Abstract
Octopuses are amongst the most fascinating animals in our oceans; however, while their intricate behaviours are often studied in laboratory settings, basic aspects of their movement ecology remain unstudied in the wild. Focusing on the socio-economically important common octopus (Octopus vulgaris), this study employs, for the first time, acoustic tracking techniques to address knowledge gaps regarding the species spatial ecology within a marine protected area. A total of 24 wild O. vulgaris (13 males, 11 females) were tagged in 2022 in the National Park Maritime-Terrestrial of the Atlantic Islands of Galicia, Spain. Acoustic transmitters were externally attached to the third arm, after testing other body parts (mantle cavity). Males were on average detected on 10 times more occasions than females (49 days in males and five in females). The average activity space in the study area was large in comparison to that determined in similar studies, with 0.16 km2. Activity space between males and females as well as day and night were comparable. Tagged octopuses displayed a crepuscular activity pattern. The location of dens could be established for 15 out of 24 individuals, from which octopuses were observed to undertake regular daytime excursions. Several individuals were also found to occupy more than one den and/or switch their main dens throughout the study duration. First implications and benefits of this approach are discussed.
Similar content being viewed by others
Background
Octopus are amongst the most fascinating inhabitants of the ocean. Their large brains, complex behaviours and their ability to learn and memorise have amazed humans for decades [1,2,3]. Octopuses have been used as a model for research in brain functioning, behaviour and to generate general biological information including growth, nutrition, evolution and behaviour [1]. Octopuses, however, also support important commercial fisheries around the globe [4]. Small-scale fisheries in Southern European countries support one of the highest average landings of octopus globally, next to Japan and the USA [5]. With Spain in the lead, Spanish, Portuguese, Italian and Greek landings combined totalled to around 25.5 thousand tonnes annually between 2013 and 2017, amounting to 90% of European octopus landings [5]. O. vulgaris is targeted by the coastal small-scale fisheries off Galicia (NW Spain) [6, 7]. Galicia has a long history of small-scale fisheries (SSFs) which play an important role in the region’s economy and culture. With a coastline stretching over 1500 km that features a large number of estuaries and provides a variety of fishing grounds, Galicia is one of the most heavily fishery-dependent regions in Europe [8,9,10,11]. Its fleet consists of ~ 4000 small-scale fishing vessels, representing ~ 89% of the total fleet in Galicia [12]. In Galician SSFs most species are caught by several gears, and only some taxa are the main target species of a specific fishery, like the octopus trap fishery [7]. Amongst cephalopods, O. vulgaris is the most landed species in Galicia, with an annual average of 2404 ± 581 t between 2002 and 2016 [7, 12]. However, along the northern and central Galician coast, the species estimated abundance and landings decreased in the past decade, while they were relatively stable along the southern coast [6, 7].
One aspect of octopus lives that has remained largely elusive to date is their movement ecology. Most of our knowledge of how octopuses move comes from studies in captivity or visual and mark-recapture observations in the wild. In a lagoon in Florida for instance, Bennice et al. (2021) observed varying activity times and feeding patterns between O. vulgaris and Macrotritopus defilippi, which allow coexistence based on observations from diving and video footage [13]. Other studies assessed activity, home range and other behaviours in several octopus species (O. vulgaris, O. cyanea) using mainly mark-recapture or visual observation methods [14,15,16]. A laboratory study by Meisel et al. (2013) complemented the available knowledge about activity timing, as O. vulgaris was found to alter its activity periods according to predator presence [17]. However, very few studies have explored the movement and behaviour of octopus in the wild over larger temporal and spatial scales using electronic tagging, i.e., acoustic telemetry. This is largely due to difficulties of tag-attachment and retention, which has widely limited the duration of studies (< 20 days), and animal handling. There are a few exceptions though, previous authors have successfully tracked other octopus species using acoustic telemetry, including Octopus bimaculatus in California [18] and Enteroctopus dofleini in Alaska [19] providing insights into tag retention, movement patterns, home range and other behaviours.
The common octopus, O. vulgaris species complex [20], supports large fisheries across its distributional range [4, 5], which spans all oceans [21]. Inhabiting the coastal waters of the continental shelf [15], it is typically targeted by small-scale fisheries in depths < 200 m [5]. Few studies have investigated the movement ecology of this species in the wild. One of the few studies that did so using pit tags found that individual home range or activity area ranged from 0.028 to 0.073 km2 [14] and another study using Peterson discs found that distances travelled ranged from 9 to 5800 m in males and 9 to 1700 m in females [22]. Like other octopods, O. vulgaris were observed to make use of dens in the wild to avoid predation and secure prey items from competitors. Females do not leave dens during spawning when they guard and ventilate eggs in shallow areas until the eggs hatch [23, 24].
In this study, we used acoustic telemetry for the first time to provide detailed insights into the movement ecology of the common octopus in the wild. Given the challenges with retention of the transmitters reported in previous studies and short study durations, we first evaluated two tagging procedures. We then tagged O. vulgaris in the wild and monitored their behaviour with the aim to investigate patterns of movement and spatial dynamics of this commercially exploited invertebrate species. In addition, the study seeks to gather insights into the diel behaviour of O. vulgaris and its utilisation of space and specifically dens during both day and night periods. Obtaining a more comprehensive understanding of the animal movement and behavioural metrics of understudied species, such as O. vulgaris in the wild, is imperative for informed decision-making and implementing effective harvest control regulations.
Methods
Study area and acoustic array
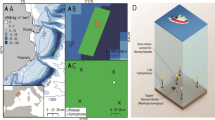
This study was conducted at the Cíes Archipelago at the mouth of the Ría de Vigo, which is part of the National Park Maritime-Terrestrial of the Atlantic Islands of Galicia (Fig. 1). Within the national park, small-scale shellfish and artisanal fishing activities are permitted, while recreational fisheries are banned [18]. Individual O. vulgaris were tracked within the boundaries of an acoustic telemetry array composed of 26 omni-directional receiver stations (Thelma Biotel TBR700L) and four reference transmitters (Thelma Biotel [n = 2 D-2LP13; n = 2 D-2LP9L] [26, 27]. The fixed telemetry array covered ~ 1.09 km2 based on an intermediate established detection radius of 250 m from receivers (maximum detection range of 400 m on hard substrates and 200 m on soft substrates for 9 mm transmitters, we thus suggest ~ 250 m to account for 6 mm transmitters) [25], with receivers located at depths ranging from 3.3 to 13.1 m. Sea bottom temperature was recorded by the Thelma Acoustic receiver temperature data loggers covering different depths and areas of the study array (Fig. 1), generating a dataset with temperatures every 30 min. The area covered by the telemetry array is characterised by granite reef (mainly covered with Cystoseira spp. and Sargassum spp. in the intertidal, and Saccorrhiza spp. and Laminaria spp. in the subtidal) interspersed with gravel valleys (mainly composed of broken bivalve shells, maerl and coarse sand) with isolated granite blocks. Octopuses excavated dens under boulders and occupied crevices in the bedrock.
