviously, this cannot be done on a bow-riding animal. Thus, we opted to design the TADpole for the likely maximum width of most dolphin dorsal fins at the point where the pin was to be inserted. Hence our choice to design the TADpole to be able to penetrate up to 26 mm of fin width. The tool will certainly tag narrower fins, however the drawback of this is that the pin/rings complex is not fully flush with the dorsal fin in smaller animals. This will lead to the potential for marginally more drag, and possibly increased snagging of debris and active fishing lines. Only once a significant number of deployments have been undertaken in areas where follow-up observations are likely will the extent of this concern be apparent. We have actively discussed a pin that could dynamically adjust to the fin width, to overcome this concern, but there was no obvious way to do this.
Tag placement
The design of the holster provides an absolute limit of the distance the tag pin can be from the trailing edge of the dorsal fin. The major vessels in the fin are anterior to this position. This distance from the trailing edge of the fin and vertical positioning was established through experience tagging 77 dolphins with single-pin radiotags, and designed to minimize the potential for dorsal fin damage [13]. Thus, despite our inability to closely examine the dorsal fin as one can with a restrained animal, the risk of the pin passing through a large vessel is very low.
Sharks
The use of the TADpole on white and whale sharks raises some questions about how to optimize the design for those two species. The white shark tagger found it possible to use the tool deeper in the water. Unfortunately, no positions were derived from these two tags. Upon consultation with the tag manufacturer, we conclude that the tags were placed too low on the dorsal fin. For this tag design (i.e., horizontal single point), virtually the entire tag must clear the water before the wet/dry sensor initiates transmission. Therefore, we recommend that the tags be placed much closer to the apex of the dorsal fin, as was the case with the whale shark. The whale shark tagging protocol departed from the deck-based tagger and used swimmers instead. Both shark events flooded the valve via the ~ 2 m exhaust tube, a design constraint that was implemented to minimize the vent tube length and, presumably, minimize pneumatic drag. A one-way exhaust valve (e.g., https://www.mcmaster.com/7933K27/) should be considered for shark tagging. While it will prolong the actuation cycle, minimizing its duration does not appear to be a concern in the sharks where touch reactions seem to be less of an issue in our field trials, given the high success rate compared to that of the dolphins. Our results suggest that additional trials with large, free-swimming sharks are a promising future avenue for tagging without capture and handling of the animal.
Conclusions
The TADpole tagging tool has the following characteristics:
-
Enables tagging of free-swimming dolphins and sharks.
-
Avoids the stress of capture/restraint to manually attach tags. Animals that are tagged while bow riding approach and leave the boat at will.
-
Trauma from the tag and attachment is comparable to that of single-pin dorsal fin tags, and hence provides the potential for longer tag durations than reported for remotely deployed, barb-retained tags.
-
Requires a smaller team and less expense than a catch-and-release expedition for tagging.
Future steps should include:
-
Further use of the TADpole tool with resident dolphin populations to undertake post-tagging follow-up observations of tag attachment sites before and after tag loss.
-
More development of the TADpole hardware and software specific for the requirements of different dolphin and shark species, especially to enhance the efficiency of dolphin tag application.
-
Use of the tool to tag offshore dolphins without the need for restraint, to establish the potential value of this novel technique.
-
Use of aluminum or brass rings to optimize tag attachment duration relative to tag battery life.
-
Development of the capability of retaining a biopsy (skin) sample during tagging, for genetic sample collection for sex determination, population structure studies, and epigenetic assessments of age and health.
Availability of data and materials
The design, operating protocol, components specifications are available in the Woods Hole Open Access Server repository https://hdl.handle.net/1912/67505 and TADpole Arduino code for the microcontroller is available at https://github.com/WHOItp/TADpole.git.
References
Andrews RD, Baird RW, Calambokidis J, Goertz CE, Gulland F, Heide-Jorgensen M-P, Hooker SK, Johnson M, Mate B, Mitani Y. Best practice guidelines for cetacean tagging. J Cetacean Res Manag. 2019;20:27–66. https://doi.org/10.47536/jcrm.v20i1.237.
