Abstract
Background
The foraging behaviour of sea ducks has always been difficult to study due to their exclusive occupancy of coastal waters during the wintering season. In this study we investigated the foraging behaviour of seven wintering velvet scoters (Melanitta fusca) in Lithuanian soft-bottom coastal waters by attaching a solar-powered external GPS‐GSM transmitter to each individual bird. This transmitter data allowed us to analyse the dive phase parameters and feeding depths during December 2021 and March 2022.
Results
The results showed that velvet scoters dived deeper than a 30-m depth, with the dive duration lasting up to 90 s on average. The bottom duration increased rapidly in the first 5 m and then levelled off at around 40 s for deeper waters. The underwater duration varied between 2 and 7.5 h a day. The dive effort (as total underwater time) and the bottom duration of velvet scoters were related to the depth and prey biomass, although both factors were demonstrated to be interdependent. Moreover, the results showed that velvet scoters were less active in December than in March in terms of both average diving duration and diving frequency. The results also confirmed the high diurnal activity of the velvet scoter, although the birds demonstrated night activity in December and not in March.
Conclusion
This information on the velvet scoter’s foraging behaviour may have important implications for the conservation of the species, and provides new insights into the improvement of conservation measures for the species in the Baltic Sea.
Similar content being viewed by others
Background
Studies employing transmitters are increasingly being used to investigate the physiology [1], spatial movements [2], migratory strategies [3], and foraging behaviour [4] of seabirds worldwide. With the help of transmitters, it is now possible to study sea duck species all year round, or for a specific period of time.
Velvet scoters (Melanitta fusca) spend the wintering season entirely on the water’s surface. In the Baltic Sea they dive at depths down to 20 m and aggregate over soft bottoms with a high density of prey [5], predominantly small bivalves [5,6,7,8]. A recent study showed Mya arenaria as the preferred prey among bivalves in the Baltic [9], although the velvet scoter demonstrates great plasticity and may feed on a wide range of taxa, such as blue mussels [10], fish [5, 8], polychaetes and crustaceans [5].
In general, sea ducks dive in bouts, with several dives followed by a long period (approximately 30 min) of resting, preening and processing food in the gizzard [11, 12]. Each single dive cycle is characterized by a time spent under water (hereafter referred as “underwater duration”) and a short pause (i.e., inter-dive) before the next dive cycle [13], but little is known about sea ducks’ foraging depth distribution. Moreover, existing information comes exclusively from studies in captivity or direct observations during calm weather and daylight conditions [14, 15]. Since the velvet scoter population in the Baltic Sea is declining and the species has been listed as vulnerable for a decade (IUCN 2013), the demand for data on its foraging behaviour in the natural environment is obviously increasing. This new knowledge could potentially provide support for the development of effective conservation measures to, e.g., protect feeding grounds and reduce by-catch risks. For this reason, this study aims to evaluate the velvet scoter’s foraging behaviour with respect to foraging depth and dive phase parameters along the exposed coastline of the southeast Baltic, an area known for being one of the most important wintering sites of the species in the Baltic region [16]. We fixed transmitters to a number of velvet scoters in mid-winter and at the end of the wintering season, and followed their foraging behaviour in an area of well-known prey diversity and distribution. This study provides the first results on the foraging behaviour of the species obtained from depth sensors employed in GPS–GSM transmitters.
Methods
Study area
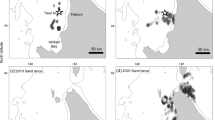
The study was performed in the Lithuanian coastal waters of the southeast Baltic Sea stretching for approximately 90 km (Fig. 1). Along the mainland coast, the waters are characterized by a highly dynamic spatio-temporal distribution of freshwater plume and heterogeneous bottom sediment from fine silt and sand to boulder fields, gravel and pebbles [17]. The seabed surface of the coastal waters along the Curonian Spit is known to be covered by a soft sediment (from silt to coarse sand), which is the preferred substrate for velvet scoter feeding [5, 9, 18].