Position of the study area within the Iberian Peninsula a. Map of the area covered by the National Park Maritime-Terrestrial of the Atlantic Islands of Galicia (PNMTIAG) shaded in blue b. Overview of the acoustic receiver array in the channel between two islands of the Cíes archipelago c. Detailed view of the study area with the acoustic receiver array and reference transmitters d
Acoustic tagging
To assess the suitability of tagging methods, five O. vulgaris specimens were tagged and observed in tanks before tagging wild individuals in the field. Of the five individuals in this trial, four specimens were reared in captivity and another one was trapped in the wild. The tagging procedures were carried out under controlled conditions in captivity. Two tagging procedures were tested: three individuals reared in captivity were externally tagged in the mantle following the procedure described by Hofmeister and Voss (2017) and the remaining two individuals, reared in captivity, were tagged in the third arm (following the procedure described by Domain et al. (2000)). For the captive trials, transmitters were fixed with epoxy to plastic Petersen discs (2 cm) that were connected to another plate which was attached on the other side of the arm/mantle by a steel filament. After trials, the discs were 3D printed with two small gutters to ensure the attachment of the transmitter to the disc (Additional file 1: Fig. S1d). These specimens were each observed over a ~ 1 month period, totalling to a 4 month pilot study, to determine unusual behaviours caused by the tagging procedure, assess tag retention and monitor potential injuries. Furthermore, the retention of T-bar plastic tags (Floy© Tag) was also evaluated with the two octopuses tagged in the third arm [27]. T-bar tags enable the recording of re-sightings or recaptures, and prevent double tagging.
A total of 24 wild O. vulgaris were tagged in May 2022 (Additional file 2). The tagging campaign was carried out on the last day of the fishing season to minimize potential captures of tagged individuals. Twenty one O. vulgaris individuals were caught using traps and tagged on board a commercial fishing vessel, while three individuals were caught one month later by hand while scuba-diving (IDs: 24, 25 and 26). Individuals that were caught by hand were released at their capture location (dens) and observed by divers immediately post-release to record behaviour. The transmitters (Thelma Biotel) were attached externally to the third arm (Fig. 2). Transmitter size varied and was selected based on the body size of the individuals, ranging between 6 and 9 mm (Additional file 2). Tagged individuals were also externally marked with T-bar plastic tags. Individuals were sexed and weighted (g) at the time of tagging.
This study complies with the corresponding experimental animal project authorization resolution of the regional government (Xunta de Galicia, reference: ES360570202001/20/FUN.01/BIOL.AN08/AGG01).
Data treatment
Detections were downloaded from receivers between October and November of 2022. The detection data underwent filtering to eliminate instances where a single transmitter was detected only once within a given day [28]. This was done due to the high degree of overlap in receiver detection capability, making it improbable for a transmitter to be detected by only one receiver, thus resulting in false detections. Centres of activity (COAs) were estimated from detections every 30 min, based on mean positions of receivers that detected a specific transmitter and the number of detections per transmitter and receiver [29]. Due to positioning inaccuracy and some inherent bias when using COAs, we estimated positions when sufficient data were available (the ping of a specific transmitter was detected by three receivers that form a triangle). Positions (Pinpoint for Thelma Biotel; hereafter referred to as ‘positions’) [30,31,32] were estimated applying a hyperbolic triangulation method from the manufacturer. Furthermore, we employed a standardised method (Additional file 1: Fig. S2) to ascertain the outcome of each tagged individual, aiming to establish the “fate date”. The term “fate date” denotes the moment when an individual was not conclusively deemed alive, leading to the removal of subsequent detections from the data set, thereby retaining only “clean detections.” The limited positions data that could be estimated for each individual (positions, COAs) led us to take this conservative approach when determining their fate and fate date (classified as: alive or unknown). Fate was determined as “unknown” if a lack of positioning data did not allow us to confirm their status as alive with certainty. This approach enabled us to ensure that detected patterns were attributed to living octopuses. Determination of fates and fate dates were performed by three independent data analysts following the same standardised method (Additional file 1: Fig. S2). Fish positioning errors were estimated using reference transmitters that were deployed at known positions inside the telemetry array. To reduce the uncertainty associated with positioning errors we followed the filtering protocol used in Freitas et al. (2016).
Estimation of movement metrics
Residency index
The residency index (RI), a measure of residency to the study site ranging between 0 (absence) to 1 (complete residency), between the first detection and the end of the study period was estimated for tagged individuals as follows [34, 35]:
Here, DD represents the count of detection-days for each individual and TD the total duration of the study period in days (150 days, expected lifetime of D-LP6 Thelma Biotel tag).
Activity space
Activity space is a measure of the space used within the acoustic telemetry array, calculated as 95% Kernel Utilisation Distribution (KUD) weekly, based on the centres of activity (COAs), estimated for every 30 min time-bin following Simpfendorfer et al. (2002) approach (adehabitatHR package) [36]. KUDs were computed only when a minimum of five COAs per week from three or more detection-days (consecutive or non-consecutive) were available, to reduce bias [37].
Activity
Trajectories, based on positions, were calculated for each individual (adehabitatLT library, version 0.3.25 in R) [38]. Sub-trajectories were then generated whenever detection gaps exceeded 12 h. Positions, including detection gaps under 12 h, were interpolated every 80 s (minimum transmission delay) and the distance travelled between each position was estimated at 80 s timesteps. Speed (m/h), which serves as a proxy for activity, was calculated as follows:
where dist represents the distance travelled between each relocation and dt is the interpolation period of 80 s.
Den-related behaviour
Dens were inferred through the utilisation of COAs and positions. A den was considered as a circular area with a radius of 5 m (accounting for positioning inaccuracies; mean positioning error: 4.58 m) where the octopus was repeatedly located throughout the study duration. The den’s location was ascertained when (1) the distance between multiple consecutive positions was zero or close to zero, suggesting that the individual was stationary and (2) individuals repeatedly returned to that position throughout the study. We then plotted the positions/trajectories (gganimate package in R and arcGIS Pro)[39] to investigate if this position in fact resembles a den. The den location was then determined using the recurse package [40] in R extracting the coordinates of the areas with the most amount of returns to a previously visited area [40].
