Abstract
Temporal accuracy is a fundamental characteristic of logging technology and is needed to correlate data streams. Single biologgers sensing animal movement (accelerometers, gyroscope, magnetometers, collectively inertial measurement unit; IMU) have been extensively used to study the ecology of animals. To better capture whole body movement and increase the accuracy of behavior classification, there is a need to deploy multiple loggers on a single individual to capture the movement of multiple body parts. Yet due to temporal drift, accurately aligning multiple IMU datasets can be problematic, especially as deployment duration increases. In this paper we quantify temporal drift and errors in commercially available IMU data loggers using a combination of robotic and animal borne experiments. The variance in drift rate within a tag is over an order of magnitude lower (σ = 0.001 s h−1) than the variance between tags (σ = 0.015 s·h−1), showing that recording frequency is a characteristic of each tag and not a random variable. Furthermore, we observed a large offset (0.54 ± 0.016 s·h−1) between two groups of tags that had differing recording frequencies, and we observed three instances of instantaneous temporal jumps within datasets introducing errors into the data streams. Finally, we show that relative drift rates can be estimated even when deployed on animals displaying various behaviors without the tags needing to be simultaneously moved. For the tags used in this study, drift rates can vary significantly between tags, are repeatable, and can be accurately measured in the field. The temporal alignment of multiple tag datasets allows researchers to deploy multiple tags on an individual animal which will greatly increase our knowledge of movement kinematics and expand the range of movement characteristics that can be used for behavioral classification.
Similar content being viewed by others
Introduction and background
Studies on the dynamics of a moving animal are classically performed by digitizing video and tracking multiple body parts through time [1, 2]. However, these studies usually examine a narrow range of locomotor behaviors in laboratory settings, and expanding estimates beyond a single individual, into three dimensions, or over longer time frames of hours to days is challenging. Researchers can also place sensors at specific locations on an animal, allowing for precise estimates of some characteristic of that location on the body. For example, the use of electromyography electrodes allow estimation of muscle activation during movement [3,4,5]. Traditionally, these sensors are tethered to an external data recorder, which provides power, data storage and enables multiple sensors to be time-synced. However, this design requires the animal to be physically connected to an external device which generally restricts such studies to laboratory settings.
To move beyond these limitations, researchers have increasingly employed animal-born biologging devices [6, 7]. These devices have an internal battery and log data from a sensor to internal memory, and they “cut the cord” and eliminate the need for an individual to be tethered, allowing for in situ measurements of behavior in the field. Biologgers using a combination of accelerometers, gyroscopes, and magnetometers (collectively inertial measurement unit; IMU) have been increasingly adopted to study a diversity of questions focusing on the movement, behavior, physiology, and ecology of animals [8,9,10,11], and provide a rich data set well beyond what can be achieved in the laboratory.
Animals are dynamic, and recording information from only one sensor at a single location presents ambiguity in determining an animal’s actions, an incomplete picture of body dynamics, and is of limited utility in studying the complex three-dimensional kinematics of most animals. Limited attempts have been made to have a single datalogger record from multiple distributed sensors, but this approach is constrained by the requirement of having the sensors tethered across the animals body and by the recording bandwidth of the logger [12]. Furthermore, the majority of these attempts have been focused on categorizing human movements and behaviors [12,13,14,15].
Placing multiple IMU dataloggers distributed across the body would resolve these issues and allow both high temporal and spatial resolution of animal body dynamics as natural behaviors are executed in the field. However, minor differences in recording frequencies between loggers can accumulate, causing sensors to drift in time from each other [16]. This drift over time obfuscates temporal patterns and thus prevents synchronous comparisons of movement across the body.
To illustrate this problem, imagine an IMU on each foot of a person walking. The IMUs will begin by recording alternating steps: one foot moves forward, while the other is stationary. As small discrepancies in their recording rates accumulate, the datasets will drift apart, and unrealistic behavior such as both feet moving forward at the same time will be recorded, changing the interpretation of the locomotor mode used. Depending on the temporal precision that is required, the accuracy of each loggers clock, and the amount of time these loggers are deployed, this temporal drift can represent a significant and unknown source of error in analysis.