Data collection
Seven adult velvet scoters were caught with night-lighting technique during new moon nights in early December and late February (for the birds’ characteristics and observation details, see Additional file 1: Table S1). In the laboratory after capture, the velvet scoters were equipped with solar-powered Global Positioning System‐Global System for Mobile Communications (GPS‐GSM) transmitters (OrniTrack-T20D 3G; Ornitela, UAB). The devices were rectangular (dimensions 58 × 25 × 14 mm) and weighed 17–20 g. They were attached to the back feathers between the scapula with a UV resin glue and Tesa tape (Tesa SE), allowing them to fall off after a few weeks. After their attachment, the velvet scoters were released into the nearest seashore area close to the capture site.
With a battery charge above 25%, the transmitters collected GPS locations every 30 min and logged diving activity. Below that threshold, GPS data were recorded only at 2-h intervals until the battery was exhausted. Depth was recorded automatically with 1 Hz frequency (1 Hz = 1 s) and ± 3 cm accuracy from the moment the bird went under water until resurfacing. Once the transmitter was on the surface, the recording ended.
Information on bottom macrofauna was collated from existing data sets based on samplings along nine soft sediment transects from 3 to 25 m depth (Fig. 1) during 2016 (November), 2020 (July), and 2021 (April). Samplings were carried out with a Van Veen grab covering a bottom area of 0.1 m2 with 1–3 replicates taken in each sampling site. All the samples were sieved with a 0.5 mm mesh size sieve and fixed with 4% formaldehyde–seawater solution on board. In the laboratory, all the taxa were identified to the lowest possible taxonomic level, individuals counted and wet weight biomass measured to a precision of 0.1 mg.
Data analysis
The bird transmitter data from the Lithuanian coastal waters were selected for analysis according to the data availability on bathymetry and sediment as well as the structure and distribution of benthic macrofauna. All seven birds demonstrated a permanent presence in the study area during the study period.
Transmitter data were pre-processed to exclude erroneous readings. Following the recommendations of the transmitter producer, diving data collected with a battery charge level < 30% were removed from the data set due to possibly non-continuous data recordings. The first day’s readings received after attaching the transmitters were also excluded from the analysis to avoid the effects of catching and handling the birds when deploying the transmitters [19]. Nevertheless, the temporary effects of manipulation and attachment of transmitters on the behaviour of the studied birds cannot be excluded [20].
The protocol used to pre-process the data consisted of the following steps: (i) removing dives shorter than 5 s; (ii) removing dives different in terms of depth and/or duration from the previous and the following dives; (iii) deleting dive records characterised by a start–end format, i.e., generated while engaging in the preening and cleaning behaviour of birds or due to wave flushing effects.
An MS Excel Pivot Table was used for counting the number of dives and for the calculation of dive cycle parameters. The dive cycle was characterized using six parameters: (a) bottom duration (s); (b) dive duration (s); (c) inter-dive duration (s); (d) number of dives (times); (e) underwater duration (s); (f) average bottom depth (m); and (g) dive efficiency.
The dive cycle in this study is defined according to [13] and consists of the time spent descending, foraging at the bottom, ascending, and the time spent on the surface. The dive duration is expressed as the underwater duration of one dive cycle. The depth was calculated by averaging the depth values recorded every second spent on the bottom. The distinction between the bottom duration and other parameters (descent, ascent) depended on the wiggle amplitude (between -0.2 m and 0.2 m per second). The inter-dive is expressed as the time up to 100 s between two consecutive dive cycles. The dive efficiency was estimated as the ratio between bottom duration and dive cycle [21].
The statistical analysis was performed using R (version 4.2.2) [22]. Non-parametric tests were used in case the data deviated significantly from the normal distribution (Shapiro–Wilk test).
