Abstract
Background
Acoustic telemetry allows detailed observations of the movement behaviour of many species and as tags get smaller, smaller organisms may be tagged. The number of studies using acoustic telemetry to evaluate marine invertebrate movement is growing, but novel attachment methods include unknowns about the effects of tagging procedures on individual survival and behaviour. This study compared methods of tag attachment on green sea urchins (Strongylocentrotus droebachiensis) to determine the feasibility of using acoustic transmitters to track echinoid movement. Four tagging methods were compared in the lab and tag retention, urchin condition, and survival analysed. Two tagging methods (Dyneema® fishing line and T-bar tags) were evaluated in the field using an existing acoustic telemetry array. Urchins were tagged and the study area revisited one week and 2 months post-release by scuba divers to estimate movement and tag retention.
Results
The best methods in the lab, with high tag retention, survival, and minimal effects on urchin condition, were fishing line methods. T-bar tags, although showing high tag retention, caused significant mortality and had deleterious long-term effects on urchin condition and behaviour. After 2 months in the field, as in the lab, fishing line was a more effective tagging method. Urchins tagged with fishing line showed increased estimates of space occupancy compared to T-bar-tagged urchins and a single fishing-line tagged individual was found by divers in good health after 80 days. Combined, these laboratory and field results demonstrate the feasibility of using acoustic telemetry to observe urchin movement.
Conclusions
Results strongly suggest that surgical attachment methods that minimize injuries at the attachment site should be prioritized for echinoid tagging studies. Together, lab and field tests indicate that acoustic telemetry is a promising method to examine marine echinoid movement over ecologically relevant spatial and temporal scales.
Similar content being viewed by others
Background
Movement behaviour is an important component of the ecology of mobile species, determining such critical interactions as foraging, reproduction and migration [1,2,3,4]. Methods to tag and follow individual organisms through time have allowed detailed observations to be made on ecological behaviours for a wide variety of species [5,6,7]. Remote tracking tags have been used on many species, particularly large mammals and birds, but ethical concerns have been raised about the impact that tags could have on tagged individuals’ survival, health, and behaviour [8,9,10]. Sub-lethal tag effects can be due to the tag itself (e.g., added weight, predator attraction) or to the tag-attachment procedure (e.g., physiological costs of healing, infection). Rules-of-thumb have developed, such as restricting tag weight to a percentage of total body weight (e.g., < 5% or < 3% for swimming or flying animals) and holding animals for a recovery period post-surgery. However, when validated, these rules do not always eliminate sub-lethal tagging effects [11] which have been clearly demonstrated for diverse vertebrate species (e.g., [10, 12]).
Many invertebrates have large ecological and economic impacts (e.g., pest insects) and information on their movement ecology has both ecological and economic implications. As transmitter tags have become smaller, tagging methods have increasingly been applied to smaller organisms, allowing hitherto impossible behavioural observations at ecologically relevant scales [13]. However, fewer evaluations of tagging effects on invertebrates have been carried out than on vertebrates [14]. Failing to consider unintended tagging effects is not only ethically concerning, but also risks drawing erroneous conclusions about behaviour that could have great consequences (e.g., changes to fishing regulations or pest control strategies based on unrealistic movement/dispersal estimates; [14]).
The difficulty of observing individual organisms over large spatial and temporal scales has historically been even greater in aquatic than terrestrial environments. Many examples exist of tagging of aquatic species [6, 15], but the development of acoustic telemetry has been critical for expanding observations of marine species [16, 17]. As in the terrestrial realm, tags were first used for larger-bodied vertebrates of economic or conservation concern such as sharks and fished species such as salmon [18, 19]. Work on invertebrates has largely been limited to species where a tag can be glued to a hard shell or carapace such as gastropods [20, 21] and crustaceans [22].
Echinoderms (Phylum Echinodermata), including sea urchins, sea stars and sea cucumbers, are ecologically and economically important species lacking a smooth external shell or carapace to which a tag can be easily glued. Many methods of attaching small identifying markers to echinoderms have been tested (Additional file 2: Echinoderm Tagging Bibliography) but if acoustic telemetry is to be applied to echinoderms, appropriate methods of attaching an acoustic tag that minimize effects on behaviour and condition of tagged individuals are essential. Several studies have begun to use acoustic telemetry on echinoderms and make management recommendations based on results, although sub-lethal effects of tagging have not been evaluated [23]. Here, we evaluate the effects of four methods of acoustic transmitter attachment, examining tag retention, survival, condition, and behaviour in a laboratory experiment, and test two methods in the field.