In this paper we demonstrate that temporal drift exists among multiple IMU data streams derived from the same controlled movement, and then (1) quantify the extent of this drift among commonly used commercially available data loggers, (2) measure the variation in drift across multiple datasets, and finally (3) illustrate a methodological approach to temporally synchronize separate IMU data loggers placed at various locations on a single individual. Demonstrating how to correct for IMU drift establishes the foundation for using multiple IMU loggers to reconstruct the three-dimensional body dynamics of animal movement in the field.
Methods
Two types of experiments were conducted: (1) robotic experiments where the movements of the tags were known, and (2) experiments on three species of live fishes in which the movement of tags was unknown. In all experiments dataloggers were manufactured by Technosmart Inc (Rome, Italy). Models used varied across the experiments and involved the AGM model, the Axy-4 model, and the Axy-5 model. The AGM model lacks a real time clock and records from an MPU-9250 (TDK InvenSense Inc.), which has a has a 3-axis accelerometer, 3-axis magnetometer, and 3-axis gyroscope. The Axy-4 and Axy-5 record 3-axis acceleration from an LIS2DH12TR (STMicroelectronics Inc.) and LSM303AGR (STMicroelectronics Inc.) respectively. In all experiments the tags were programmed to record at 100 Hz. AGM models were purchased at three different times with 6 tags having a larger 400 mAh battery and 6 tags having a 100 mAh battery, while the Axy4 and Axy-5 models each had a 100 mAh battery.
Robotic experiments
Experiments were conducted using an automated robotic flapping mechanism that has been extensively used to study dynamics of fish movement in the past [17,18,19] The robotic flapping system has two degrees of freedom and can alter the heading (yaw, or pitch) as well as induce lateral translative movements (sway, or heave), with the user programming the frequency and amplitude of these movements. Multiple dataloggers were rigidly affixed to the flapping mechanism so that all tags experienced the same movement simultaneously (Fig. 1A). The flapping mechanism was turned on for 1 min in duration and the tags were then oscillated laterally back and forth at 0.75 Hz and experienced a programmed 3 cm lateral amplitude and a 25 degree heading oscillation. Flapping was repeatedly conducted over a ~ 24 h period or until the tag batteries were drained. This entire procedure was repeated so that each tag’s drift rate could be estimated at least 3 times.
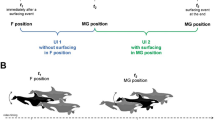
Pictures of multiple tags during each experimental set up. During robotic experiments (A) multiple tags were affixed to a rigid metal rod and simultaneously moved sinusoidally in heave and pitch to provide known a input movement pattern to all tags using a robotic flapping mechanism. Multiple tags were additionally attached along the body onto live animals including a smooth dogfish (B), white sucker (C) and sea lamprey (D)
After an experiment, tag data was downloaded, and the data were imported into R for analysis. In this experiment we had no “true” time, and thus Tag 1 was used as the baseline and all other tags were compared to this tag. The starting and stopping point of each flapping period was identified and a buffer of 60 s was added before and after the flapping period. Temporal lag between each tag and Tag 1 was estimated using a cross correlation function of the two tags Z-axis gyroscope data (change in heading). The lag that maximized the correlation between the two datasets was identified as the temporal lag during each flapping period. The lag for each tag was regressed against time in to estimate the drift rate and converted to seconds per hour. Linear mixed effects models were used to estimate the variance in drift rate within and between tags using the lme4 package in R [20]. Tag ID was identified as a random effect and was used to estimate variance between tags, while residual variance was used to estimate the within tag variation. After observing large discrepancies between groups of tags, a “fast-slow” recording group was also incorporated as a fixed effect.
Animal based experiments
While estimating drift under controlled laboratory conditions is beneficial and provides an accurate estimate of among-tag drift, ideally we want to directly estimate the drift rates of tags in the field during an actual deployment. To determine if this was possible, we placed multiple tags on three species of fishes (Fig. 1B–D) and allowed them to volitionally swim. Data loggers were fixed to the animal’s body using two sutures, one at the anterior side of the tag and a second at the posterior side of the tag. A smooth dogfish (Mustelus canis, TL = 99 cm, Fig. 1B) was tagged at three locations (anterior-above gills, mid:below first dorsal fin, and posterior: at caudal peduncle) using AGM tags. The shark was then placed into a small tank (2.5 m diameter) and allowed to swim freely for 26 h. White sucker (Catostomus commersonii, TL = 50 cm, Fig. 1C) were tagged with an AGM tag placed close to their center of mass with a second Axy-5 tag placed at the caudal peduncle. Finally, lamprey (Petromyzon marinus, TL = 65 cm, Fig. 1D) were tagged at three locations (anterior, mid body, and peduncle) with a combination of AGM, Axy-5, and Axy-4 tags. Lamprey and white sucker were released into a large holding tank (8 × 7 m) that was connected to a flume (36 m long and 0.635 m wide) inducing flow that has been used to investigate volitional bursting behavior in migrating fish [21, 22].