A generalized linear model (Rcmdr package) was used to explain the variation of the bottom duration (dependent variable) by the differences in total biomass of the preferred prey taxa (Mya arenaria, Macoma balthica, Cerastoderma glaucum and polychaetes), dive depth, and individual velvet scoters (independent variables). The original data set was randomly subsampled to select 100 dives per individual, ensuring an equal number of records within five depth intervals between 5 and 25 m. By subsampling, the data set was reduced to a total sample size of 588 dives.
Collinearity among predictor variables was measured by the variance inflation factor (VIF). Since the biomass of M. balthica, M. arenaria, C. glaucum and polychaetes was highly correlated with the bottom depth, only the total biomass of these preferred prey taxa was used for analysis due to its lower VIF. The dependent variables were normally distributed, and therefore, the Gaussian family with the link-function identity was used. Residuals were also normally distributed.
Results
Macrofauna data
The total macrofauna diversity comprised of 21 taxa, belonging mainly to crustaceans (10 taxa), polychaetes (four taxa), and molluscs (four taxa) (Additional file 2). The biomass ranged between 0.5 and 227 g m−2, accounting for 540–9140 ind m−2 distributed across 1–13 taxa per sampling site depending on depth. The variability between years and seasons was negligible (e.g., 1.9 ± 1.2 g m−2 and 2.1 ± 1.1 g m−2 for July 2020 and April 2021, respectively), but there was a clear pattern of increasing taxonomic diversity, density and biomass with the depth (Additional file 2). The diversity of the velvet scoters’ prey constituted five taxa only (three bivalves M. balthica, C. glaucum and M. arenaria, and two polychaete taxa: Hediste diversicolor and Marenzelleria complex), although these taxa comprised up to 99% (68.2% ± 5.3% on average) of the total macrofauna biomass. Therefore, similar to the general macrofauna parameters, the biomass of the main prey taxa also had a distinct distribution at depths from 3 to 25 m. Prey taxa were missing at around 3 m depth, where infaunal Pygospio elegans and/or nectobenthic Bathyporeia pilosa were recorded. The biomass of prey increased with the depth, but also its proportion in the total macrofauna increased from 39.0% at 5 m depth to 72.9% and 91–92% at 10 m and 15–25 m depth, respectively. Polychaetes occurred at low biomass levels within the entire depth interval, but an increasing biomass of M. arenaria with the depth and substitution of C. glaucum biomass by M. balthica at 20–25 m depth ensured a consistent increase of the total prey biomass along the depth gradient (Additional file 1: Table S2).
Velvet scoters distribution
Velvet scoters were observed for a total of 77 days during 6–15 December 2021, and 1–25 March 2022, covering all soft-bottom coastal waters in the study area (Fig. 1). The birds showed a general preference for shallow soft-bottom environments, with 97% of the dives performed exclusively in homogenous soft-bottom areas along the Curonian Spit. Only one individual (transmitter code 203597) fed in deeper and heterogenous seabed areas along the mainland coast (Fig. 1).
In December, five velvet scoters were segregated well between areas and depth ranges along the entire studied coastline (Fig. 1). In March, the other two observed individuals (transmitter codes 203605 and 203595, Fig. 1) showed fidelity to the northern area off the Curonian Spit for the whole period (24–25 days). The GPS fixes (locations) of one of these individuals was restricted to a small area of approximately 17 km2, while the other one covered a larger area of approximately 42 km2.
Overall, the collected data set accounted for 21,679 dives in depths down to 36 m, with the highest frequency of dives around 12 m depth (Fig. 2). Three individuals spent most of the studied period (> 80% of the dives) in depths of less than 9 m, and two individuals fed exclusively in deep waters (20–36 m depth). The remaining two individuals showed a less distinct depth distribution and fed down to 20 m depth.
