Abstract
Background
Hawksbill turtles (Eretmochelys imbricata) are Critically Endangered throughout their global range, and concerningly little is known about this species in the Red Sea. With large-scale coastal development projects underway in the northern Red Sea, it is critical to understand the movement and habitat use patterns of hawksbill turtles in this environmentally unique region, so that effective conservation strategies can be implemented. We satellite tagged three hawksbill turtles, one 63 cm curved carapace length adult male captured near Wahlei Island, one 55 cm turtle captured in the Gulf of Aqaba, and one 56 cm turtle suffering from a floating syndrome which was captured at Waqqadi Island, rehabilitated, and released at Waqqadi Island. Turtles were tracked for 156, 199, and 372 days between October 2020 and November 2021.
Results
We calculated the home ranges and core use areas of hawksbill turtles using kernel-density estimations and found that each turtle showed high fidelity to their foraging sites. Home ranges calculated with GPS-derived locations ranged between 13.6 and 2.86 km2, whereas home ranges calculated with Argos-derived locations ranged from 38.98 to 286.45 km2. GPS-derived locations also revealed a higher proportion of time spent in coral and rock habitats compared to Argos, based on location overlap with the Allen Coral Reef Atlas. We also found that turtles were making shallow dives, usually remaining between 0 and 5 m.
Conclusions
While the number of tracked turtles in this study was small, it represents an important contribution to the current understanding of spatial ecology among foraging hawksbill turtles globally, and provides the first-ever reported hawksbill turtle tracking data from the Red Sea. Our results suggest that protecting coral reef habitats and implementing boating speed limits near reefs could be effective conservation measures for foraging hawksbill turtles in the face of rapid coastal development.
Similar content being viewed by others
Background
Sea turtle populations have undergone substantial declines in many areas of the world (e.g., [1]), with every assessed sea turtle species considered Vulnerable, Endangered, or Critically Endangered [2]. These declines have largely been associated with anthropogenic activities [1], but our ability to effectively manage sea turtle populations has been hindered by the lack of data in many regions. Recoveries of sea turtle populations have been successful in some locations (e.g., [3]), demonstrating the value of scientific data sets for enacting effective conservation measures and reaffirming the need for information on particularly imperiled species in data-poor regions.
Hawksbill turtles (Eretmochelys imbricata) are one of the least abundant sea turtle species globally [1, 4]. Current estimates suggest that there are fewer than 25,000 nesting females, which is a 90% decline in abundance from the previous century [1]. This loss is largely due to targeted hunting for the species’ ornate carapaces [5], with mortality estimates ranging up to nine million hawksbill turtles killed over the past 150 years to sustain this trade [5]. Worldwide, the hawksbill turtle has been classified as Critically Endangered on the International Union for Conservation of Nature (IUCN) Red List of Threatened Species, though recoveries in some areas (e.g., [6]) suggest that effective management strategies could be successful if applied elsewhere. Failing to conserve hawksbill turtles could have far-reaching ecological implications, as they are an important predator of sponges, providing an indirect benefit to reef-building corals through top-down regulation of their competitors [7].
Hawksbill turtles have a patchy distribution across their circumglobal range, with semi-isolated populations forming in loose proximity to critical breeding habitats [8]. Regional management plans, accounting for the conditions and threats faced by each rookery-group, are critical to the conservation of this species as a whole [8]. Developing these plans requires information on local hawksbill habitat use and movement behavior [8], making turtle tracking via satellite telemetry a research priority. Unfortunately, tracking research on hawksbill turtles has lagged behind other species, with only 4.3% of sea turtle tag deployments occurring on this species [9]. In addition, many tagging studies to date have focused on nesting females, resulting in a lack of information on the movement ecology of both males and immature hawksbill turtles [10].