Data processing
Acceleration or angular velocity data from the latitudinal direction was used to estimate the drift in tag sensor data from live fishes as these animals primarily locomote using lateral body oscillation. Data from the two sensors was sequentially cross-correlated with each other using sliding and overlapping windows. Window size was 10 min and windows overlapped by 50 percent. The lag that represented the maximum cross correlation was selected as the lag for each analysis window. Windows that represented low cross correlation scores (ACF < 0.4) were excluded from further analysis. The lag for each window was regressed against time and any outlier with a Cooks distance of greater than 3 was excluded. To examine the sensitivity of drift estimate to window size, the analysis was rerun using window sizes that varied from 1 to 40 min (Additional file 1: Figs. S2–S4).
Results
Robotic experiments
Data from each tag were manually shifted to correct for initial starting time so that the tags produced similar and overlapping data during the first flapping time period (Fig. 2A). However, 24 h after the experiment started, there were noticeable temporal shifts in the flapping patterns in tag data (Fig. 2B), indicating that the data streams from the different tags had drifted with respect to each other. Tags that were purchased at the same time as Tag1 (Tags 1–4) experienced temporal drift between + 0.2 and − 0.5 s over a 24-h period (Fig. 3A), an average a drift rate of − 0.011 ± 0.015 s·h−1 across all trials. Tags 5–12 had a significantly greater drift rates than tags 2–4 (t = − 55.5, p < 0.001) and drifted from Tag1 by 4.0–5.3 s over a 9-h duration (Fig. 2B), with an average drift rate of 0.545 ± 0.014 s·h−1. Once this large difference in drift rate between tags 1–4 and tags 5–12 was accounted for, the variance between tags in each group was not significantly different (Tags 1–4: σ = 0.015 s·h−1, Tags 5–12: σ = 0.014 s·h−1). In addition, variance within a tag was an order of magnitude smaller (σ = 0.001 s h−1) than the variance across tags, representing only 0.5% of the total variance in drift estimates. This suggests that each tag was highly consistent in drift rate across all flapping trials, even as the average temperature between trials ranged from 21.1 to 27.0 C.
Raw angular velocity data from 4 AGM tags that were simultaneously moved over a 24 h period using a robotic flapping mechanism. Immediately after the tags were corrected for their initial temporal offset, all 4 tags show identical and overlapping gyroscope data (A). However, after 24 h, small differences in the clock frequency among tags led to considerable drift among the loggers (B)
The absolute amount of drift in seconds among Tags 1–4 over a 25 h period (A), and among tags 7–12 (B) over a 9 h period during one trial run. Each point represents an individual flapping trial, with the lines showing a linear model and the slope being the estimated drift rate. Tag 7 in B experienced a large instantaneous jump in the drift at ~ 3 h as noticed by the light blue points. All drift rate estimates for each tag, with each datapoint representing drift estimates from each trial (C). There was a large discrepancy in drift rate between the two sets of tags and thus the y axis is broken, but the original axis can be seen in the inset
Within an experiment, tags recorded low variation in temperature (< 1C) and drift rates were highly linear and remained constant over time (r2 > 0.99, Fig. 3). However, during trials we observed three noticeable exceptions (e.g. Tag 7, Fig. 3B) during which a tag experienced an unrecorded error that introduced an instantaneous 1 s shift. On one of these occasions, 100 datapoints were missing from the dataset. On the other two occasions, 100 extra datapoints were added into the dataset. As these errors were significantly greater than the drift rate, we were able to isolate when these shifts occurred and estimate the drift rate excluding this large error (Additional file 1: Fig. S1).