Dive phase
Individually, velvet scoters performed 285 ± 32 dives per day on average and spent around 4.1 ± 0.2 h a day under water. The mean dive duration was 51.6 ± 0.1 s, and dives of 45–60 s had the highest frequency (53%) (Additional file 1: Fig. S1). Individually, birds had highly variable mean dive duration values differing up to three times, but a clear grouping around 49–53 s and 79–86 s was characteristic of six out of seven birds (Kruskal–Wallis test, H(2) = 7850.1, df = 6, p < 0.001) (Additional file 1: Table S4). In contrast to this, the mean bottom duration was consistent for the same birds, and varied between 37.2 s and 43.9 s, regardless of the depth range exploited (Kruskal–Wallis test, H(2) = 4745.2, df = 6, p < 0.001). Shallow-feeding bird 203598, however, had significantly shorter bottom durations and dive durations (Dunn post-hoc test).
The mean inter-dive duration was 37.6 ± 0.1 s (Additional file 1: Table S4), and 90% of inter-dives lasted between 15 and 65 s (Additional file 1: Fig. S1).
Dive duration was dependent on depth (Fig. 3A). Major changes in dive duration occurred at depths down to 5 m, where a dive took 48.0 ± 0.2 s on average. It increased with the depth and levelled off at 86.0 ± 0.3 s at 25–30 m depth.
Bottom duration accounted on average for 71% of the total dive duration, but varied considerably across the studied depth range (Fig. 3C). Similar to the dive duration, it increased rapidly in the first five metres, and then remained relatively constant down to 22–23 m depth, with an average bottom duration of 43.6 ± 0.2 s. The bottom duration changes below this depth remain less certain, but a pattern of a weak decrease at an increasing depth is possible (Fig. 3).
Inter-dive duration was largely influenced by the dive duration (Fig. 3D), but was low and relatively constant (22.9 ± 0.2 s on average) for dives up to 35 s long. Such short inter-dives (less than 25 s) were rare and did not exceed 4% of observations (73 cases in total out of 1871). Inter-dive duration increased rapidly up to 75–80 s on average for dives of 75 s, and then levelled off for longer dives.
The dive efficiency was relatively stable around 0.53 ± (< 0.01) in the first 5-m depth interval and decreased down to 0.25 at depths below 15 m (Fig. 3B).
Modelling of feeding duration at the bottom
The generalized linear model showed a significant effect of depth on the time spent at the bottom for each dive, while prey biomass had a marginal significance (Fig. 4; Additional file 1: Table S3). The bottom time of three individuals was significantly different, whereas the remaining four velvet scoters spent a similar time on the bottom. Almost two-thirds of the total variability (62.3%) in the bottom time of velvet scoters was explained by depth, prey biomass and individual differences.
Temporal variation in foraging behaviour
The average daily number of dives was higher in March compared to December (305.9 ± 20.1 and 249.5 ± 30.9, respectively) (Mann–Whitney U test, U = 507.5, p = 0.059). The underwater duration differed significantly between December and March (Mann–Whitney U test, U = 412, p = 0.003). In December, the underwater duration was 3.4 ± 0.3 h on average (from 2 to 6.7 h per day−1). In a few cases (two out of 28 recorded days) the diving activity in December was low, i.e., 15 and 50 min under water per day. In March, the underwater duration was approximately 32% higher than in December, and varied between 2.5 and 7.5 h per day (4.5 ± 0.2 h d−1). There were no days with low diving activity observed in March.
The velvet scoters’ preference for shallow areas down to 12 m depth was stable between the two studied months with a similar overall dive frequency (81% in December and 88% in March). In spite of this, important differences occurred in the feeding depth distribution between the months on different birds. Feeding in deeper areas of 20–30 m depth was observed during December (13% of total dives), but not in March. Next to these depth preferences, the observed diving activity at night was also more frequent in December than in March (14% and 1% of the total number of dives, respectively; Fig. 5).
Discussion
Our study provides the first data on the foraging behaviour of velvet scoters in 2 month stretching over mid-winter and early spring in the exposed soft bottoms of southeast Baltic Sea coastal waters.