The Red Sea is generally understudied compared to other ocean basins [11, 12]. Five of the seven extant turtle species are at least occasional visitors to the basin, but only hawksbill and green turtles (Chelonia mydas) are known to nest along the Red Sea coast [11, 13]. Most Red Sea turtle research has focused on green turtles, with little information currently available on the population abundance, movement patterns, or key foraging locations of hawksbill turtles [11, 14]. To date, there have been no studies on the genetic composition of Red Sea hawksbill turtles, though the green turtle rookery in the Saudi Arabian Red Sea presents a significantly different genetic structure than the nearby Arabian (Persian) Gulf and northwestern Indian Ocean populations [15]. Because hawksbill turtles generally have shorter migrations compared to green turtles [16], Red Sea hawksbill turtles may also be genetically isolated from the global population due to the semi-enclosed nature of the Red Sea basin and the highly philopatric nature of the species. It also remains unclear whether hawksbill turtles within the Red Sea exhibit similar patterns of space and habitat use to that of the species elsewhere, particularly considering the unique environmental conditions present in the Red Sea [17].
Anthropogenic pressures on hawksbill turtles may increase in the Saudi Arabian Red Sea as part of the country’s economic diversification plan, which includes greater numbers of tourists and coastal developments [18]. In particular, two large developments (known locally as “giga-projects”) are currently being constructed. For example, NEOM is proposed to span 450 km of the northern Red Sea and Gulf of Aqaba coastlines, and includes plans for a 170 km linear city (The Line), a manufacturing hub (Oxagon), and a mountain resort (Trojena) [18]. In addition, The Red Sea Project (TRSP) plans to develop an airport, 50 hotels, and 1,000 residential properties on more than 20 islands between the cities of Umluj and Al Wajh as well as 6 inland sites [18]. Both giga-projects are committed to high environmental standards and the protection of endangered species within their boundaries. Identifying and protecting key hawksbill foraging habitats along the Saudi Red Sea coast is thus vital to conserving this species in the face of rapid regional development.
In this study, we assessed the home range and corresponding benthic habitats based on the Allen Coral Atlas [19], as well as the depth of dives for three hawksbill turtles in the northern Red Sea. We assessed movement patterns and residency behavior of hawksbill turtles in the Red Sea, and discuss how these may relate to conservation challenges arising from development projects in the region.
Methods
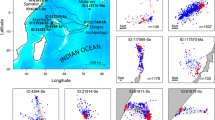
Hawksbill turtles were opportunistically captured in the Red Sea between October 2020 and April 2021. Two turtles were hand captured in NEOM waters, while free-diving as part of dedicated manta-tow surveys. The turtles were fitted with SPLASH10 FastLoc tags (Wildlife Computers, Redmond, WA, USA), and released at the site of capture (Fig. 1). A third turtle was found at TRSP struggling at the surface with a buoyancy disorder. It was transferred to the Fakieh Aquarium, rehabilitated, then released at the site of capture (Fig. 1) with an Argos positioning SPOT5 tag (also produced by Wildlife Computers). Both the SPLASH10 and SPOT5 tags transmit lower resolution Argos-derived geopositioning data when at the surface (error radius 250–1500+ m [20]), and the SPLASH10 tag also collects FastLoc GPS-derived locations (error radius < 100 m, [20]). Fastloc technology allows locations to be recorded when the tags are at the surface for mere milliseconds, providing high-resolution locations for animals that surface briefly [20]. SPLASH10 tags also recorded time at depth (TAD) data. Following recommendations from the manufacturer, depth bins were programmed with upper limits of 0, 5, 10, 30, 50, 60, 70, and 80 m.
Release locations of tagged hawksbill turtles (Eretmochelys imbricata) (green dots Ei1, Ei2, and Ei3), in the Saudi Arabian Red Sea. The dashed lines refer to the areas of ongoing coastal development giga-projects. The purple polygon refers to NEOM borders, and the red polygon refers to The Red Sea Project (TRSP) borders; these projects aim to attract visitors through eco-friendly luxury hotels and residential areas on islands and coastal areas
The tagging procedure was similar in all cases. After capture, each animal’s carapace was cleared of algae and other epibiota. Satellite transmitters were attached to the second vertebral scute of the carapace using a two-part epoxy (HILTI, www.hilti.sa). After the epoxy had dried, both the satellite tag and the epoxy were coated in marine anti-fouling paint (Hempels, www.hempels.com). The curved carapace length and width (CCL and CCW, respectively) were measured. Sex determination was attempted by assessing secondary sex characteristics (tail length > 20 cm plastron-to-cloaca in males) but this was only successful for one turtle. The other two turtles might have been juveniles based on their CCL, but we are unable to definitively determine their state of maturity. Each turtle was also equipped with two individually numbered titanium flipper tags (Stockbrand, Perth, Australia), one on the trailing edge of each front flipper.