Animal experiments
The sliding window cross correlation during natural behavior was able to identify clear trends in temporal drift across both the shark (Fig. 4), the lamprey (Fig. 5) and the white sucker (Fig. 6) data sets. The shark and lamprey were tagged with the same AGM tags that were used in the robotic based experiments and allow us to directly compare drift estimates. Drift estimates from animal borne deployments were in the same direction and differed by less than 0.02 s·h−1, close to the drift rate we observed when all tags were moved with the same motion during the robotic flapping experiments in the laboratory. However, due to variation in behavior and subsequent temporal shifts in movement among the different tags, there was significantly more variation in animal datasets than in controlled simultaneous flapping. Furthermore, distance between the tags significantly impacted the mean cross correlation value between signals for the shark (ANOVA F1,728 = 35.8, p < 0.001) and for the lamprey (ANOVA F1,248 = 466, p < 0.001). In both cases the anterior and mid position of the body had a higher mean cross correlation than between the head and the posterior tag. Drift estimates were moderately sensitive to the window size used for the cross correlation; however, a window size of approximately 10 min was adequate across all three species (Additional file 1: Figs. S2–S4).
Estimation of the time lag between Tag 2 (A) and Tag 3 (B) compared to Tag 1, when deployed on a captive shark over a 30 h period. Solid lines represent the drift rate estimation calculated while deployed on the animal, while the dashed lines represent the estimate from the robotic flapping in the laboratory
Estimation of the time lag between the anterior (Tag 8) and middle (Tag 7) tags while deployed on a lamprey (A). The solid line represents the drift rate based on the wild deployment and the dashed line in A represents the estimate from robotic flapping in the laboratory. We are also able to estimate the drift between a previously untested Axy-4 tag deployed on the posterior tail of the lamprey and Tag 7 (B)
Discussion
The attachment of multiple data loggers along the body of individual animals permits a detailed analysis of body dynamics during field-based locomotion going beyond what is possible to observe in the laboratory. In addition, data streams from IMU loggers provide an extremely rich source of information on animal movement that does not require the digitization and analysis of video recordings. Here we document the temporal drift in the data stream between multiple dataloggers, that if uncorrected, would result in erroneous analyses of animal body movement patterns. We document a methodology to measure temporal drift among independent dataloggers that are deployed on animals when it is not possible to externally validate their temporal offsets. We also demonstrate that different tags log time at different intervals from each either, and we show that the drift rate between two tags varies little over short periods of time (< 1 month). The standard deviation in drift rates between the tags used in these experiments was 0.015 s·h−1. While this deviation is small, when the error is extrapolated over a 24-h period, 33% of tag pairs will drift by more than 0.5 s from each other. While seemingly a minor quantity for the reconstruction of broader ecological movement patterns, for analyses of the movement of different parts of the body and studies of locomotor biomechanics in the field, a 0.5 s shift is substantial. For example, with a fish beating its tail at 1 Hz, this offset represents 50% of the total duration of the behavior of interest, and > 50% of the phase differences between parts of the body. If tags are applied to both a fish’s head and tail, the tail tag will gradually drift out of sync with the head, leading to a misalignment of behaviors and erroneous reconstruction of the phase relationships between head and tail movement, a key variable of interest in studying the biomechanics of fish locomotion.
While we found a drift estimate of 0.5 s per day to be common, it is not the maximal drift variation measured between tags. In our study, two groups of tag drift behaviors emerged: a “low-drift” group, with an average drift rate of − 0.011 ± 0.015 s·h−1, and a “high-drift” group, with an average drift rate of 0.545 ± 0.014 s·h−1. When comparing these two groups, the drift is large, with tags in group 1 drifting over 10 s from tags in group 2 over a 24-h period. This drift is an order of magnitude more than the difference in phase shift along a body, with the different tags representing entirely different tailbeats at the same point in time, solely as a result of drift. Lastly, we observed large instantaneous errors that offset datasets from each other by one second, again causing datasets to shift from each other by an entire tailbeat.