The spatial distribution of velvet scoters showed a general preference for shallow waters down to 12 m depth. Although a similar depth distribution in offshore and nearshore waters has been demonstrated for other wintering sea duck species in the Baltic Sea [23] and in North America [24], this contradicts the overall prey biomass distribution along the depth gradient. Our results showed that prey organisms are at least temporally absent at depths of 3 m, and the biomass below 10 m depth is 5–15 times higher compared to shallower areas. Nevertheless, shallow areas remain important, and high foraging activity here might be related to two main factors: (1) a temporary increase in the biomass of mobile prey species aggregated after stormy events (personal observations); and (2) the larger proportion of easier located surface and sub-surface dwellers at high sediment density compared to the predominance of deeper burrowing infaunal species in lower-density sediment at greater depths.
High prey biomass and reduced bird exposure to waves may at least partly compensate for increased diving costs and explain individual foraging activity in depths of 20–30 m observed during December. However, this is not applicable for March, when the lower frequency of storms increases the suitability of shallow waters for foraging, and hence our study does not provide sufficient data to fully understand and justify the role of deeper water for species foraging.
Alongside the predominant diving in shallow waters, some individuals moved between different depth areas in 1 day, while others showed a fidelity to the same depth range for several days. Four individuals out of seven were always feeding in the same depth range between 2 and 12 m for a total of 38 days, while the others exploited different soft-bottom sites in depths ranging between 14 and 36 m. Individual differences in feeding strategies and depth preferences have also been reported for other diving ducks (e.g., tufted duck [Aythya fuligula] [25]), attributing foraging site fidelity to the patchy distribution or temporary aggregations of prey [24]. Less likely, though, is the influence of competitors in the individual behaviour of velvet scoters. Among the seabirds wintering in Lithuanian waters, the only possible competitor might be the common scoter (Melanitta nigra; Morisita index = 0.53) [26], but the density of this species is low on the Lithuanian coast [27, 28].
The dive duration and bottom duration increased linearly with depth, but the bottom duration in areas deeper than 5 m remained stable at around 40 s. It seems that such a timespan is most preferable for the velvet scoter, and exceeding this bottom duration or diving less intensively may not be energetically profitable in the context of the existing environment and prey availability. Estimated dive efficiency changes along the depth gradient indicated that the bottom duration accounted for 50% of the dive cycle in shallow waters down to 5 m depth, and it was considerably longer compared to depths below 15 m. This was probably due to scarcer, smaller, and more mobile prey, together with higher costs to compensate for the buoyancy force.
The inter-dive was affected by the dive duration, and hence was primarily dependent on depth. Velvet scoters needed longer recovery after long dives and intense activity under water. The long inter-dive depending on the depth was also observed in other studies [29,30,31].
We recorded noticeable changes in diving behaviour between 2 months in the middle and at the end of the wintering season. Although this observation is based on a relatively low number of birds unequally distributed between the months (five birds in December, and two in March), we consider the number of recorded dives and the number of observation days as being sufficient to indicate the principal differences (28 and 49 observation days, with 6,988 and 14,979 dives in December and March, respectively). Our results on the increased foraging effort in March, i.e., the higher number of dives and longer underwater duration, might be explained by the 29% longer daylight during this month compared to the first half of December [12, 32]. On the other hand, considerably shorter daylight in mid-winter likely resulted in an attempt to compensate for the winter energy demand with night foraging, as was observed in this study. In addition, extreme environmental conditions (i.e., low temperatures, a high frequency of storms, strong winds, and high waves) can impact the energy costs of wintering and affect their behaviour [33, 34]. During both months studied, approximately 50% of the time, the prevailing wind blew from directions of the highest wind exposure (i.e., north, northwest and west), but the frequency of wind speeds higher than 5 m s−1 was 85% in December compared to 29% in March (daily data collection from Windy.com). Furthermore, the increase in foraging effort during March might be related to the necessity to accumulate body reserves before migration and reproduction, as was observed in other sea duck species in a similar period (March–April) [35, 36]. All these factors (daylight length, wind speed and physiological response to the approaching migration and reproduction period) could likely affect the foraging behaviour and cause changes in underwater duration or in the number of dives per day. To conclude, in this study we observed the dive effort and the bottom duration of velvet scoters related to the depth and prey biomass, although both factors were demonstrated to be dependent. We observed a predominant feeding down to 12 m depth, in spite of pronounced low prey biomass compared to greater depths, but individual differences in foraging depths were also obvious. In addition, we found noticeable differences in the foraging behaviour between mid-winter and early spring: the number of dives and the underwater duration, together with higher nocturnal diving activity, were recorded in December compared to March. Although this can be explained well by the body reserve accumulation before the migration period and differences in daylight and meteorological conditions, studies involving more individuals will certainly help to understand better the physiological and climatic effects on foraging behaviour, as well as the role of deviations in individual behaviour.