Renshaw S, Hammerschlag N, Gallagher AJ, Lubitz N, Sims DW. Global tracking of shark movements, behaviour and ecology: a review of the renaissance years of satellite tagging studies, 2010–2020. J Exp Mar Biol Ecol. 2023;560:151841. https://doi.org/10.1016/j.jembe.2022.151841.
Johnson M, Tyack P. A digital acoustic recording tag for measuring the response of wild marine mammals to sound. IEEE J Ocean Eng. 2003;28:3–12.
Shaff JF, Baird RW. Diel and lunar variation in diving behavior of rough-toothed dolphins (Steno bredanensis) off Kauaʻi Hawaiʻi. Mar Mamm Sci. 2021;37:1261–76. https://doi.org/10.1111/mms.12811.
Schorr GS, Falcone EA, Moretti DJ, Andrews RD. First long-term behavioral records from Cuvier’s beaked whales (Ziphius cavirostris) reveal record-breaking dives. PLoS ONE. 2014;9: e92633. https://doi.org/10.1371/journal.pone.0092633.
Baird RW, Hanson MB, Schorr GS, Webster DL, McSweeney DJ, Gorgone AM, Mahaffy SD, Holzer DM, Oleson EM, Andrews RD. Range and primary habitats of Hawaiian insular false killer whales: informing determination of critical habitat. Endanger Spec Res. 2012;18:47–61. https://doi.org/10.3354/esr00435.
Moore MJ, Zerbini AN. Dolphin blubber/axial muscle shear: implications for rigid trans-dermal intra-muscular tracking tag trauma in whales. J Exp Biol. 2017;220:3717–23. https://doi.org/10.1242/jeb.165282.
Norman SA, Flynn KR, Zerbini AN, Gulland F, Moore MJ, Raverty S, Rotstein DS, Mate BR, Hayslip C, Gendron D. Assessment of wound healing of tagged gray (Eschrichtius robustus) and blue (Balaenoptera musculus) whales in the eastern North Pacific using long-term series of photographs. Mar Mamm Sci. 2018;34:27–53. https://doi.org/10.1111/mms.12443.
Gendron D, Martinez Serrano I, de Ugalde la Cruz A, Calambokidis J, Mate B. Long-term individual sighting history database: an effective tool to monitor satellite tag effects on cetaceans. Endang Spec Res. 2015;26:235–41. https://doi.org/10.3354/esr00644.
Balmer BC, Wells RS, Schwacke LH, Rowles TK, Hunter C, Zolman ES, Townsend FI, Danielson B, Westgate AJ, McLellan WA. Evaluation of a single-pin, satellite-linked transmitter deployed on bottlenose dolphins (Tursiops truncatus) along the coast of Georgia, USA. Aquat Mamm. 2011;37:187–92.
Wells RS, Fougeres EM, Cooper AG, Stevens RO, Brodsky M, Lingenfelser R, Dold C, Douglas DC. Movements and dive patterns of short-finned pilot whales (Globicephala macrorhynchus) released from a mass stranding in the Florida Keys. Aquat Mamm. 2013;39:61–72. https://doi.org/10.1578/AM.39.1.2013.61.
Balmer BC, Schwacke LH, Wells RS. Linking dive behavior to satellite-linked tag condition for a bottlenose dolphin (Tursiops truncatus) along Florida’s Northern Gulf of Mexico Coast. Aquat Mamm. 2010. https://doi.org/10.1578/AM.36.1.2010.1.
Balmer BC, Wells RS, Howle LE, Barleycorn AA, McLellan WA, Ann Pabst D, Rowles TK, Schwacke LH, Townsend FI, Westgate AJ. Advances in cetacean telemetry: a review of single-pin transmitter attachment techniques on small cetaceans and development of a new satellite-linked transmitter design. Mar Mamm Sci. 2014;30:656–73. https://doi.org/10.1111/mms.12072.
Deming AC, Wingers NL, Moore DP, Rotstein D, Wells RS, Ewing R, Hodanbosi MR, Carmichael RH. Health impacts and recovery from prolonged freshwater exposure in a common bottlenose dolphin (Tursiops truncatus). Front Vet Sci. 2020;7:235. https://doi.org/10.3389/fvets.2020.00235.