The AGM tags used in this study lack a real time clock, and thus the tags temporal accuracy is dependent on a temperature compensated oscillator stabilized by external capacitors. After discussions with the tag manufacturer, it was suggested variation in the batch of capacitors, quality of capacitors, or model of capacitor of the oscillator might account for the large discrepancy between the “fast” and “slow” groups drift rates. Furthermore, the instantaneous jumps were identified as an issue with the tags firmware, which has since been updated. These findings highlight the importance of testing and measuring clock drift when using multiple data loggers. In addition to multiple IMU tags deployed on a focal animal, biologging data streams are frequently referenced against additional datasets, such as additional tags on the same animal collecting different data using separate internal clocks (e.g. video loggers, swim speed sensors, muscle temperature loggers), or loggers simultaneously deployed on separate animals. Biologging datasets are also often compared to external environmental datasets (e.g. weather stations). As each of these datasets are independently collected, knowing the extent of drift among data streams becomes a key factor in data analysis and interpretation.
In addition to the precision of the components, tag recording frequencies are known to vary with device age, manufacturing tolerances, supplied voltage, and temperature [13, 23]. Temperature is known to be one of the biggest drivers of variation in clock frequency in electronics and usually has a nonlinear impact. As our study is aimed at deploying multiple tags on one animal, all tags are in roughly the same temperature environment and thus simultaneously experience temperature changes. However, as temperature deviates from the electronics designed operating temperatures differences in frequency between tags are expected to increase. We observed linear relationships in drift rate during this experiment, but field and lab experiments were also held at near constant temperatures. As the variation in temperature increases, such as animals moving through thermally stratified environments, variation in drift rates might also be expected to increase, potentially introducing non-linear impacts that are more difficult to correct. Future work should investigate and quantify the potential for temperature-based influences on tag drift rates.
We also chose to focus on the drift rate of tags with respect to each other, and not between tags and the “true” time. Compared to a highly accurate clock, all our tags could have drifted a substantial amount. Thus, the drift values within this paper should not be taken to represent the clocks’ absolute error. As many data products are indexed to “true”, global standardized times, additional experiments are needed to compare how biologgers drift from these time standards. Increasing clock accuracy will likewise decrease the drift rates between tags [13]. For tags purpose-built for simultaneous measurement, developers could incorporate temperature compensating internal crystals of higher accuracy (< < 10 ppm, ~ 0.86 s·h−1) to limit the degree of temporal drift. Throughout our robotic experiments, there was little variation in drift estimates within a tag. However, the stability of these estimates through time is unknown, future work is needed to understand the stability of the characteristic drift rate between tags over time.
We show that animal borne estimates of drift are comparable to controlled simultaneous estimates of drift. However, for animals displaying volitional behavior in the field, care should be employed before uncritically estimating the drift rates. After release, animals are known to exhibit shifts in behavior over time, such as large changes in tail beat frequency immediately post release [24, 25]. This change in behavior could make it difficult to separate the change in timing due to the tag’s temporal drift and the variation due to the animals’ movement. In these cases, alternative algorithms could be employed that would focus on similar or high intensity behaviors only that would be less susceptible variations due to animal behavior [13].
Standardizing time between multiple devices is a commonly encountered problem in electronics [26]. For circuits that are hard wired, time can easily be transferred in real time, or use an external synchronization signal to mark the temporal difference between recording units. Systems have been developed to have multiple data loggers synchronized using an external wire that sends pulses that can be simultaneously received by all loggers [15]. Alternatively, the data recording function can be centralized and where all sensors send information to a single device [12]. However, the size of the central recording station can be large and quite power intensive to have the bandwidth needed to record at a high frequency simultaneously from multiple dataloggers. In these situations, the devices used need to be custom designed and still require physical connections between the loggers, which can be difficult to manage while an animal is performing behaviors involving substantial body deformation. Furthermore, these connections risk altering the movement of each tag due to motion of the connecting wire. Increasingly, communication between tags is becoming wireless. Frameworks have been developed that allow for multiple independent sensors to wirelessly stream to a central data recorder/ processor, which could alleviate the problem of the tags being physically connected [26] or allow tags to periodically synchronize their times during deployments [27]. However, radio frequencies are rapidly attenuated in marine and aquatic environments, limiting wireless communication to acoustic transmission, which lacks the bandwidth to transfer data at a sufficiently high rate needed for recording higher-frequency animal body movements.