Raw Argos and GPS-derived locations were manually filtered to remove locations on land. There were no “haul-out” events recorded which suggests that these were not nesting events, but were erroneous locations. Argos positions with location class Z (indicating an invalid location) were removed, retaining location classes 3, 2, 1, 0, A, and B (following [21,22,23]). GPS-derived positions with residual error value greater than 35 were also removed (following [21,22,23]). The Douglas filter was then applied to the data in Movebank (www.movebank.org) [24]. The hybrid filter was selected, which combines the “maximum redundant distance” filter and “distance angle rate” filter to remove unrealistic locations resulting in swimming speeds > 5 km/h or turning angles > 12.5° (following [22, 23]). The home range of each turtle was calculated with the kernel density estimation in R Studio version 2021.09.0 + 351 [25] using the AdehabitatHR package [26]. The smoothing parameter (href) was used to generate 95% and 50% utilization distributions (UD) for each turtle using the bivariate normal mode. The 95% and 50% UDs were then mapped on ArcGIS Pro v.2.6.0. For turtles Ei1 and Ei2, which had both FastLoc and Argos data, home ranges were calculated separately for each data set. For turtle Ei3, only Argos locations were used, because its tag type did not have FastLoc capabilities.
To determine the habitat type within the home ranges and core areas of each turtle, we used the Allen Coral Atlas (allencoralatlas.org, [18]) benthic cover types in ArcGIS Pro. The Allen Coral Atlas maps benthic habitats in the world’s shallow coral reefs from remotely sensed imagery at a resolution of 5 m [18]. Benthic cover types (i.e., coral/algae, microalgal mats, seagrass, rock, rubble, sand) were characterized from remotely sensed imagery only in waters shallower than 10 m; therefore, we used an additional benthic cover type of ‘depth > 10 m’. We cross-tabulated areas of benthic cover types within each turtle’s 50% and 95% UD from Argos and GPS-derived locations and then converted the areas into the respective proportions. Finally, the average time spent at various depth bins for the entire tracking duration was plotted for the two turtles equipped with FastLoc tags.
Results
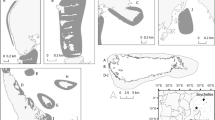
Three hawksbill turtles were satellite tagged, including an adult male (63 cm curved carapace length (CCL), labelled Ei1) and two turtles of unknown sex (55 and 56 cm CCL, labelled Ei2 and Ei3, respectively) (Table 1). Tag deployments lasted 156, 199, and 371 days, yielding a total of 2,826 transmissions from both Argos and GPS-derived locations prior to filtering. The FastLoc GPS and Argos location data were simultaneously active throughout the time periods shown in Table 1. Of the 1,960 Argos transmissions, 99% were location class A or B. After all filtering, 1,520 locations were available for analysis from the three turtles. Based on Argos data, turtle Ei1 had a core area (50% UD) of 17.89 km2 and a home range (95%) of 203.94 km2 (Fig. 2a), whereas Ei2 had a smaller core area of 3.77 km2 and a home range area of 38.97 km2 (Fig. 2b). Finally, Ei3 (the rehabilitated turtle) had the largest core area of 24.52 km2 and a home range area of 286.45 km2 (Fig. 2c). The vast majority (97.8%) of GPS-derived transmissions had residuals < 24. After filtering, 838 Fastloc GPS-derived locations were available to be included in analyses. From these GPS-derived locations, Ei1 (male turtle tagged off of Wahlei Island in the northern Red Sea) had a tracking duration of 371 days, with a core area (50% UD) of 2.86 km2 and a home range area (95% UD) of 13.64 km2 (Fig. 2d). In comparison, Ei2 (the turtle tagged in the Gulf of Aqaba) had a tracking duration of 156 days, with a much smaller core area of 0.16 km2 and a home range area of 0.80 km2 (Fig. 2e). Turtles Ei1 and Ei2 showed more than a tenfold difference in home range and core use estimations between GPS-derived locations compared to Argos-derived (Fig. 2).