Methods
Laboratory
Four methods to externally tag green sea urchins (Strongylocentrotus droebachiensis O.F. Müller, 1776) with acoustic transmitters (V7 tags from VEMCO/Innovasea) were tested over a 3-month lab experiment. The green sea urchin is a regular echinoid in the family Strongylocentrotidae, with a wide distribution in the northern hemisphere, including both the east and west Atlantic, the Pacific and the Arctic [24]. The green sea urchin is a subtidal herbivore, found from the intertidal down to hundreds of metres, has enormous impacts on their preferred food resource, brown macroalgae and kelp, and has been extensively studied for its role in driving regime shifts in kelp forest habitats [25]. It is also an economically important species, fished throughout much of its range [26].
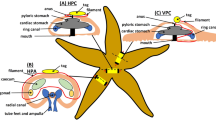
Divers collected 600 + urchins in December 2017 at water depths of 8 to 12 m near Mont-Joli (Quebec), Canada. Urchins were fed ad libitum (Saccharina latissima and Fucus sp.) at the Maurice-Lamontagne Institute (MLI) until tagging experiments started on February 6, 2018. The four methods tested were: Dyneema® fishing line (1—FL1), nickel titanium fishing line (2—FL2), T-bar tags (3—TB), and nylon screws (4—NS) (Fig. 1 and Additional file 1: Details of tagging methods). The two fishing line methods (FL1 and FL2) consisted of threading a length of fishing line directly through the test to form a loop (sensu [27]). Two types of fishing line were tested: Dyneema® nylon fishing line (0.18 mm diameter) and a more durable but less flexible nickel titanium fishing line (0.254 mm diameter). The urchin’s test was pierced with a 0.69-mm-diameter hypodermic needle, first on the aboral side about 1 cm from the mouth in an interambulacral plate to enter the test and then on the oral side to exit the test, again through an interambulacral plate. The fishing line was maintained in place as the needle was withdrawn, the two ends of line knotted together, and the tag fixed to the resulting loop using a combination of electrical tape and glue (Lepage UltraGel super glue). The nickel titanium fishing line was evaluated because of concerns that urchins might graze on the Dyneema fishing line, thereby detaching tags over longer temporal scales and at higher urchin densities [27].
The third method (TB) used commercially available plastic T-bar tags (sensu [28]). A tagging gun for clothing was used to pierce the test and insert a T-bar tag on the aboral side of the urchin about 1 cm from the anus in an interambulacral plate and the printed tag was attached to the stalk of the plastic tag using electrical tape and glue. The fourth method (NS) used a nylon screw cut to a length of 6 mm (diameter: 3 mm; sensu [29]). A hole slightly smaller in diameter than the gauge of the screw was made on the aboral side of the urchin using a dissection needle and the nylon screw carefully rotated into the hole. The tag was then glued on the head of the screw. To minimize costs, “tags” were printed with a 3D printer to have the same length (18 mm), weight (0.7 g) and volume in water as V7 tags.
A total of 288 urchins (80 each of FL1, TB and NS and 48 FL2) with test diameters > 35 mm were tagged from February 6–13. Before tagging, test diameter (mm) and condition (righting time sensu [30]) of all urchins were measured. Urchins were randomly distributed among eight separate 1-m2 tanks (each with an independent supply of flow-through seawater to a depth of 50 cm), with 10 individuals of each of three tagging methods (FL1, TB, and NS), 6 FL2 individuals, and 30 non-tagged individuals in each tank to match densities observed in the field trial (51 + 15 individuals m−2). Tanks were located inside the aquaculture facility at MLI and seawater was at ambient conditions, drawn from the adjacent intake at a water depth of 15 m; temperature ranged from − 0.1 to 4.3 ℃ and salinity from 25.0 to 30.2 PSU over the course of the experiment. Tanks were examined weekly to evaluate tag loss and mortality, and dead individuals removed when found.