Once the drift between two tags is known, there are multiple computational methods that researchers can employ so that the datasets are temporally synchronized. For analytical methods that do not require simultaneous measurements, one of the tags timestamp can simply be recalculated. However, when simultaneous measurements are required for temporal comparison, a commonly employed method is that one, or both, of the datasets can be resampled with interpolated datapoints [28, 29]. However, when data are interpolated, the resulting values are ones that were never actually recorded by the tag. Depending on the method of interpolation, an increase or systematic decrease in the amplitude of recorded signals can be observed. Often, it is preferable to limit data manipulation to retain as much of the original data quality as possible. A simple method that limits data manipulation is to periodically delete or duplicate a single datapoint in one of the tag data streams so that the data are temporally synchronized. This method limits tag data manipulation to single localized time points, with the rest of the data stream being the true recorded values and temporal error between two tags is maximally half the recording frequency.
For future studies that employ multiple independent dataloggers on the same focal individual as shown in Fig. 1, we suggest that prior to field deployments, the tags be tested under controlled conditions of simultaneous movement to calculate drift estimates. In this study we employed a robotic flapping machine with repeated periodic flapping, although simpler methods would likely suffice. Attaching multiple tags to a rigid object, and then shaking the object at the beginning and then several hours later, would provide two estimates of lag. By regressing these two estimates of lag across time a simple estimate of drift can be calculated. Repeating this procedure would provide confidence that the drift estimates are repeatable and provide a baseline estimate of drift. However, we recommend that when deployed on an animal, researchers should still calculate the lag using moving window cross correlation to account for minor variation due to time or temperature. Laboratory based estimates can act as a prior and give confidence to understand the expected magnitude and direction of drift observed in the field among tags.
The use of multiple data loggers on one animal provides researchers with a key method to wholistically capture the movement of animals and significantly expand the ability of natural movement data to inform biomechanical studies. The use of multiple data loggers to capture the kinematics of animals is a rapidly advancing field and has been used to classify gait in horses [29]. However, deploying multiple motion sensing dataloggers has utility beyond biomechanics, as motion sensing data loggers are commonly used to estimate discrete animal behaviors and generate ethograms using both supervised and unsupervised classification [30, 31]. However, classifying all behaviors from a single point on the body can be difficult, especially for behaviors that are similar (e.g. prey capture attempt vs predator avoidance) [30]. Incorporating additional sensors (e.g. gyroscopes and magnetometers) into a single datalogger has improved accuracy of behavioral classification [32]. But by placing multiple sensors across an animal’s body, researchers will be able to add additional features to these datasets further increasing the ability to accurately identify discrete behaviors [33]. For example, placing a datalogger on an animal’s jaw in addition to the body midpoint would aid in the ability to separate prey capture attempts from escape behaviors not involving rapid jaw movements [34, 35].
Conclusions
We demonstrate the magnitude of temporal drift between multiple commercially available tags while deployed on free swimming animals. The methodology we present here allows researchers to use generic, commercially available tags to monitor multiple locations on an animal simultaneously. By employing multiple dataloggers, we can begin to understand movements at separate locations on the body simultaneously to better analyze and discretize specific behaviors. Studies on the complex three-dimensional kinematics of animals can now progress from the laboratory into the wild by capturing volitional behavior with ecologically relevant stimuli [36], and move toward analyzing thousands of locomotor behaviors (i.e. tailbeats and body kinematics) over long time periods to better understand the variability and dynamics of animal biomechanics and behavior.
Availability of data and materials
All data and scripts used within the current study are available on request from the corresponding author.
Abbreviations
- IMU:
-
Inertial measurement unit
References
Gray J. Studies in animal locomotion: I. The movement of fish with special reference to the eel. J Exp Biol. 1933;10:88–104.
Marey E-J. Le mouvement. Paris: G. Masson, Libraire De L'Academie De Medecine; 1894.
Basmajian JV. Electromyography comes of age: the conscious control of individual motor units in man may be used to improve his physical performance. Science. 1972;176:603–9.
Jayne B, Lauder G. How swimming fish use slow and fast muscle fibers: implications for models of vertebrate muscle recruitment. J Comp Physiol A. 1994;175:123–31.
Rome LC, Swank D, Corda D. How fish power swimming. Science. 1993;261:340–3.
Rutz C, Hays GC. New frontiers in biologging science. London: The Royal Society London; 2009.