For individuals with a sufficiently high number of positions, we further examined their excursions from the estimated den (Fig. 3). An excursion’s initiation was defined as the moment when the octopus left its den (when the distance from the den centre exceeded 5 m and including the last position within the den) and concluded when it returned to the den (when the distance from the den’s centre was less than 5 m). We recorded various excursion attributes, including excursion duration, distance travelled per excursion, the overall bearing of the excursion, and the maximum distance from the den center for each excursion.
Schematic representation of a an excursion, b den visits c a den transfer. The metrics generated from this analysis were: a the maximum displacement from the den (blue segment); the maximum distance from the den (black arrow); the bearing of the excursion (green arrow); duration of the excursion (in minutes), the percentage of day and night time during the excursion; b the time spent inside the den for each visit (in minutes); the percentage of day and night time spent inside the den; c the duration of the travel between dens (in minutes) and the percentage of day and night time spent during the travel between dens
Data analysis
Generalised additive mixed effect models (GAMMs, mgcv library) [41] were used to evaluate dependencies between activity space, activity and temporal and biological factors. The hour of the day [1–24] was included in the models and fitted with a cyclic cubic spline. Furthermore, the biological factors sex (female, male) and biomass (in grams) were included. All models included individual ID as a random effect and an autoregressive term (corAR1) to account for the correlation between the sequential time series of observations and the corresponding individual [42].
Results
Tag retention and recaptures
During tagging trials, we observed variations in the tag retention and animal behaviour. The captivity-reared octopuses that were tagged in the mantle were observed manipulating the transmitters right after tagging and during the following days. Two out of three specimens were able to remove the tag after two weeks, leaving a circular wound (healing) in the mantle, so this tagging method was discarded for the study in the wild. The alternative tagging method in the third arm resulted in increased secureness of the tag and almost no signs of discomfort (Additional file 1: Fig. S1). The arm tag was manipulated during the first hour after tagging and ignored thereafter. All octopuses accepted food in less than two hours after tagging, and mating behaviour was also observed for two individuals just minutes after returning into the tank. The tag was successfully retained during one month with no external visual damage at the tissue or muscular level. The t-bars were also retained during the whole month, and no signs of discomfort or manipulation were observed. Despite the differences observed in tag retention, the feeding behaviour and growth (based on weight data) of the five specimens was not affected by the two tagging methods.
Four (2 males and 2 females) out of the 24 individuals tagged in the wild were recaptured by fishers using octopus traps. Three of them were recaptured on the release day (IDs: 05, 07, 12), and the other one the day after (IDs: 13). In addition, two individuals (IDs: 24, 25) were observed by researchers while diving 11 days after release (June 3rd, 2022) in the same crevices they were spotted in immediately after release (post-tagging). All recaptures (fisheries and scuba-diving) took place within the Cíes archipelago (in and outside the array).
Movement behaviour
Out of the total 24 wild O. vulgaris that were tagged for this study, 11 individuals were females and 13 were males. Individuals had an average body mass of 2205 ± 686 g, ranging between 550 and 3280 g. A total of 215,927 clean detections were recorded between May 19th 2022 and October 16th 2022, with considerable variation in the number of detections between individuals ranging from just four to 83,480 (mean = 11,996 ± 22,608). Positions (n = 9527) could be estimated for 11 out of 24 individuals (mean = 866 ± 2273).
Residency
Of the 24 tagged O. vulgaris, 18 individuals were detected in the acoustic array during the study duration. Six individuals (IDs: 04, 05, 07, 08, 12 and 15) were never detected amongst which three individuals (IDs: 05, 07 and 12) were fished a few hours after tagging (Fig. 4). Six individuals (four females and two males) dispersed from the study area, as inferred from movements towards the edge of the acoustic array followed by a cessation in the detections. Dispersal occurred through the western part (connecting with open ocean) of the array in all six cases, suggesting movements toward the continental shelf (Additional file 1: Fig. S3). Tagged individuals were detected in the area on average for 35 days (median = 7), with considerable variation in the average number of days males (n = 49) and females (n = 5) were detected. Three males were detected for an extended period (between 3 and 6 months, max.: 148 days; Fig. 4) in the array. The population had a residency index of 0.23 ± 0.35, with males being more residents (RI = 0.33 ± 0.11) than females (RI = 0.03 ± 0.01).
Abacus plot showing the daily presence of Octopus vulgaris in the study area. Detections for females and males are represented by light and dark blue, respectively. Light red dots represent detections past the fate date, when the status of the individual (live vs dead) is uncertain. The two grey lozenge symbols at the start and end of each individual time series denote the tagging date and the end of the study based on the predicted battery life. Yellow and orange lozenges represent recapture events during diving and fishing events when individuals were found to be respectively alive and dead. Daily presence is also shown for the reference transmitter (in red). RT—Reference Transmitter
Activity space and activity
Only 16 individuals had enough COAs to estimate their activity space. The mean weekly activity space was 0.16 km2 (range: 0.08–0.29 km2, Additional file 3). Activity space estimates were similar for females (0.17 ± 0.031 km2, n = 4) and males (0.16 ± 0.020 km2, n = 12) as well as between day (0.16 ± 0.018 km2, n = 15) and night (0.11 ± 0.013 km2, n = 13). Individuals predominantly occupied an area close to the small islet in the north–east of the study area (Illa dos Viños; Fig. 5), which is in proximity to the release location (for the individual caught while diving).
Total activity space areas (based on centres of activity) for the four Octopus vulgaris with the highest residency indices. Yellow dots represent the acoustic telemetry receiver locations and the shaded colour areas represent the activity space (KUD = kernel utilisation distribution). Blue dots show the diving area where specimens 24 and 26 were captured and released by scuba divers, while specimens 19 and 21 were captured by fishery traps
Model results showed a significant reduction in activity space of O. vulgaris after tagging, where activity space reached its minimum 136 days after the tagging date, i.e., October (Table 1 and Fig. 6). Despite the low number of individuals captured by hand (n = 3) as compared to traps (n = 21) we detected that individuals captured using traps had an activity space 1.7 times larger than individuals caught by hand while scuba diving (Table 1 and Fig. 6).