Urchin health and condition were measured four times during the experiment (February 21, March 21, April 23, and May 22). Test diameter (mm), wet weight (g), righting time, and visible injuries were reported at each time for all individuals still retaining their tag, all urchins that had lost their tags (‘hole’ urchins), and ten control urchins from each tank. Righting time was measured by placing urchins individually, upside down, in a floating chamber large enough to ensure they were unable to touch the sides and measuring the time necessary for the urchin to flip itself back over. Trials were limited to 15 min and urchins not righting themselves in that period were assigned this value. Injuries were coded using four categories: 0—no injury; 1—small injury with minimal loss of spines and some discolouration; 2—apparent injury with dark or discoloured plaque or significant loss of spines in an area more than 2 mm in diameter (necrotic, very dark borders); and 3—very apparent injury with dark or discoloured plaque more than 5 mm in diameter (often with an apparent hole). The experiment finished at the end of May 2018 and all remaining tagged individuals, ‘hole’ urchins, plus 10 control urchins from each tank were dissected to determine gonad wet weight.
Field
In August 2017, 30 green sea urchins (Strongylocentrotus droebachiensis) were tagged with V7 acoustic telemetry tags (VEMCO/Innovasea) using two methods: FL1 and TB. Urchins were collected by SCUBA divers near a salmon farm in Doctor’s Cove (southwest New Brunswick, Canada) where an existing acoustic receiver array was deployed (Fig. 2) to evaluate decapod movements. The urchin population at the site was described by collecting all urchins from 5 quadrats (0.25 m2) from the tagging site.
Acoustic telemetry array surrounding a salmon aquaculture site in Doctor’s Cove (NB, Canada). Receivers are shown as black triangles, urchin release points ‘a’ and ‘b’ are shown labelled in red, the extent of the salmon farm is shown as a polygon, and all detections of urchins during the study are shown as orange points
Urchins (test diameter > 45 mm) were tagged on-site in two batches (16 at ‘a’ and 14 at ‘b’) with equal numbers of both tagging methods at each location (Fig. 3). Total manipulation time was limited, with divers replacing the urchins as soon as tagging was completed, and all tagging was done by the same person (KAM). At ‘a’, two markers separated by 280 cm were set up on the bottom and all 16 urchins released at known positions in relation to these markers. An unattached tag was also placed on the bottom at this release location to mimic a lost or detached tag. Two days later, SCUBA divers returned to ‘a’ and measured positions of all located tagged urchins to calculate 2-day net displacement. Divers revisited ‘a’ again on November 8 and recorded tag presence and status (attached or lost) for all tags found.
Data analysis
Laboratory
Tag retention and survival were analysed with Cox proportional hazards models (right-censored data) using the R packages survival, MASS and nnet [31,32,33]. The nylon screw tagging method was dropped from the analysis of survival because most urchins lost their tag within the first 10 days, leaving few individuals for which mortality could be observed. Differences between tagging treatments were evaluated using log-rank statistics.
Urchins that lost their tags were easily separated from control urchins due to the presence, placement, and form of the injury resulting from tag loss. These were almost entirely NS urchins (only 3 TB and no FL1 or FL2 urchins lost their tags throughout the entire study, whereas 75 NS tags were lost: Additional file 1: Fig. S1). These urchins were included in subsequent analyses as a separate group (‘hole’ urchins).
Condition was log-transformed because of heterogeneity of residuals and analysed using a linear mixed effects model including three fixed effects (tagging method, days since tagging, and urchin diameter), the two-way interaction between tagging method and days since tagging and two random effects to account for repeated measures on the same individual and for urchins in the same tank (Table 1; Eq. 1). The fixed factor ‘Tag’ had 6 levels: control, FL1, FL2, TB, NS, and ‘hole’.
Variation in diameter was analysed using a linear mixed effects model, as in Eq. 1, but with (1|Tank) removed from the final model because it accounted for almost zero variance in the response, causing singularity issues during model fitting (Table 1; Eq. 2). One outlier observation was removed because the difference between measures was clearly beyond possibility (increase of > 1 cm in test diameter) and represented a recording error.
Differences in wet weight (final–initial) were analysed using a linear mixed effects model with a single fixed effect and a random effect for urchins in the same tank (Table 1; Eq. 3). The fixed factor for tagging treatment had only three levels (FL1, FL2 and TB). The NS treatment was dropped from this analysis because only 4 individuals remained tagged at the end of the experiment; control and ‘hole’ treatments were excluded because data on individual urchins could not be calculated as they were not individually identifiable. Gonad wet weight at the end of the experiment was log-transformed because of heterogeneity of residuals and was analysed using a linear model. The random effect to account for urchins in the same tank again accounted for almost zero variance in the response, causing singularity issues, and was removed from the model (Table 1; Eq. 4). The fixed factor ‘Tag’ had 5 levels: control, FL1, FL2, TB, and ‘hole’; the NS tagging method was excluded because two few urchins remained at the end of the experiment (n = 4) when dissections for gonad weight were done.