Wilmers CC, Nickel B, Bryce CM, Smith JA, Wheat RE, Yovovich V. The golden age of bio-logging: how animal-borne sensors are advancing the frontiers of ecology. Ecology. 2015;96:1741–53.
Brown DD, Kays R, Wikelski M, Wilson R, Klimley AP. Observing the unwatchable through acceleration logging of animal behavior. Anim Biotelem. 2013;1:1–16.
Payne NL, Taylor MD, Watanabe YY, Semmens JM. From physiology to physics: are we recognizing the flexibility of biologging tools? J Exp Biol. 2014;217:317–22.
Whitney NM, Lear KO, Gleiss AC, Payne N, White CF. Advances in the application of high-resolution biologgers to elasmobranch fishes. In: Carrier JC, Heithaus MR, Simpfendorfer CA, editors. Shark research: Emerging technologies and applications for the field and laboratory. Boca Raton (FL): CRC Press; 2018. p. 45–70.
Williams HJ, Taylor LA, Benhamou S, Bijleveld AI, Clay TA, de Grissac S, Demšar U, English HM, Franconi N, Gómez-Laich A. Optimizing the use of biologgers for movement ecology research. J Anim Ecol. 2020;89:186–206.
Cutti AG, Ferrari A, Garofalo P, Raggi M, Cappello A, Ferrari A. ‘Outwalk’: a protocol for clinical gait analysis based on inertial and magnetic sensors. Med Biol Eng Comput. 2010;48:17–25.
Brønd JC, Pedersen NH, Larsen KT, Grøntved A. Temporal alignment of dual monitor accelerometry recordings. Sensors. 2021;21:4777.
Stewart T, Narayanan A, Hedayatrad L, Neville J, Mackay L, Duncan S. A dual-accelerometer system for classifying physical activity in children and adults. Med Sci Sports Exerc. 2018;50:2595–602.
Zhang K, Werner P, Sun M, Pi-Sunyer FX, Boozer CN. Measurement of human daily physical activity. Obes Res. 2003;11:33–40.
Shipley JR, Kapoor J, Dreelin RA, Winkler DW. An open-source sensor-logger for recording vertical movement in free-living organisms. Methods Ecol Evol. 2018;9:465–71.
Quinn DB, Lauder GV, Smits AJ. Scaling the propulsive performance of heaving flexible panels. J Fluid Mech. 2014;738:250–67.
Shelton RM, Thornycroft PJ, Lauder GV. Undulatory locomotion of flexible foils as biomimetic models for understanding fish propulsion. J Exp Biol. 2014;217:2110–20.
Thandiackal R, Lauder G. In-line swimming dynamics revealed by fish interacting with a robotic mechanism. Elife. 2023;12: e81392.
Bates D, Maechler M, Bolker B, Walker S, Christensen RHB, Singmann H, Dai B, Scheipl F, Grothendieck G, Green P. 2009. Package ‘lme4’. http://lme4.r-forge.r-project.org.
Castro-Santos T, Sanz-Ronda FJ, Ruiz-Legazpi J. Breaking the speed limit—comparative sprinting performance of brook trout (Salvelinus fontinalis) and brown trout (Salmo trutta). Can J Fish Aquat Sci. 2013;70:280–93.
Duguay JM, Lacey RJ, Castro-Santos T. Influence of baffles on upstream passage of brook trout and brown trout in an experimental box culvert. Can J Fish Aquat Sci. 2019;76:28–41.
Vig JR. Introduction to quartz frequency standards. Army Research Laboratory Electronics and Power Sources Directorate, Tech. Rep. SLCETTR-92-1. 1992.
Whitney NM, White CF, Anderson PA, Hueter RE, Skomal GB. The physiological stress response, postrelease behavior, and mortality of blacktip sharks (Carcharhinus limbatus) caught on circle and J-hooks in the Florida recreational fishery. Fish Bull. 2017;115(4):532–43. https://doi.org/10.7755/FB.115.4.9.
Whitney NM, White CF, Gleiss AC, Schwieterman GD, Anderson P, Hueter RE, Skomal GB. A novel method for determining post-release mortality, behavior, and recovery period using acceleration data loggers. Fish Res. 2016;183:210–21.