References
Elliott KH. Measurement of flying and diving metabolic rate in wild animals: review and recommendations. CompBiochemPhysiol. 2016. https://doi.org/10.1016/j.cbpa.2016.05.025.
Matsumoto S, Yamamoto T, Yamamoto M, et al. Sex-related differences in the foraging movement of streaked shearwaters Calonectris leucomelas breeding on Awashima Island in the Sea of Japan. Ornithol Sci. 2017. https://doi.org/10.2326/osj.16.23.
Karwinkel T, Pollet IL, Vardeh S, Kruckenberg H, Glazov P, Loshchagina J, Quillfeldt P. Year-round spatiotemporal distribution pattern of a threatened sea duck species breeding on Kolguev Island, south-eastern Barents Sea. BMC Ecol. 2020;20(1):1–13.
Lewis TL, Esler D, Sean BW. Effects of predation by sea ducks on clam abundance in soft-bottom intertidal habitats. Mar Ecol Prog. 2007. https://doi.org/10.3354/meps329131.
Fox AD. Diet and habitat use of scoters Melanitta in the Western Palearctic-a brief overview. Wildfowl. 2003;54(54):163–84.
Durinck J, Christensen KD, Skov H, Danielsen F. Diet of the common scoter Melanitta nigra and velvet scoter Melanitta fusca wintering in the North Sea. Ornis Fenn. 1993;70(4):215–8.
Palm EC, Esler D, Anderson EM, Wilson MT. Geographic and temporal variation in diet of wintering white-winged scoters. Waterbirds. 2012. https://doi.org/10.1675/063.035.0407.
Stempniewicz LECH. The food intake of two Scoters Melanitta fusca and M. nigra wintering in the Gulf of Gdańsk, Polish Baltic coast. Vår Fågelv. 1986;11:211–4.
Morkūnė R, Lesutienė J, Morkūnas J, Barisevičiūtė R. Triple stable isotope analysis to estimate the diet of the Velvet Scoter (Melanitta fusca) in the Baltic Sea. PeerJ. 2018. https://doi.org/10.7717/peerj.5128.
Nilsson L. Habitat selection, food choice, and feeding habits of diving ducks in coastal water of South Sweden during the non-breeding season. Ornis Scand. 1972. https://doi.org/10.2307/3676166.
Žydelis R, Richman SE. Foraging behavior, ecology, and energetics of sea ducks. Ecology and conservation of North American sea ducks. Stud Avian Biol. 2015;46:241–66.
Guillemette M. Digestion rate constraint in wintering common eider (Somateria mollissima) - implications for feeding capabilities. Auk. 1994. https://doi.org/10.2307/4088822.
Heath JP, Gilchrist HG. When foraging becomes unprofitable: energetics of diving in tidal currents by common eiders wintering in the Arctic. Mar Ecol Prog. 2010. https://doi.org/10.3354/meps08482.
Lovvorn JR, Gillingham MP. Food dispersion and foraging energetics: a mechanistic synthesis for field studies of avian benthivores. Ecology. 1996. https://doi.org/10.2307/2265620.
Richman SE, Lovvorn JR. Costs of diving by wing and foot propulsion in a sea duck, the white-winged scoter. J Comp Physiol B. 2008. https://doi.org/10.1007/s00360-007-0225-9.