Severity of injury for tagged urchins was analysed using ordinal logistic regression with a 5-level ordered factor response variable and a log–log link using the polr function from the MASS package (Table 1; Eq. 5). The fixed factor ‘Tag’ had 4 levels: FL1, FL2, TB, and NS. Both control and ‘hole’ treatments were dropped from this analysis because each showed no change for all urchins across all time points, causing problems with model fit (‘hole’ urchins always had very apparent injuries and control urchins had no injuries except for 2 individuals with small injuries). Probabilities of injury severity through time were predicted using the fit model.
Model fit was verified graphically in all linear and linear mixed effects models by plotting residuals versus fitted values, qqplots, histograms of residuals, and plots of residuals against all factors included in the model. Multicollinearity was verified by calculating variance inflation factors (< 4). Type II Wald F-tests with degrees of freedom calculated using the Kenward–Roger method were used to evaluate significance of interactions and main effects because of the unbalanced nature of the experimental design due to tag losses and mortalities (see above), except in the analysis of injury severity, where log-likelihood ratio Chi-squared type II tests were used. Tukey HSD post hoc comparisons were used, where appropriate, to compare tagging treatments (function emmeans; [34]).
Field
Variation in observed net displacement explained by tagging method was analysed using a linear model with a single fixed factor (tagging method, with two levels; Table 1; Eq. 6).
To evaluate if movement behaviour differed between tagging treatments, we calculated Minimum Convex Polygon (MCP) estimates of area occupied per month for each urchin; detections were first filtered by Position Error Sensitivity values (HPE) < 200 to eliminate several extremely high-error positions and urchin–month combinations with fewer than 5 detections were discarded. Because HPE values for this array were inconsistently related to error in distance due to interference and reflections from the aquaculture gear in the water, the pattern of increase in MCP area as a function of the percentage of detections included was used to choose a cut-off percentage of points to discount high-error detections but capture movement behaviour (Additional file 1: Fig. S2). A threshold value of 75% was identified that excluded the steep increase in area associated with including the few points far from the majority and this was assumed to represent an area with a high probability of actual occupation. 75% MCPs were then calculated for each urchin and the detached reference tag for every month. Due to the overall low numbers of detections at release point ‘b’ and the fact that the receiver array was removed from the water in mid-November, only urchins from release point ‘a’ and only estimates for September and October were included in the analysis of MCP area. 75% MCP area was analysed using a linear model to test for the effect of tagging method, month and their interaction. The interaction was not significant and was dropped from the final model (Table 1; Eq. 7). Model fit and multicollinearity were verified as above.
Results
Laboratory
Three of the four methods tested resulted in greater than 90% tag retention throughout the experiment (Fig. 4a). Only NS resulted in significant tag loss, with 50% loss after only 8 days (95% CI 6, 13). Both fishing line methods resulted in low mortality, with only 7 and 5 mortalities observed during the experiment for FL1 and FL2 (8.8% and 10.4%), respectively (Fig. 4b). T-bar tags, by contrast, resulted in much higher mortality, with 50% mortality after 79 days (95% CI 78, 80).
a Tag retention and b survival probabilities for tagging methods. Results shown are fitted survival models (with 95% profiled confidence intervals). Vertical black dashed line shows the median Kaplan–Meier value (when 50% of urchins have lost their tag or died) for the single tagging method where more than 50% of urchins lost their tag (NS—a) or died (TB—b)
Condition measured as righting time increased days post-tagging as a function of tagging method and with diameter (Table 2, Additional file 1: Fig. S3 and Table S1), driven by increasingly longer righting times for TB and NS urchins as days post-tagging increased (Fig. 5). Notice the large number of individuals at 75 days post-tagging with T-bar tags that have the maximum righting time (15 min) indicating that they failed to right themselves during the 15-min trial. These individuals (all except 1) then died or lost their tag before the final measurement time.