Predicted activity space of Octopus vulgaris within the study area as a function of the a days after tagging and b catch type (traps n = 21, dive n = 3). Light blue shaded areas a and black error bars b represent a 95% confidence interval, orange dots represent the original data points. Values used for predictions: catch type = traps in a; days after tagging = 120 in b
The activity of O. vulgaris ranged between 0 m/h and 440.45 m/h with a mean speed of 15.49 ± 0.14 m/h (Additional file 3). Males were less active (mean = 15.41 ± 0.14 m/h) than females (mean = 20.31 ± 1.70 m/h); however, differences were not significant. Individuals displayed a higher activity level over a larger activity space during the day compared to during the night (Additional file 1: Fig. S4). Model results revealed significant effects of the hour of the day and sea bottom temperature on the activity of O. vulgaris. The activity was predicted to peak twice a day during early mornings (5 am) and late afternoons (6 pm), while the lowest activity occurred roughly at 12 am and 12 pm. Predicted activity was at its highest at 15 °C and decreased with both higher and lower temperatures (Fig. 7).
Predicted activity of Octopus vulgaris in the study area as a function of a hour of day and b sea bottom temperature. Light blue areas represent a 95% confidence interval, and orange dots represent average values of the raw data. Predictions were made using the following fixed values: sea bottom temperature = 14 ºC in a; hour = 5 in b
Excursions
We were able to identify at least one den for 15 out of the 24 tagged O. vulgaris. Only six individuals yielded enough positions to enable the detailed analysis of excursions from the den (Additional file 4). One octopus (ID: 19) moved on a very small area and did not seem to exhibit any den-related behaviour (Fig. 8). The other five octopuses exhibited repeated behaviours of returning to 1–4 fixed locations that, based on the available ecological knowledge about the species, are thought to represent dens (Fig. 8; Additional file 1: Fig. S5.1–5). Five individuals undertook between 6 and 663 excursions (Table 2), which had a mean duration of 07 h 15 min 28 s (range: 80 s–54.09 days). The maximum distances from the den during the excursions ranged between 0.29 m and 273.01 m (mean = 10.45 m), while the total distances travelled per excursion ranged between 0.25 m and 1438.87 m. Excursions and den changes primarily took place during the daytime (Fig. 9). The time it took an individual to first find a den after release varied greatly between individuals (mean = 19.74 h, range: 0 s.—2.82 days) with three individuals being released directly in their dens (IDs: 24, 25, 26). An even larger variation was observed for the last unfinished excursion recorded “Out of the den” (mean: 1.14 months, 2 min. 40 s.—5.73 months). Most of the den exchanges, excursions as well as the time it took an individual to find a first den occurred during daytime, whereas the last unfinished excursion (“out of den”) took place both during day and night-time (Fig. 9).
Map displaying the positions of the six tagged individual Octopus vulgaris (ID: 13, 19, 20, 21 and 26) that yielded enough data to investigate excursions in detail. Yellow dots = daytime positions, black dots = night-time positions, grey lines = movement between positions, red circles = den (radius = 5 m)
Panel displaying an example of the four different behaviours of Octopus vulgaris, as well as a box plot of the mean percentage of day and night-time of these behaviours. Two separate excursions as well as den changes are displayed within the panel. Yellow dots = daytime positions, black dots = night time positions, red circles = den (radius = 5 meters)
Discussion
In this study, we used acoustic telemetry to study the movement behaviour of O. vulgaris in a small national park in Galicia, NW Spain. Overall, males were found to be more resident in the study area than females. O. vulgaris was found to exhibit a crepuscular activity pattern characterised by increased swimming speeds during mornings and evenings, compared to reduced speeds during day and night-time. On average, individuals used a space of ~ 0.16 km2 around one or several dens, to which they regularly returned. In addition, some individuals were observed to change dens throughout the study duration. To our knowledge, this is the first approach to the spatial behaviour of this species in the wild using telemetry methods.
Tag retention
Our acoustic transmitter retention study revealed that the third left arm, (but not the mantle), is a suitable area of attachment for this species, at least at the time scale at which we evaluated retention. This is in accordance with the previous observation of successful retention of Peterson discs attached also to the third arm for at least a month in common octopus [27]. In addition, Barry et al. (2011) speculated that tagging in the third arm may extend the time until tissue expels tags compared to tagging in the mantle, as the muscle here is the thickest in the octopus body. Tagging on the third arm provided an extended period of observation in the field to just under 5 months (for five individuals spanned over 40 days) on which presence inside the acoustic array was recorded, with a maximum of 148 days (ID 24). To our knowledge, this is the longest retention period recorded for any octopus species using acoustic telemetry and exceeds the retention time of previous studies such as the 0–15 days in O. cyanea [44], 2–20 days in E. dofleini [19] and 0–19 days in O. bimaculatus [18], tagged in the mantle, or 0–34 days for transmitters implanted under the skin of the third arm, 0–51 days for transmitters attached by cable tie bands to the third arm, and 0–88 days for mantle-mounted transmitters on modified Peterson disks in E. dofleini [19]. In addition, tagging in the third arm renders a suitable compromise between ease of observing the transmitter (allowing re-sightings of released individuals) and minimising potential impacts on wild animals and their behaviour (e.g. predators spotting camouflaged octopuses due to a visible transmitter on the first or second arm, obscuring of the eyes or impacting reproduction).
Detection patterns
The detection time series of several individuals included multiple gaps, i.e. days without any detections. Such gaps are thought to primarily result from individuals being inside a den, or potentially from octopuses navigating through complex habitats including a mix between rocky reefs, large seaweeds, scattered small rocks and sand which block signal transmission [18, 19]. Detection gaps were also observed in other telemetry studies on octopus. For instance, in giant Pacific octopus (E. dofleini), gaps (undetected inside den) accounted for 86% of the time [19]. We observed that males were more resident in the study area than females (RI males = 0.33, RI female = 0.03). One possible explanation is the use of signal warping dens by females for egg laying and parental care [23], which for O. vulgaris typically are rocky crevices and caves closed off with an artificial wall of small stones and shells [2, 23]. Acoustic transmissions would then be blocked, as females are known to stay inside the den and die after brooding as a consequence of increased metabolic effort and starvation [2, 45]. The period of the tagging experiment (May–September) coincides with the known spawning season of O. vulgaris [46]. The females in this study might have been sexually mature, since the mean weight (2349 ± 553 g) was above the weight of sexually mature O. vulgaris between 1350 and 1788 g [46, 47]. Due to the short duration of this study, we were unable to make inferences about any seasonal trends of presence and space use of the species in our study area.