Skov HS, Žydelis R, Bellebaum J, Bzoma S, Dagys M, Durinck J, Garthe S, Grishanov G, Hario M, Kieckbusch JJ, et al. Waterbird populations and pressures in the Baltic Sea. Copenhagen, Denmark: TemaNord Nordic Council of Ministers; 2011. p. 550.
Olenin S, Daunys D. Coastal typology based on benthic biotope and community data: the Lithuanian case study. Coastline Reports. 2004;4:65–84.
Byrkjedal I. Identifying inter-dive intervals in time-activity budget studies of diving ducks. Wildlife Biol. 1997. https://doi.org/10.2981/wlb.1997.007.
Duckworth J, O’Brien S, Petersen IK, Petersen A, Benediktsson G, Johnson L, Green JA. Spatial and temporal variation in foraging of breeding red-throated divers. J Avian Biol. 2021. https://doi.org/10.1111/jav.02702.
Vandenabeele SP, Wilson RP, Grogan A. Tags on seabirds: how seriously are instrument-induced behaviours considered? Anim Welf. 2011. https://doi.org/10.1017/S0962728600003195.
Zimmer I, Wilson RP, Beaulieu M, Ropert-Coudert Y, Kato A, Ancel A, Plötz J. Dive efficiency versus depth in foraging emperor penguins. Aquat Biol. 2010. https://doi.org/10.3354/ab00213.
R Core Team. R. A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2023.
Bräger S, Meißner J, Thiel M. Temporal and spatial abundance of wintering Common Eider Somateria mollissima, Long-tailed Duck Clangula hyemalis, and Common Scoter Melanitta nigra in shallow water areas of the southwestern Baltic Sea. Ornis Fenn. 1995. https://doi.org/10.1016/j.ecss.2022.108007.
Guillemette M. The effect of timing and digestion constraints in Common Eiders while feeding and diving over Blue Mussel beds. Funct Ecol. 1998. https://doi.org/10.1046/j.1365-2435.1998.00164.x.
Nilsson L. Local and seasonal variation in sex-ratios of diving ducks in South Sweden dur- ing the non-breeding season. Ornis Scand. 1970;1:115–28.
Žydelis R. Habitat selection of waterbirds wintering in Lithuanian coastal zone of the Baltic Sea. Doctoral dissertation, Biomedical Sciences: Ecology and Environmental Research (03B), Vilnius University. 2002
Šniaukšta L. Žiemojančių vandens paukščių apskaitų rezultatai lietuvoje 2021 metais. Paukščiai. 2021;49:6–11.
Šniaukšta L. Žiemojančių vandens paukščių apskaitų rezultatai lietuvoje 2022 metais. Paukščiai. 2022;53:10–6.
Crook SL, Conway WC, Mason CD, Kraai KJ. Winter time-activity budgets of diving ducks on eastern Texas reservoirs. Waterbirds. 2009. https://doi.org/10.1675/063.032.0408.
Grunst AS, Grunst ML, Grémillet D, Kato A, Bustamante P, Albert C, Fort J. Mercury contamination challenges the behavioral response of a keystone species to Arctic climate change. ES&T. 2023. https://doi.org/10.1021/acs.est.2c08893.
Osterrieder SK, Weston MA, Robinson RW, Guay PJ. Sex-specific dive characteristics in a sexually size dimorphic duck. Wildfowl. 2014;64(64):126–31.
Systad GH, Bustnes JO, Erikstad KE. Behavioral responses to decreasing day length in wintering sea ducks. Auk. 2000;117(1):33–40.
Brodsky LM, Weatherhead PJ. Variability in behavioural response of wintering Black Ducks to increased energy demands. Can J Zool. 1985. https://doi.org/10.1139/z85-246.
Jorde DG, Krapu GL, Crawford RD, Hay MA. Effects of weather on habitat selection and behavior of mallards wintering in Nebraska. The Condor. 1984. https://doi.org/10.2307/1366993.