Righting time of urchins. Control and hole urchins were not individually identified (points are not connected between successive measures), but for tagging treatments, consecutive measures on the same individual are connected. Points are scaled by the number of overlapping observations. Model results (predicted values and 95% CI) are shown
Urchin test diameter differed among tagging treatments (Table 2 and Additional file 1: Table S1), with the FL2 treatment tagging marginally larger urchins (Additional file 1: Fig. S4). Wet weight decreased significantly from the beginning to the end of the study for TB urchins whereas FL1 and FL2 showed no decrease (Table 2, Additional file 1: Table S1 and Fig. 6). Gonad weight, however, showed no effect of tagging treatment although larger urchins clearly had a greater gonad weight (Table 2 and Additional file 1: Table S1, Figs S5 and S6).
Difference in weight between last and first measures. Values below zero indicate weight loss over the study period. Boxplots (line is median, whiskers to 1.5*interquartile range, and outliers as filled triangles), individual datapoints (unfilled jittered points), and mean values ± 1 standard error (filled points in the right of each box). Significant differences are indicated by different letters
Both fishing line methods resulted in a gradual increase in the number of small injuries (Fig. 7). TB urchins had high numbers of injuries, beginning almost immediately post-tagging and increasing in severity through time; by 60 days post-tagging, these urchins had less than a 25% probability of no injury. NS urchins had even more severe and rapid injury progression, with the probability of no injury falling to less than 15% after only 30 days (Fig. 7). Control urchins had almost no injuries throughout the entire experiment (Additional file 1: Fig. S7).
Field
Diver observations
Urchin density at the field site was 51 ± 15 ind·m−2 (mean ± 1 se) with a mean test diameter of 36 mm. Diver-measured urchin displacement indicated that urchins tagged using either method moved during the initial 2 days post-tagging. We relocated 11 tagged urchins (5 FL1 and 6 TB). Both the minimum and maximum observed displacement were TB urchins, indicating greater variability in movement behaviour (0.2 and 3.1 m of displacement in 2 days: Additional file 1: Fig. S8). Overall, FL1 urchins moved an average of 1.1 ± 0.23 m and TB urchins 1.5 ± 0.45 m, but this difference is not statistically significant (F(1, 9) = 0.08; p = 0.78). No detached tags were seen. All urchins located appeared in good health and the reference tag was present at the site and remained in the same location. During the diver search on November 8, we found one tagged urchin, which was in good shape and showed no apparent injury (FL1), and four detached tags on the seafloor (all TB).
Acoustic telemetry
Low rates of movement by urchins over the experimental period, low numbers of detections, particularly at release point ‘b’ (Additional file 1: Fig. S9) combined with high error in positioning and reflectance due to aquaculture gear (HPE values ranging from a minimum of 10.5 to a maximum of 939.9 with a mean of 37.8 and median of 28.1) complicated detailed analysis of individual movement tracks. However, analysis of 75% MCPs showed that TB urchins occupied significantly less space than FL1 urchins over the study period (Table 3, Fig. 8 and Additional file 1: Fig. S10). The area occupied by the detached reference tag falls within the range of areas calculated for tagged urchins of both treatments, however.
75% Minimum Convex Polygon (MCP) areas; individual datapoints (unfilled jittered points), and mean values ± 1 standard error (filled points in the right of each box). Different letters indicate a significant difference in Tukey post hoc tests. Estimates for the detached reference tag are also shown as blue labels indicating an estimate for September and October, respectively. These reference estimates provide an indication of estimated area occupied by a stationary tag on the bottom during a month of detections in this array
Discussion
Acoustic telemetry is a promising method to acquire long-term in situ observations of marine invertebrate movement behaviour. Our results indicate that if care is taken to use tagging methods that minimize sub-lethal effects, observations of movement behaviour over large temporal scales have the potential to provide reliable information to answer key ecological questions. Tagging urchins using fishing line (sensu [27]) caused minimal mortality, very low tag loss, almost no attachment injuries, and no or small effects on condition and behaviour. There were no clear differences between the two types of fishing line used, although the more flexible Dyneema monofilament (FL1) appeared to have marginally lesser impacts and was considerably easier to manipulate during the tagging process. These results provide encouraging guidelines to develop tagging methods for echinoids to allow the application of acoustic telemetry to address key ecological questions in coastal marine habitats.