Space use and activity
While the fate of 14 individuals was unclear (no clear dispersal pattern, no known casualty), six individuals (4 females, 2 males) were observed to permanently exit the acoustic array towards the west (i.e. towards the open ocean), suggesting that individuals use an area larger than the extent of our telemetry array. A previous mark and recapture study of O. vulgaris carried out in the Ría de Vigo, recaptured 80.5% of individuals within 5 km from the release site, 13.9% between 5 and 20 km, and 5.5% more than 20 km [48]. Together, these results suggest that in the Ría de Vigo which encompasses our telemetry array, the species does not undertake long-distance movement; however, their home range likely exceeds the coverage of our telemetry array. Similarly, the longest movement recorded in E. dofleini in Alaska was 4,8 km for a 16.5 kg female in three months using acoustic telemetry [19]. In addition, since octopuses can be highly mobile and do not exhibit territoriality nor aggregate as a response to conspecific movement (with the exception for mating) it was not possible to determine whether observed activity spaces are permanent or temporary [18]. We estimated an average activity space of 0.16 km2 in tagged O. vulgaris, which is larger than previous estimations for the species based on mark-recapture data with 0.028–0.073 km2 [14]. However, the coverage of the research site in their study was less than half of the size of our telemetry array (0.42 km2), which has likely artificially restricted the activity space size. Similarly, tracking studies of O. bimaculatus and E. dofleini had smaller average activity spaces of 0.00614 km2 ± 0.00317 km2 (research area 4 km2) [18] and 0.0043–0.05 km2 (research area 0.055 km2) [19], respectively. It is likely that the estimated areas for these studies are comparatively small considering the relatively short period of time over which data were collected (maximum tracking duration: 19 and 20 days, respectively) and smaller time-bins (< 6 h and hourly-daily, respectively) in which activity space was estimated.
Of all the variables considered as potential drivers of activity space, only catch type yielded significant results. Individuals caught during dives were released back at the location of their capture, which is expected to have reduced the search effort and time spent by octopus to locate a new suitable den compared to trapped individuals (that were released further away from the capture areas). The initial search for dens in the latter may account for our finding of an increased activity space size in the time after tagging, since they need to explore new areas. Similar findings were obtained in E. dofleini where activity space use increased rapidly in the first 48 h, indicating post-handling relocation [19]. To reduce the impact on the spatial behaviour, we advise future studies to consider catching octopuses by hand and releasing them back to their known dens. Neither sex nor weight affected the activity space of octopuses, the second of which is likely due to the limited size range of the tagged individuals (550–3280 g). This finding partially contradicts previous studies [18, 19], which found that larger individuals move further and use greater areas than small individuals. Our daytime variable (day/night), too, did not affect activity space estimates. This may be surprising, as activity was found to be dependent on the hour of the day. However, as O. vulgaris exhibits crepuscular activity, the day/night variable in the activity space model does not represent this behavioural pattern and the dusk and dawn phases are included in both, day and night phases. Female presence in the study area was significantly smaller than that of males, thus yielding a smaller amount of data on their activity space and activity. This lack of data for female O. vulgaris prevented testing of interactive effects between sex and the other variables on the different behavioural traits. A comparison of activity space models (GAMMs) including day of the year or day after tagging, was effectuated prior to the publication of this study. Furthermore, between releasing the first individual (ID: 02) in January 2022 and the end of the study, the data has a total of 18 weeks of gaps (weeks 1–3 and weeks 5–19) with zero detections, and additionally O. vulgaris are short lived animals (< 2 years) with possibly high variation in their behaviour between months and years [49]. Accordingly, we removed any seasonal variable from analyses as it could not account for potential seasonal and life-history effects and included day after tagging fitted with a non-parametric smoothing function, instead.
Tagged octopuses displayed a crepuscular activity pattern thus being the most active during dusk and dawn. O. vulgaris has previously been described as being nocturnal in the Mediterranean [50] and diurnal in the North Atlantic [51]. Although observations were previously made in the Western Atlantic, where juvenile O. vulgaris activity was initially described as diurnal [52] and later as crepuscular in Bermuda [53], as well as nocturnal in Florida [13], recent research suggests that O. vulgaris is restricted to the Northeastern Atlantic, and that those observations might correspond to Octopus americanus, a member of the O. vulgaris species complex [54]. Other octopod species like E. dofleini are active during night and dawn in Alaska [19]. Predator presence, together with the influence of light cues [51] and prey/resource availability (observed e.g. in E. dofleini [18, 55]), might be the primary drivers of activity patterns of O. vulgaris. Adaptive behavioural shifts e.g. in activity timing, in response to predator presence were previously observed in laboratory tests in captive juveniles of O. vulgaris [17]. Both, nocturnal and diurnal predators of O. vulgaris may be present in the area, including the nocturnal European conger (Conger conger) [17, 56,57,58] and elasmobranchs including tope (Galeorhinus galeus) [24, 59] as well as diurnally feeding bottlenose dolphins (Tursiops truncatus) [60,61,62,63], among other marine mammals. Reports of within-population variation of nocturnal behaviour towards crepuscular activity as a consequence of the developmental stage, potentially to avoid cannibalism by conspecifics [64], have been proposed [13]. However, no variation in activity according to the body mass of individuals was observed in our study. We found considerable intraspecific variation in average activity, ranging from 2.59 m/h to a maximum of 163.64 m/h. This could be due to the ability of octopuses to learn based on experience, allowing them to adapt their behaviours accordingly [18, 65, 66]. A comparable intraspecific variation in speed was found in O. cyanea (mean = 43.8 m/h, range: 3.6–117) in Hawaiʻi [44]. Octopuses are ectothermic, and thus cannot thermoregulate, leaving them susceptible to changes in environmental temperature [67]. Ectothermic animals often use adaptive behaviours to modify their body temperature [67]. In addition, not only their fitness but also their metabolism is reliant on environmental temperatures [67, 68], which could explain behavioural responses to temperature. It is likely that the activity patterns of O. vulgaris are influenced by many complex and interrelated factors such as predator activity and density, prey availability and hunting behaviour, mate searching, habitat complexity and shelter availability. Consequently, octopuses may not be easily placed into the traditional diurnal, nocturnal or crepuscular activity patterns.
Den-related behaviours
In accordance with the ecological knowledge, tagged octopuses appeared to use protective dens to avoid predation, defend prey and for parental care [24]. We identified a minimum number of one den to which octopuses returned to for 15 out of 24 tagged individuals. For individuals with a high abundance of positions, a regular return to between one and a maximum of four dens was observed.