Home range (95% utilization distribution, teal) and core use areas (50% utilization distribution, white) of hawksbill turtles (Eretmochelys imbricata) tagged in the northern Red Sea and Gulf of Aqaba. The first row (panels a, b, and c) includes areas calculated using Argos-derived locations, and the second row includes areas calculated using FastLoc GPS-derived locations. The small white rings show the locations of each transmission. Panel d is a close-up view of the area indicated by the yellow rectangle inset in panel a; panel e is a close-up view of the area indicated by the yellow rectangle in panel b. Panel f shows the regional context for the specific areas of focus (indicated in red) for the three tagged turtles. Note the differing scale bars in panels a–e
All three turtles had different habitat uses, with coral and rock being the most common habitats based off FastLoc data, whereas sand and rock were more prevalent with the Argos data (Additional file 1: Fig. S2). Seagrass and microalgal mats were consistently the least common habitat type used. Of the two turtles fitted with depth sensors, the adult male turtle Ei1 spent on average only 23.6% of its time in waters deeper than 5 m, and turtle Ei2 spent only 6.12% of its time deeper than 5 m (Additional file 1: Fig. S2). The deepest recorded dive was from Ei1 at 80 m, whereas Ei2 had a maximum depth of 30 m (Additional file 1: Fig. S2).
Discussion
This study presents the first published data on the movement patterns, home range size, and habitat use of foraging hawksbill turtles in the Red Sea. All three tracked turtles maintained localized home ranges, though their habitat selection within those ranges was varied, particularly within their respective core use areas. Despite the small sample size, the data presented here provide the first insight into hawksbill turtle habitat use in the Red Sea and represents an important step toward improved local management of key foraging habitats.
Restricted movement patterns have been commonly reported from foraging hawksbill turtles tracked in other areas [27,28,29,30]. The animals tracked in the present study exhibited similar restricted movements (Additional file 1: Table S1). The Red Sea hawksbill turtles exhibited similarly sized home range and core use areas as from other regions, with some smaller and some larger (Additional file 1: Table S1) [27,28,29,30]. Home ranges may simply reflect the physical constraints where each animal resides. For instance, the reef habitat in Aqaba is often restricted to a narrow (a few 10 s of meters) band of fringing reef that rapidly drops off into deep waters exceeding 100 m. On the other hand, the northern Red Sea increases in depth more gradually, potentially providing increased foraging habitat over wider vertical and horizontal ranges. The smaller home range used by the hawksbill tracked in the Gulf of Aqaba corresponded to a smaller vertical range (0–30 m) than its counterpart in the northern Red Sea (0–80 m). This difference in diving depths may be explained by the differences in size and potentially sex of the turtles [31]. Nonetheless, both tracked hawksbill turtles spent most of their time in waters shallower than 5 m (Additional file 1: Fig. S2). While turtles made predominantly shallow dives, habitat analysis revealed that the most common category associated with locations was “ > 10 m depth”. While this suggests that turtles may spend time in the top 5 m of the water column over deeper habitats, this pattern may result from a mismatch between location accuracy and the habitat data set resolution, or the Allen Coral Atlas not having high enough accuracy in this region.
Extended use of surface or near-surface waters could make animals vulnerable to boat strikes, a major source of mortality in sea turtles, especially in locations with high amounts of tourism or boat use (e.g., [24]). Currently, the main threat of boat strikes is for the turtles migrating through deeper waters. The Red Sea is relatively narrow with a high number of shipping vessels, exasperating the risk of collision [33]. The developing tourism industry may increase the number of boats visiting reefs for recreational use, which could increase the risk of collision at these foraging sites. Conservation managers working for the relevant giga-projects should consider implementing coastal boating speed limits to minimize this threat. While the differences in bathymetry and habitat distribution between the Gulf of Aqaba and the northern Red Sea could explain corresponding differences in the home range sizes between tagged turtles, more data are required to draw broad, population-level conclusions. More tracking studies on foraging hawksbill turtles in the Red Sea are clearly needed to generate actionable information for the effective conservation of local populations and the key foraging grounds on which they depend.