Rigou Y, Guillemette M. foraging effort and pre-laying strategy in breeding common eiders. Waterbirds. 2010. https://doi.org/10.1675/063.033.0307.
Laursen K, Møller AP, Haugaard L, Öst M, Vainio J. Allocation of body reserves during winter in eider Somateria mollissima as preparation for spring migration and reproduction. J Sea Res. 2019. https://doi.org/10.1016/j.seares.2018.11.005.
Guillemette M, Himmelman JH, Barette C, Reed A. Habitat selection by common eider in winter an is interaction with flock size. Can J Zool. 1993. https://doi.org/10.1139/z93-172.
Acknowledgements
We are grateful to project No 28T-2021-71 funded by the Lithuanian Environmental Protection Agency and the project “Understanding and Mitigating Seabird Bycatch in Static Fishing Nets” (UNCATCH) funded by the German Federal Agency for Nature Conservation (BfN). We thank all the fieldworkers who helped in catching the birds and attaching the transmitters. We want to thank Andrius Šiaulys (Marine Research Institute, Klaipėda University) for his help during the benthic macrofauna samplings, and Ramunas Žydelis (UAB Ornitela) for his suggestions and comments during the early and final stages of the manuscript. We appreciate the help from Petra Quillfeldt (Giessen University) in the early stage of the data analysis, and we are also grateful to Martynas Bučas and Laura Šaltytė-Vaisiauskė (Klaipėda University) for their advice on statistical data analysis.
Funding
This work was funded by the Lithuanian Environmental Protection Agency, and the project “Understanding and Mitigating Seabird Bycatch in Static Fishing Nets” (UNCATCH) funded by the German Federal Agency for Nature Conservation (BfN).
Author information
Authors and Affiliations
Contributions
PF, JM and DD conceived the study; PF designed the methodology; PF and JM collected the data; PF and DD analysed the data; PF led the writing of the manuscript. All the authors contributed to the drafts and gave final approval for publication. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed with the approval of the Environmental Protection Agency of Lithuania (Permission No (26)-SR-124).
Consent for publication
Not applicable.
Competing interests
The authors declare they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Frequency distribution of the dive duration (left) and inter-dive duration (right) for seven Velvet scoters tracked in the Lithuanian soft-bottom coastal waters (southeast Baltic Sea). Table S1. Information on velvet scoter individuals tagged during the wintering season 2021/2022 along the Lithuanian coastal waters. Each transmitter code identifies one individual of velvet scoter tagged. Table S2. Average biomass (g m-2; ± SE) of the main prey taxa of velvet scoter in the coastal waters off Juodkrantė area (Fig. 1 in the text). n represents the number of samples. Table S3. Results of generalized linear model on the effects of depth, the total biomass of benthic prey and individual variation on the feeding time at the bottom (bottom duration) in the Lithuanian soft-bottom coastal waters (southeast Baltic Sea). Null deviance of the model: 51723; residual deviance: 31264. Table S4. Individual diving parameters (bottom duration, dive duration, inter-dive duration and bottom depth), reported as average value ± standard error and the maximum value recorded.
Additional file 2.
Description of data: biomass (expressed in g m-2) of benthic macrofauna collated from existing data sets from 2016, 2020 and 2021. Benthic macrofauna samples were collected in soft-sediment transect from 5 to 25 m depth. Sampling was carried out with Van Veen grab covering a bottom area of 0.1 m2. All samples irrespective of sampling were sieved with 0.5 mm mesh size sieve and fixed with 4% formaldehyde–seawater solution on board. In the laboratory all the taxa were identified to the lowest possible taxonomic level, individuals counted and wet weight biomass measured with precision of 0.1 mg.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Forni, P., Morkunas, J. & Daunys, D. Case report: first data on the foraging behaviour of velvet scoters (Melanitta fusca) in soft-bottom coastal waters of the southeast Baltic Sea. Anim Biotelemetry 11, 43 (2023). https://doi.org/10.1186/s40317-023-00355-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40317-023-00355-w