Octopuses, including O. vulgaris, are known to be highly mobile, non-territorial animals [18] that aggregate depending on the available habitat structure and resources [69]. Individuals strongly varied in the total excursions they undertook from their dens (n = 9–663), number of dens (0–4), as well as in the mean duration of the excursion (1.33 min–54 days) and maximum distances from the den (0.29–273.01 m). Excursions from dens predominantly took place during daytime. Even though assessed at the categorical day/night level rather than per hour, the timing of excursions is expected to mirror the crepuscular activity patterns in our study population. In their 1997 study, Forsythe & Hanlon [16] observed two O. cyanea to undertake excursions mainly during dusk and dawn from their dens to forage. We propose that the multiple excursions per day observed in tagged O. vulgaris in our study, were motivated primarily by foraging. Foraging or hunting behaviour as a primary motivation for excursions was further supported by the exhibition of rapid changes from high to low speeds and vice versa during excursions. A similar “stop and go” (saltatory) search strategy was observed in an O. vulgaris population in the western Atlantic ocean [13] as well as in O. cyanea [16]. Furthermore, as in their study, our octopuses often did not follow the same route in consecutive excursions [16]. This behaviour was observed previously in foraging octopus and is thought to increase the foraging efficiency [16]. The mean duration of trips was 16 h and 17 min per trip, with a mean excursion length of 49.3 m. In comparison to previous observations in Tahiti which were made by eye, our observed excursions are of considerably shorter mean duration of 7 h 15 min 28 s, while distances travelled were slightly longer (59.06 m compared to 49.3 m) [16]. We hypothesise that tagged O. vulgaris spent periods of time resting or hiding in locations to which they did not regularly return and which consequently were not identified as dens or that transmissions were lost as individuals hid or left the array.
Conclusions
In summary, this study demonstrated that female and male Octopus vulgaris exhibit variable presence/absence patterns in a small acoustic telemetry array. Furthermore, tagged individuals were found to occupy a large activity space of 0.16 km2, while displaying a crepuscular activity pattern in the North-Eastern Atlantic. Finally, tagged individuals were found to utilize 1–4 dens, from which they regularly undertook excursions during the day. Although more research is needed to understand seasonal patterns of behaviours including presence/absence as well as the species movements outside of our telemetry array, our results add to the limited knowledge we have on this commercially relevant species and can be readily used to inform spatial management, and a starting point for new spatial research.
Availability of data and materials
The data sets supporting the conclusions of this article is available at: Alexandre Alonso-Fernández, David Villegas-Ríos, Gonzalo Mucientes (2019). Spatial ecology and behaviour of coastal fish species off Galician coast (NW Spain), European Tracking Network (ETN) repository, https://marineinfo.org/id/dataset/6605.
Abbreviations
- SSFs:
-
Small-scale fisheries
- RI:
-
Residency index
- KUD:
-
Kernel utilisation distribution
- COAs:
-
Centres of activity
References
Di Cosmo A, Pinelli C, Scandurra A, Aria M, D’Aniello B. Research trends in octopus biological studies. Animals. 2021;11(6):1808.
Hanlon RT, Messenger JB. Cephalopod Behaviour. Cambridge: Cambridge University Press; 2018.
Hochner B, Glanzman DL. Evolution of highly diverse forms of behavior in molluscs. Curr Biol. 2016;26(20):R965–71.
Sauer WHH, Gleadall IG, Downey-Breedt N, Doubleday Z, Gillespie G, Haimovici M, et al. World Octopus fisheries. Rev Fish Sci Aquac. 2019;29(3):279–429.
Pita C, Roumbedakis K, Fonseca T, Matos FL, Pereira J, Villasante S, et al. Fisheries for common octopus in Europe: socioeconomic importance and management. Fish Res. 2021;1(235): 105820.
Alonso-Fernández A, Otero J, Bañón R, Campelos JM, Quintero F, Ribó J, et al. Inferring abundance trends of key species from a highly developed small-scale fishery off NE Atlantic. Fish Res. 2019;1(209):101–16.
Bañón R, Otero J, Campelos-Álvarez JM, Garazo A, Alonso-Fernández A. The traditional small-scale octopus trap fishery off the Galician coast (Northeastern Atlantic): historical notes and current fishery dynamics. Fish Res. 2018;1(206):115–28.
Freire J, Garcı́a-Allut A. Socioeconomic and biological causes of management failures in European artisanal fisheries: the case of Galicia (NW Spain). Mar Policy. 2000;24(5):375–84.
Graeme Macfadyen, Poseidon, Pavel Salz, Framian. Characteristics of small-scale coastal fisheries in Europe. 2011. https://www.europarl.europa.eu/cmsdata/62947/att_20111019ATT29772-3081506159436905403.pdf
Natale F, Carvalho N, Harrop M, Guillen J, Frangoudes K. Identifying fisheries dependent communities in EU coastal areas. Mar Policy. 2013;1(42):245–52.
Surís-Regueiro JC, Santiago JL. Characterization of fisheries dependence in Galicia (Spain). Mar Policy. 2014;1(47):99–109.
Xunta de Galicia. Pescado, Marisco, Galicia. https://www.pescadegalicia.gal/gl/publicacions
Bennice CO, Brooks WR, Hanlon RT. Behavioral dynamics provide insight into resource exploitation and habitat coexistence of two octopus species in a shallow Florida lagoon. J Exp Mar Biol Ecol. 2021;1(542–543): 151592.
Arechavala-Lopez P, Minguito-Frutos M, Follana-Berná G, Palmer M. Common octopus settled in human-altered Mediterranean coastal waters: from individual home range to population dynamics. ICES J Mar Sci. 2019;76(2):585–97.
de Beer C, Potts W. Behavioural observations of the common octopus Octopus vulgaris in Baía dos Tigres, southern Angola. Afr J Mar Sci. 2013;35(4):579–83.
Forsythe JW, Hanlon RT. Foraging and associated behavior by Octopus cyanea Gray, 1849 on a coral atoll, French Polynesia. J Exp Mar Biol Ecol. 1997;209(1):15–31.
Meisel DV, Kuba M, Byrne RA, Mather J. The effect of predatory presence on the temporal organization of activity in Octopus vulgaris. J Exp Mar Biol Ecol. 2013;1(447):75–9.
Hofmeister JKK, Voss KM. Activity space and movement patterns of Octopus bimaculatus (Verrill, 1883) around Santa Catalina Island. California J Exp Mar Biol Ecol. 2017;1(486):344–51.
Scheel D, Bisson L. Movement patterns of giant Pacific octopuses, Enteroctopus dofleini (Wülker, 1910). J Exp Mar Biol Ecol. 2012;15(416–417):21–31.
Amor MD, Norman MD, Roura A, Leite TS, Gleadall IG, Reid A, et al. Morphological assessment of the Octopus vulgaris species complex evaluated in light of molecular-based phylogenetic inferences. Zool Scr. 2017;46(3):275–88.