Rhee I-K, Lee J, Kim J, Serpedin E, Wu Y-C. Clock synchronization in wireless sensor networks: an overview. Sensors. 2009;9:56–85.
Wild TA, Wikelski M, Tyndel S, Alarcón-Nieto G, Klump BC, Aplin LM, Meboldt M, Williams HJ. Internet on animals: Wi-Fi-enabled devices provide a solution for big data transmission in biologging. Methods Ecol Evol. 2023;14:87–102.
Bosch S, Serra Bragança F, Marin-Perianu M, Marin-Perianu R, Van Der Zwaag BJ, Voskamp J, Back W, Van Weeren R, Havinga P. Equimoves: a wireless networked inertial measurement system for objective examination of horse gait. Sensors. 2018;18:850.
Bragança F, Bosch S, Voskamp J, Marin-Perianu M, van der Zwaag B, Vernooij J, van Weeren P, Back W. Validation of distal limb mounted inertial measurement unit sensors for stride detection in Warmblood horses at walk and trot. Equine Vet J. 2017;49:545–51.
Brewster L, Dale J, Guttridge T, Gruber S, Hansell A, Elliott M, Cowx I, Whitney N, Gleiss A. Development and application of a machine learning algorithm for classification of elasmobranch behaviour from accelerometry data. Mar Biol. 2018;165:1–19.
Sakamoto KQ, Sato K, Ishizuka M, Watanuki Y, Takahashi A, Daunt F, Wanless S. Can ethograms be automatically generated using body acceleration data from free-ranging birds? PLoS ONE. 2009;4: e5379.
Noda T, Kawabata Y, Arai N, Mitamura H, Watanabe S. Monitoring escape and feeding behaviours of cruiser fish by inertial and magnetic sensors. PLoS ONE. 2013;8: e79392.
Ellis K, Kerr J, Godbole S, Staudenmayer J, Lanckriet G. Hip and wrist accelerometer algorithms for free-living behavior classification. Med Sci Sports Exerc. 2016;48:933.
Ding L, Lv Y, Jiang R, Zhao W, Li Q, Yang B, Yu L, Ma W, Gao R, Yu Q. Predicting the feed intake of cattle based on jaw movement using a triaxial accelerometer. Agriculture. 2022;12:899.
Liebsch N, Wilson RP, Bornemann H, Adelung D, Plötz J. Mouthing off about fish capture: jaw movement in pinnipeds reveals the real secrets of ingestion. Deep Sea Res Part II. 2007;54:256–69.
Wilson AM, Lowe J, Roskilly K, Hudson PE, Golabek K, McNutt J. Locomotion dynamics of hunting in wild cheetahs. Nature. 2013;498:185–9.
Acknowledgements
We would like to thank the United States Geological Survey for providing access to the facilities at the S.O. Conte Anadromous Fish Research laboratory. Special thanks go out to E. Goerig for helping coordinate and assisting in operations, and to Dr. T. Castos-Santos for collecting fish, and assisting in all operations at the AbiKis flume at the Conte Lab. We also thank M. Blumstein for helpful comments on early versions of the manuscript, and members of the Lauder Lab for helpful comments and discussion of these data. Finally, we would like to thank two anonymous reviewers who provided valuable insights and comments that greatly improved this manuscript.
Funding
This work was supported by the National Science Foundation (Grant number EFRI-830881), the Office of Naval Research (Grants N00014-18-1-2673 and N00014-22-1-2187), and the Robert A. Chapman Fund from the Museum of Comparative Zoology. Published by a grant from the Wetmore Colles fund of the Museum of Comparative Zoology, Harvard University.
Author information
Authors and Affiliations
Contributions
All authors (CFW & GVL) participated in idea conception and data collection. Data analysis was performed by CFW, and both authors wrote, edited and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All research procedures were reviewed and approved under protocols #20-03-3 and #20-03-4 by The Harvard University Institutional Animal Care and Use Committee (IACUC).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no financial or non-financial competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Shows one of the instantaneous jump errors that we recorded, while Figures S2-S4. Show the sensitivities to window size on drift rate estimates.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
White, C.F., Lauder, G.V. Studying animal locomotion with multiple data loggers: quantifying time drift between tags. Anim Biotelemetry 12, 5 (2024). https://doi.org/10.1186/s40317-024-00363-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40317-024-00363-4