Dunn C, Claridge D, Herzing D, Volker C, Melillo-Sweeting K, Wells RS, Turner T, O’Sullivan K. Satellite-linked telemetry study of a rehabilitated and released Atlantic spotted dolphin in the Bahamas provides insights into broader ranging patterns and conservation needs. Aquat Mamm. 2020;46:633–9. https://doi.org/10.1578/AM.46.6.2020.633.
Moore RBT, Douglas DC, Nollens HH, Croft L, Wells RS. Post-release monitoring of a stranded and rehabilitated short-finned pilot whale (Globicephala macrorhynchus) reveals current-assisted travel. Aquat Mamm. 2020;46:200–14. https://doi.org/10.1578/AM.46.2.2020.200.
Wells RS: Evaluation of tag attachments on small cetaceans. Final Report to the Office of Naval Research for Award Number: N000141210391. 5 pp. 2013
Wells RS, Fauquier DA, Gulland FMD, Townsend FI, DiGiovanni RA. Evaluating postintervention survival of free-ranging odontocete cetaceans. Mar Mamm Sci. 2013;29:E463–83. https://doi.org/10.1111/mms.12007.
Wells RS, Schwacke LH, Rowles TK, Balmer BC, Zolman E, Speakman T, Townsend FI, Tumlin MC, Barleycorn A, Wilkinson KA. Ranging patterns of common bottlenose dolphins Tursiops truncatus in Barataria Bay, Louisiana, following the Deepwater Horizon oil spill. Endanger Spec Res. 2017;33:159–80. https://doi.org/10.3354/esr00732.
Pulis E, Wells RS, Schorr GS, Douglas DC, Samuelson MM, Solangi M. Movements and dive patterns of pygmy killer whales (Feresa attenuata) released in the Gulf of Mexico following rehabilitation. Aquat Mamm. 2018;44:555–67. https://doi.org/10.1578/AM.44.5.2018.555.
Wells RS, Cremer MJ, Berninsone LG, Albareda D, Wilkinson KA, Stamper MA, Paitach RL, Bordino P. Tagging, ranging patterns, and behavior of franciscana dolphins (Pontoporia blainvillei) off Argentina and Brazil: considerations for conservation. Mar Mamm Sci. 2022;38:571–605. https://doi.org/10.1111/mms.12879.
Nasby-Lucas N, Domeier ML. Impact of satellite linked radio transmitting (SLRT) tags on the dorsal fin of subadult and adult white sharks (Carcharodon carcharias). Bull Mar Sci. 2020;96:23–30. https://doi.org/10.5343/bms.2019.0019.
Skomal G. Evaluating the physiological and physical consequences of capture on post-release survivorship in large pelagic fishes. Fish Manage Ecol. 2007;14:81–9. https://doi.org/10.1111/j.1365-2400.2007.00528.x.
Jewell OJ, Wcisel MA, Gennari E, Towner AV, Bester MN, Johnson RL, Singh S. Effects of smart position only (SPOT) tag deployment on white sharks Carcharodon carcharias in South Africa. PLoS ONE. 2011;6: e27242. https://doi.org/10.1371/journal.pone.0027242.
Chin A, Mourier J, Rummer JL. Blacktip reef sharks (Carcharhinus melanopterus) show high capacity for wound healing and recovery following injury. Conserv Physiol. 2015;3:cov062. https://doi.org/10.1093/conphys/cov062.
TADpole—CAD drawings of a device to tag dolphins, parts list, and operating instructions. https://doi.org/10.26025/1912/67505 [https://hdl.handle.net/1912/67505]. Accesed 9 Apr 2024.
TADpole—Arduino code for TADpole. [https://github.com/WHOItp/TADpole.git]. Accesed 9 Apr 2024.
Ploner M, Gross J, Timmermann L, Schnitzler A. Pain processing is faster than tactile processing in the human brain. J Neurosci. 2006;26:10879–82. https://doi.org/10.1523/JNEUROSCI.2386-06.2006.