Allcock L, Headlam J, Allen G. IUCN Red List of Threatened Species: Octopus vulgaris. IUCN Red List Threat Species. 2016. https://www.iucnredlist.org/en
Mereu M, Agus B, Addis P, Cabiddu S, Cau A, Follesa MC, et al. Movement estimation of Octopus vulgaris Cuvier, 1797 from mark recapture experiment. J Exp Mar Biol Ecol. 2015;1(470):64–9.
Garci ME, Hernández-Urcera J, Gilcoto M, Fernández-Gago R, González ÁF, Guerra Á. From brooding to hatching: new insights from a female Octopus vulgaris in the wild. J Mar Biol Assoc U K. 2016;96(6):1341–6.
Guerra Á, Hernández-Urcera J, Garci ME, Sestelo M, Regueira M, González ÁF, et al. Dwellers in dens on sandy bottoms: ecological and behavioural traits of Octopus vulgaris. Sci Mar. 2014;78(3):405–14.
Papadopoulo K, Villegas-Ríos D, Mucientes G, Hillinger A, Alonso-Fernández A. Drivers of behaviour and spatial ecology of the small spotted catshark (Scyliorhinus canicula). Aquat Conserv Mar Freshw Ecosyst. 2023. https://doi.org/10.1002/aqc.3943.
Domain F, Jouffre D, Caverivière A. Growth of Octopus vulgaris from tagging in Senegalese waters. J Mar Biol Assoc U K. 2000;80(4):699–705.
Fuentes L, Otero JJ, Moxica C, Sánchez FJ, Iglesias J. 2006. Application of different external tagging methods to Octopus vulgaris Cuvier, 1797, with special reference to T-bar anchor tags and Petersen disks.
Meyer C, Holland K, Papastamatiou Y. Seasonal and diel movements of giant trevally Caranx ignobilis at remote Hawaiian atolls: implications for the design of marine protected areas. Mar Ecol-Prog Ser. 2007;12(333):13–25.
Simpfendorfer CA, Heupel MR, Hueter RE. Estimation of short-term centers of activity from an array of omnidirectional hydrophones and its use in studying animal movements. Can J Fish Aquat Sci. 2002;59(1):23–32.
Muñoz L, Aspillaga E, Palmer M, Saraiva JL. A tool to monitor fish swimming behavior in sea-cage aquaculture. Front Mar Sci. 2020;7:645.
Lennox RJ, Aarestrup K, Alós J, Arlinghaus R, Aspillaga E, Bertram MG, et al. Positioning aquatic animals with acoustic transmitters. Methods Ecol Evol. 2023;14(10):2514–30.
Smith F. Understanding HPE in the VEMCO positioning system (VPS). 2013.
Freitas C, Olsen EM, Knutsen H, Albretsen J, Moland E. Temperature-associated habitat selection in a cold-water marine fish. J Anim Ecol. 2016;85(3):628–37.
Espinoza M, Farrugia TJ, Lowe CG. Habitat use, movements and site fidelity of the gray smooth-hound shark (Mustelus californicus Gill 1863) in a newly restored southern California estuary. J Exp Mar Biol Ecol. 2011;401(1):63–74.
Papastamatiou YP, Friedlander AM, Caselle JE, Lowe CG. Long-term movement patterns and trophic ecology of blacktip reef sharks (Carcharhinus melanopterus) at Palmyra Atoll. J Exp Mar Biol Ecol. 2010;386(1):94–102.
Calenge C. The package “adehabitat” for the R software: a tool for the analysis of space and habitat use by animals. Ecol Model. 2006;197(3):516–9.
Villegas-Ríos D, Réale D, Freitas C, Moland E, Olsen EM. Individual level consistency and correlations of fish spatial behaviour assessed from aquatic animal telemetry. Anim Behav. 2017;1(124):83–94.
Calenge C. Package ‘adehabitatLT’. 2020. https://cran.microsoft.com/snapshot/2020-06-09/web/packages/adehabitatLT/adehabitatLT.pdf
Pedersen T, Robinson D. gganimate: A Grammar of Animated Graphics. R package version 1.0.9.9000, 2024. https://github.com/thomasp85/gganimate, https://gganimate.com.
Bracis C, Bildstein KL, Mueller T. Revisitation analysis uncovers spatio-temporal patterns in animal movement data. Ecography. 2018;41(11):1801–11.
Wood SN. Generalized additive models: an introduction with R, second edition. 2nd ed. New York: Chapman and Hall/CRC; 2017. p. 496.
Dormann CF. Assessing the validity of autologistic regression. Ecol Model. 2007;207(2):234–42.
Barry PD, Tamone SL, Tallmon DA. A comparison of tagging methodology for North Pacific giant octopus Enteroctopus dofleini. Fish Res. 2011;109(2):370–2.
Ivey GL. Acoustic telemetry of the short-term movements of Octopus cyanea (Gray, 1849) in Kaneohe Bay, Hawaiʻi. 2007. http://hdl.handle.net/10125/20927
Anderson RC, Wood JB, Byrne RA. Octopus senescence: the beginning of the end. J Appl Anim Welf Sci. 2002;5(4):275–83.
Otero J, González ÁF, Sieiro MP, Guerra Á. Reproductive cycle and energy allocation of Octopus vulgaris in Galician waters. NE Atlantic Fish Res. 2007;85(1):122–9.
Carvalho JMN, Reis CS. Contributions to knowledge on the maturation and fertility of the common octopus Octopus vulgaris Cuvier, 1797 on the Portuguese coast. Bol Inst Esp Oceanogr. 2003;9(1–4):473–81.
Fuentes L, Iglesias J. Release experiments with Octopus vulgaris Cuvier, 1797 in Galicia Nw Spain first results on recapture rate, distribution and growth. Vie Milieu. 2010;60(1):65–71.
Jereb P, Allcock AL, Lefkaditou E, Piatkowski U, Hastie LC, Pierce GJ. Cephalopod biology and fisheries in Europe: II. Species Acc. 2015. https://doi.org/10.1080/10236247309386935.
Kayes RJ. The daily activity pattern of Octopus vulgaris in a natural habitat. Mar Behav Physiol. 1973;2(1–4):337–43.
Meisel DV, Byrne RA, Kuba M, Mather J, Ploberger W, Reschenhofer E. Contrasting activity patterns of two related octopus species, Octopus macropus and Octopus vulgaris. J Comp Psychol. 2006;120(3):191–7.
Mather J. 1988. Daytime activity of juvenile Octopus vulgaris in Bermuda. Malacologia. https://www.semanticscholar.org/paper/Daytime-activity-of-juvenile-Octopus-vulgaris-in-Mather/2c39726dbeccdc4d86c5266eddfa8fa4d8aefb2a
Mather JA, O’Dor RK. Foraging strategies and predation risk shape the natural history of juvenile Octopus vulgaris. Bull Mar Sci. 1991;49(1–2):256–69.