Our results showed more than a tenfold difference in the home range and core area estimations when using GPS-derived locations compared to the Argos-derived locations (see also [27]). While home range estimations from both data types indicated highly constrained foraging grounds when compared to the available habitat, the difference in area estimation had a pronounced effect on finer-scale analyses, such as the benthic habitat classification (see Additional file 1: Fig. S1). Benthic habitat use based on FastLoc data indicated that turtles are using rock and reef habitats (Additional file 1: Fig. S1), thus, these ecosystems should be protected in the face of upcoming coastal developments. Specifically, conservation managers should avoid introducing pollutants into the water, avoid sedimentation, and create regulations for tourism to decrease the impact on the marine ecosystem. Though FastLoc technology is more costly compared to Argos, it provides higher resolution location data, allowing managers to refine spatial protections to truly critical habitats [27, 34]. In the absence of other data, Argos locations can still be used, but managers should understand that the home ranges derived from these data are likely an overestimation.
Telemetry is a crucial tool for quickly understanding the horizontal and vertical space-use of marine megafauna. Hawksbill turtles spend most of their time foraging on coral reef ecosystems [27], yet most tracking studies on sea turtles to date have focused on the migratory behavior of nesting females [10]. The Red Sea has an estimated 4,000 km of coral reefs along the shoreline [35], which could be suitable habitats for hawksbill turtles. More research efforts are needed to identify “hotspots” used by the species. Hawksbill turtle sightings are commonly reported by recreational divers and boaters throughout the Red Sea. However, at present, there is only one systematic study that includes in-water observations of hawksbill turtles in the Red Sea, which found a high number of turtles in the same area, where turtle Ei2 was tagged [36]. While the number of tracked turtles in this study was small, it still represents an important contribution to the current understanding of spatial ecology among foraging hawksbill turtles globally and provides the first ever reported hawksbill turtle tracking data from the Red Sea (Additional file 1: Table S1). More tagging studies are needed to add to the preliminary findings presented here and guide the implementation of effective spatial management for hawksbill turtles in the Red Sea and throughout this Critically Endangered species’ circumglobal range.
Availability of data and materials
The data sets used and/or analyzed during the current study are available from the corresponding author on request.
References
Mortimer JA, Donnelly M. 2007 IUCN Red List status assessment: Hawksbill turtle (Eretmochelys imbricata). International Union for Conservation of Nature and Natural Resources. 2008. www.iucnredlist.org
IUCN red list of threatened species. 2022. http://www.redlist.org. Accessed 20 Jan 2022.
Balazs GH, Chaloupka M. Thirty-year recovery trend in the once depleted Hawaiian green sea turtle stock. Biol Conserv. 2004;117:491–8.
Meylan A, Donnelly M. Status justification for listing the hawksbill turtle (Eretmochelys imbricata) as critically endangered on the 1996 IUCN red list of threatened animals. Chelonian Conserv Biol. 1999;3:200–24.
Miller EA, McClenachan L, Uni Y, Phocas G, Hagemann ME, Van Houtan KS. The historical development of complex global trafficking networks for marine wildlife. Sci Adv. 2019. https://doi.org/10.1126/sciadv.aav5948.
Hamilton RJ, Bird T, Gereniu C, Pita J, Ramohia PC, Walter R, et al. Solomon islands largest hawksbill turtle rookery shows signs of recovery after 150 years of excessive exploitation. PLoS ONE. 2015;10:1–16.
León YM, Bjorndal KA. Selective feeding in the hawksbill turtle, an important predator in coral reef ecosystems. Mar Ecol Prog Ser. 2002;245:249–58.
Wallace BP, DiMatteo AD, Hurley BJ, Finkbeiner EM, Bolten AB, Chaloupka MY, et al. Regional management units for marine turtles: a novel framework for prioritizing conservation and research across multiple scales. PLoS ONE. 2010;5:1–11.
Hays GC, Hawkes LA. Satellite tracking sea turtles: opportunities and challenges to address key questions. Front Mar Sci. 2018;5:1–12.
Godley BJ, Blumenthal JM, Broderick AC, Coyne MS, Godfrey MH, Hawkes LA, et al. Satellite tracking of sea turtles: where have we been and where do we go next? Endanger Species Res. 2008;4:3–22.
Mancini A, Elsadek I, El-alwany MAN. Marine turtles of the Red Sea. In: Rasul NM, Stewart ICF, editors. The Red Sea. Berlin: Springer Earth System Sciences; 2015.