Caldwell DJ, Cronin JA, Wu J, Weaver KE, Ko AL, Rao RPN, Ojemann JG. Direct stimulation of somatosensory cortex results in slower reaction times compared to peripheral touch in humans. Sci Rep. 2019;9:3292. https://doi.org/10.1038/s41598-019-38619-2.
Lele P, Sinclair D, Weddell G. The reaction time to touch. J physiol. 1954;123:187. https://doi.org/10.1113/jphysiol.1954.sp005042.
Woodworth RS, Barber B, Schlosberg H. Experimental psychology. Oxford: Oxford and IBH Publishing; 1954.
Palmer E, Weddell G. The relationship between structure, innervation and function of the skin of the bottle nose dolphin (Tursiops truncatus). Proc Zool Soc Lond. 1964;143:553-568 557. https://doi.org/10.1111/.1469-7998.1964.tb03881.x.
Eldridge S, Mortazavi F, Rosene D. The hydrodynamic sensory system in the skin of cetaceans. FASEB J. 2020;34:1–1. https://doi.org/10.1096/fasebj.2020.34.s1.00356.
Acknowledgements
Jay Sweeney and Rae Stone enabled critical initial support from Dolphin Quest. Gretchen Lovewell, Misty Niemeyer, and William McLellan collected and supplied dolphin cadaver dorsal fins from stranded, deceased dolphins. Michael Scott, Brian Balmer, Teri Rowles, Lori Schwacke, Cynthia Smith, and Andrew Westgate contributed to the project. We thank the Mote Marine Laboratory Marine Operations staff for field support. White shark tagging was funded by the Atlantic White Sharks Conservancy and conducted off the F/V Aleutian Dream with the assistance of John King. Brian Hanson, Megan Winton, and spotter pilot Wayne Davis. Whale shark tagging was conducted off the F/V Endurance and made possible by Eric Savetsky and Dr. Tom Burns, DVM. We thank Krystan Wilkinson for tracking map preparation.
Funding
Funding for initial TADpole development and testing was provided to WHOI by Dolphin Quest, Inc. Support for refinements and further testing was provided by Dolphin Biology Research Institute, Mote Scientific Foundation and the NOAA RESTORE Program via WHOI CINAR NA19OAR4320074. Fieldwork in Hawaiʻi was supported by a grant from the Tides Foundation to Cascadia Research Collective. White shark tagging was supported by the Atlantic White Shark Conservancy and the Massachusetts Division of Marine Fisheries. Whale shark tagging was supported by internal funding at WHOI. Support for preparation of the manuscript was provided by the Independent Research & Development Program at WHOI and the NOAA RESTORE program.
Author information
Authors and Affiliations
Contributions
MJM coordinated the tool development process, assisted with field testing, and drafted the manuscript. TML led the design, engineering and iterative development of the hardware and software. RSW conceived of the tool, secured initial funding, recruited the team, and led the field testing. JK oversaw the engineering project and provided critical input as to how to evolve and enhance the system. AAB and JBA operated the tag tool and worked with the Mote Marine Laboratory staff to enable the dolphin field tests. RWB enabled and supported the field tests in Hawaiʻi. CDB conceived of and undertook the whale shark tagging. GBS conceived of and undertook the white shark tagging. SRT supported the planning for both shark tagging projects. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Dolphin field tests in Florida were conducted under National Marine Fisheries Service Scientific Research Permits No. 15543, 20455, and 26622, and under annually renewed IACUC approvals from Mote Marine Laboratory, issued to Wells. Field tests in Hawaiʻi were conducted under National Marine Fisheries Service Scientific Research Permit No. 20605, issued to Baird, and under IACUC approval from Cascadia Research Collective. Sharks were tagged under IACUC approval from the Woods Hole Oceanographic Institution.
Consent for publication
Not applicable.
Competing interests
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Moore, M.J., Lanagan, T.M., Wells, R.S. et al. Development of single-pin, un-barbed, pole-tagging of free-swimming dolphins and sharks with satellite-linked transmitters. Anim Biotelemetry 12, 6 (2024). https://doi.org/10.1186/s40317-024-00364-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40317-024-00364-3