Avendaño O, Roura Á, Cedillo-Robles CE, González ÁF, Rodríguez-Canul R, Velázquez-Abunader I, et al. Octopus americanus: a cryptic species of the O vulgaris species complex redescribed from the Caribbean. Aquat Ecol. 2020;54(4):909–25.
Rigby PR, Sakurai Y. Multidimensional tracking of giant Pacific Octopuses in Northern Japan reveals unexpected foraging behaviour. Mar Technol Soc J. 2005;39(1):64–7.
Correia AT, Manso S, Coimbra J. Age, growth and reproductive biology of the European conger eel (Conger conger) from the Atlantic Iberian waters. Fish Res. 2009;99(3):196–202.
Matić-Skoko S, Tutman P, Petrić M, Skaramuca D, Đikić D, Lisičić D, et al. Mediterranean moray eel Muraena helena (Pisces: Muraenidae): biological indices for life history. Aquat Biol. 2011;13(3):275–84.
Pierce GJ, Allcock L, Bruno I, Bustamante P, González Á, Guerra Á, et al. 2010. Cephalopod biology and fisheries in Europe. Report No. 303.
Schaber M, Gastauer S, Cisewski B, Hielscher N, Janke M, Peña M, et al. Extensive oceanic mesopelagic habitat use of a migratory continental shark species. Sci Rep. 2022;12(1):2047.
Coscarella MA, Crespo EA. Feeding aggregation and aggressive interaction between bottlenose (Tursiops truncatus) and Commerson’s dolphins (Cephalorhynchus commersonii) in Patagonia. Argentina J Ethol. 2010;28(1):183–7.
Mèndez-Fernandez P, Bustamante P, Bode A, Chouvelon T, Ferreira M, López A, et al. Foraging ecology of five toothed whale species in the Northwest Iberian Peninsula, inferred using carbon and nitrogen isotope ratios. J Exp Mar Biol Ecol. 2012;413:150–8.
Miller LJ, Solangi M, Kuczaj SA II. Seasonal and Diurnal patterns of behavior exhibited by atlantic bottlenose dolphins (Tursiops truncatus) in the Mississippi Sound. Ethology. 2010;116(12):1127–37.
Wells R, McHugh K, Douglas D, Shippee S, Berens McCabe E, Barros N, et al. Evaluation of potential protective factors against metabolic syndrome in bottlenose dolphins: feeding and activity patterns of dolphins in sarasota bay Florida. Front Endocrinol. 2013. https://doi.org/10.3389/fendo.2013.00139.
Smith CD, Griffiths CL. Aspects of the population biology of Octopus vulgaris in false Bay, South Africa. South Afr J Mar Sci. 2002;24(1):185–92.
Alves C, Boal JG, Dickel L. Short-distance navigation in cephalopods: a review and synthesis. Cogn Process. 2008;9(4):239–47.
Mather JA. ‘Home’ choice and modification by juvenile Octopus vulgaris (Mollusca: Cephalopoda): specialized intelligence and tool use? J Zool. 1994;233(3):359–68.
Noyola J, Caamal-Monsreal C, Díaz F, Re D, Sánchez A, Rosas C. Thermopreference, tolerance and metabolic rate of early stages juvenile Octopus maya acclimated to different temperatures. J Therm Biol. 2013;38(1):14–9.
Noyola J, Mascaró M, Caamal-Monsreal C, Noreña-Barroso E, Díaz F, Re D, et al. Effect of temperature on energetic balance and fatty acid composition of early juveniles of Octopus maya. J Exp Mar Biol Ecol. 2013;1(445):156–65.
Caldwell RL, Ross R, Rodaniche A, Huffard CL. Behavior and body patterns of the larger pacific striped Octopus. PLoS ONE. 2015;10(8): e0134152.
Acknowledgements
We would like to thank the National Park Maritime-Terrestrial of the Atlantic Islands of Galicia as well as the local artisanal fisheries sector for their support and collaboration. We would also like to thank Alexandra Castro and Miguel Martínez for helping in octopus maintenance. Furthermore, we would like to thank Alexandre Martínez Schönemann for supporting us by 3D printing essential parts for tagging octopus.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. Ecology of wild Common Octopus: towards Sustainable Management and Aquaculture (ECOSUMA), Proyectos de I + D + I (Generación de Conocimimento y Retos de Investigación), Ministerio de Ciencia e Innovación (RTI2018-099868-B-I00). PI: Dr. Ángel González. The project that gave rise to these results received the support of a fellowship from the”la Caixa” Foundation (ID 100010434). The fellowship code is LCF/BQ/DR23/12000027.
Author information
Authors and Affiliations
Contributions
GM and AR lead the study design and execution with support from AAF, AFG and JI; KP lead the data evaluation with support from DVR and AH; AH lead the data interpretation and manuscript writing with support from all authors, especially KP and GM. Due to the partitioned tasks and expertise specific to this study, KP, AH and GM are considered to be corresponding authors of this study.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Authors who performed practical works complied with the legislation in the country and region where fieldwork was conducted. This study complies with the corresponding experimental animal project authorization resolution of the regional government (Xunta de Galicia, reference: ES360570202001/20/FUN.01/BIOL.AN08/AGG01).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: This file includes five additional figures, namely: Figure S1. Panel displaying the different elements of the tagging trials, Figure S2. Flow chart of the standardised method used to infer the fate date of individual Octopus vulgaris, Fig. S3. Map showing the trajectories (based on centres of activity) of six Octopus vulgaris individuals and their movement towards the western edge of the array during the study, Figure S4. Map of the mean speed of Octopus vulgaris over the whole study area, at day versus night based on estimated positions and Figure S5. Summary of all the excursions to and from dens executed by tagged individuals based on positions data.
Additional file 2
: Table S1. Tagging information for all Octopus vulgaris individuals.
Additional file 3:
Table S2. Octopus vulgaris biological data and estimations of behavioural characteristics. Activity space is based on centers of activity and speed was calculated from positions. AS: Activity space; “−”: not enough data to calculate variable, study time in days, Position: number of high precision position generated for each individuals. Clean detections: filtered detections
Additional file 4:
Table S3. Excursion data for all Octopus vulgaris individuals.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Papadopoulo, K., Hillinger, A., Mucientes, G. et al. First insights into the spatial behaviour of Octopus vulgaris in the wild using acoustic telemetry. Anim Biotelemetry 12, 16 (2024). https://doi.org/10.1186/s40317-024-00361-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40317-024-00361-6