Ameri H, Al Harthi S, Al Kiyumi A, Al Sariri T, Al-Zaidan A, Antonopoulou M, et al. Biology and conservation of marine turtles in the North-Western Indian Ocean: a review. Endanger Species Res. 2022;48:67–86.
Shimada T, Duarte CM, Al-Suwailem AM, Tanabe LK, Meekan MG. Satellite tracking reveals nesting patterns, site fidelity, and potential impacts of warming on major green turtle rookeries in the Red Sea. Front Mar Sci. 2021;8. Article number: 633814.
Hamann M, Flavell F, Frazier J, Limpus CJ, Miller JD, Mortimer JA. Assessment of the conservation status of the hawksbill turtle in the Indian Ocean and South-East Asia. IOSEA Species Assess. 2022. I.
Jensen MP, Miller JD, FitzSimmons NN, Al-Merghani M. Identification of Chelonia mydas populations in the Kingdom of Saudi Arabia through regional genetic analyses. Mar Turt Newsl. 2019;156:16–20.
Hays GC, Scott R. Global patterns for upper ceilings on migration distance in sea turtles and comparisons with fish, birds and mammals. Funct Ecol. 2013;27:748–56.
Jensen MP, FitzSimmons NN, Dutton PH. Molecular genetics of sea turtles. In: Wyneken J, Lohmann KJ, Musick JA, editors. Biol Sea Turtles, vol. III. Boca Raton, FL: CRC Press; 2013.
PIF. Giga-project initiatives, public investment fund program document. 2017. http:// www.pif.gov.sa. Accessed 2 Dec 2021.
Allen Coral Atlas. Imagery, maps and monitoring of the world's tropical coral reefs. 2022. https://doi.org/10.5281/zenodo.3833242
CLS. Argos User Manual. 2016. https://www.argos-system.org/wp-content/uploads/2016/08/r363_9_argos_users_manual-v1.6.6.pdf. Accessed 13 Jan 2022.
Witt MJ, Åkesson S, Broderick AC, Coyne MS, Ellick J, Formia A, et al. Assessing accuracy and utility of satellite-tracking data using Argos-linked Fastloc-GPS. Anim Behav. 2010;80:571–81.
Gaos AR, Lewison RL, Wallace BP, Yañez IL, Liles MJ, Nichols WJ, et al. Spatial ecology of critically endangered hawksbill turtles Eretmochelys imbricata: implications for management and conservation. Mar Ecol Prog Ser. 2012;450:181–94.
Kranstauber B, Cameron A, Weinzerl R, Fountain T, Tilak S, Wikelski M, et al. The movebank data model for animal tracking. Environ Model Softw. 2011;26:834–5.
Douglas DC, Weinzierl R, Davidson SC, Kays R, Wikelski M, Bohrer G. Moderating Argos location errors in animal tracking data. Methods Ecol Evol. 2012;3:999–1007.
R Core Team. R. a Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2022.
Calenge C. Home Range Estimation in R: the adehabitatHR Package. 2011. https://cran.r-project.org/web/packages/adehabitatHR/vignettes/adehabitatHR.pdf. Accessed 19 Sept 2021
Thomson JA, Börger L, Christianen MJA, Esteban N, Laloë JO, Hays GC. Implications of location accuracy and data volume for home range estimation and fine-scale movement analysis: comparing Argos and Fastloc-GPS tracking data. Mar Biol. 2017;164:1–9.
Berube MD, Dunbar SG, Rutzler K, Hayes WK. Home range and foraging ecology of juvenile hawksbill sea turtles (Eretmochelys imbricata) on inshore reefs of Honduras. Chelonian Conserv Biol. 2012;11:33–43.
Blumenthal JM, Austin TJ, Bell CDL, Bothwell JB, Broderick AC, Ebanks-Petrie G, et al. Ecology of hawksbill turtles, Eretmochelys imbricata, on a western caribbean foraging ground. Chelonian Conserv Biol. 2009;8:1–10.
Martinez-Estevez L, Amador JPC, Amador FC, Zilliacus KM, Pacheco AM, Seminoff JA, et al. Spatial ecology of hawksbill sea turtles (Eretmochelys imbricata) in foraging habitats of the Gulf of California Mexico. Glob Ecol Conserv. 2021;27:e01540.
Nivière M, Chambault P, Pérez T, Etienne D, Bonola M, Martin J, et al. Identification of marine key areas across the Caribbean to ensure the conservation of the Critically Endangered hawksbill turtle. Biol Conserv. 2018;223:170–80.
Casale P, Affronte M, Insacco G, Freggi D, Vallini C, D’Astore PP, et al. Sea turtle strandings reveal high anthropogenic mortality in Italian waters. Aquat Conserv Mar Freshw Ecosyst. 2010;20:611–20.
Womersley FC, Humphries NE, Queiroz N, Vedor M, Furtado M, Tyminski JP, et al. Global collision-risk hotspots of marine traffic and the world’s largest fish, the whale shark. PNAS. 2022;119:1–10.
Tomkiewicz SM, Fuller MR, Kie JG, Bates KK. Global positioning system and associated technologies in animal behaviour and ecological research. Philos Trans R Soc B Biol Sci. 2010;365:2163–76.
Voolstra CR, Berumen ML, editors. Coral Reefs of the Red Sea, vol. 11. Berlin/Heidelberg, Germany: Springer; 2019.
Garzon F, Williams CT, Cochran JEM, Tanabe LK, Abdulla A, Berumen ML, et al. A multi-method characterization of Elasmobranch & Cheloniidae communities of the north-eastern Red Sea and Gulf of Aqaba. PLoS ONE. 2022;17:1–17.
Acknowledgements
We would like to thank OceanX and the crew of OceanXplorer for their operational and logistical support for the duration of this expedition. We would also like to thank OceanX Media, for documenting and communicating this work to the public. We would also like to thank the Fakieh Aquarium for their assistance tagging the rehabilitated turtle.
Funding
This work was supported by King Abdullah University of Science and Technology (baseline research funds to MLB).
Author information
Authors and Affiliations
Contributions
LT, LH, MB, RB, PM, and AE developed the initial aims and methods of the study. LT, JC, CW, FG, LW, and LW conducted the fieldwork. LT and JC wrote the first draft of the manuscript. LT and UL analyzed the data. RH and UL created the maps and LT made the remaining figures. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Research was conducted under King Abdullah University of Science and Technology (KAUST) Institutional Animal Care and Use Committee (IACUC) approval #19IACUC07.
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no potential competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Figure S1. Comparison of benthic categories (>10 m depth, coral/algae, microalgal mats, rock, rubble, sand, and seagrass; indicated by color in the legend) that each of three tagged hawksbill turtles (Eretmochelys imbricata) (individuals numbered as Ei1, Ei2, and Ei3) used in their core use areas (50% utilization distribution) and their home ranges (95% utilization distribution). Panels a–c show results using Argos-derived locations, and panels d and e show results using FastLoc GPS-derived locations. Figure S2. Proportion of time Ei1, the adult male hawksbill (Eretmochelys imbricata) tagged near Wahlei island, and Ei2, the hawksbill tagged in the Gulf of Aqaba, spent in shallow (<5m) and deep (>5m) depths (panel a). The comparison of benthic habitat use (coral/algae, rock, rubble, and sand) by Ei1 and Ei2 (panel b). For panels a and b, the center lines denote the median, the boxes contain the inner quartiles of the sampled values, the whiskers extend to the most extreme data point which is within 1.5 times above the 75th percentile or below the 25th percentile, and the black dots are outliers that are >1.5 times the interquartile range above the 75th percentile and below the 25th percentile. The proportion of time Ei1 and Ei2 spent at each depth bin (surface, 0–5, 5–10, 10–30, 30–50, 50–60, and 70–80 m) is shown in panel c. Table S1. Estimated foraging core use area (utilization distribution 50%) and home range (95% utilization distribution) of hawksbill turtles. The study site, name of turtle, sensor type (FastLoc or Argos), number of days of satellite tag deployment, sex (M = male, F = Female, and U = unknown), and turtle’s life stage if noted in the paper.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tanabe, L.K., Cochran, J.E.M., Williams, C.T. et al. Case report: tracking data from foraging hawksbill turtles in the northern Red Sea. Anim Biotelemetry 11, 1 (2023). https://doi.org/10.1186/s40317-022-00314-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40317-022-00314-x