Adapting echinoid tagging methods for acoustic telemetry
A key issue with adapting tagging methods for use in acoustic telemetry studies is that adding the transmitter to previous external tagging methods alters the effects of tagging by changing the dynamics of how the tag moves, hangs, or drags upon the attachment point. Nylon screws were the least successful method tested in our study, with most tags being lost in a very short period. This contrasts with the results of Duggan and Miller [29], who showed the nylon screw tagging method to have > 50% retention after 3 months. Another concern with evaluating sub-lethal tagging effects is ensuring that the temporal scale of the evaluation corresponds to the proposed scale of movement observations. For example, although T-bar tags showed minimal tag losses, they caused persistent injuries, significant mortality and clearly affected condition and behaviour in the second and third months of the study. Several previous non-telemetry studies have also remarked that T-bar tags prevent recalcification of the insertion hole in the test and can cause persistent injury [28, 29, 35]. Our lab results were confirmed in the field where T-bar tags were both lost at a higher rate and altered movement behaviour.
Tagging effects: who to tag and what to measure?
Evaluations of sub-lethal effects must be clearly thought out to be applicable and have a chance of detecting negative effects. An indicator of condition that is often relied upon for marine invertebrates is investment in reproduction through a measure of gonad mass or gonad index. However, tagged urchins maintained investment in reproduction while showing evidence of physiological stress through reductions in total wet weight in the present study. Organisms must allocate available energy to many possible processes and when resources are limited or individual condition impacted, the allocation of available resources to different aspects of physiology and behaviour can change. The observed continued investment in reproduction concurrent with increased righting times and decreased total weight indicate that urchins were physiologically stressed but maintained investment in gonad mass, at the expense of other processes. Metrics chosen to assess tagging effects should be evaluated with care and explicitly justified in any evaluation of sub-lethal effects. Our lab results show that measuring only tag retention and mortality failed to capture the effect that some tagging methods had on health and behaviour. We recommend that multiple metrics be assessed to cover both short and long-term effects, reproductive allocation, injury, and behaviour.
A comprehensive review of urchin tagging methods used to measure growth concludes that internal tagging should be avoided [35]. We agree that, where it is possible to obtain observations pertaining to a particular question without using intrusive internal tagging methods, other approaches should be prioritized. However, there are questions about behaviour and movement, particularly on large temporal and spatial scales, that do not lend themselves to direct observations or external or chemical tagging methods. We argue that surgical attachment of an acoustic transmitting tag, as in the present study, has minimal negative effects on the movement behaviour of individuals and is an acceptable compromise between impact on individuals and detailed large-scale behavioural observations of echinoderms such as urchins. It is critical that future acoustic telemetry work on marine invertebrates be conscious of non-lethal tagging effects, but the benefit of high-resolution temporal data is worth exploiting.
Extrapolating these results to other echinoderms
Differences between urchins, which have a test, and more soft-bodied echinoderms, such as sea stars and sea cucumbers, will require adjustments in tagging procedures. However, previous work using surgically implanted PIT tags [36, 37] indicates that surgical tagging of other echinoderms is possible. Our results suggest that methods which minimize persistent injuries will be the least impactful in terms of sub-lethal effects. If possible, the method of attachment should rely on something flexible which does not inhibit healing, such as the fishing line we used, which remained in place and did not rub or otherwise irritate the entry and exit points where it passed through the test. A variation on the fishing line method of tagging applied here could be adapted for other echinoderms. Indeed, several studies have already successfully used similar surgical methods for short-term studies of sea star behaviour [23, 38, 39]. A concern when adapting this method to other species will be ensuring that the tag is held in a position that remains above the substratum as much as possible. Green sea urchins are active benthic herbivores, and are found widely distributed across the benthic substratum. Acoustic telemetry relies upon the receiver array being able to hear the signal emitted by the tag and any physical obstruction between the tag and the receiver can block the signal to a greater or lesser extent. This will be of particular concern when adapting this tagging method for echinoderms which hide in crevices for a large proportion of the time or burrow into the bottom. When adapting this method for species displaying cryptic or endobenthic habits, care should be taken to test the detectability of tags in representative situations.
Applying acoustic telemetry to slow-moving invertebrate populations in the field: a question of scale
Unlike for species with much larger ranges of movement, marine invertebrates often move on scales that are close to the positioning error acoustic telemetry arrays provide in challenging field conditions. For example, previous estimates of green sea urchin movement range from up to 3 m in 24 h [40] to 4 m in one month [41] and many factors, including urchin size [42], food availability and substrata composition [43], have been shown to influence rates of movement. Net displacement for tagged urchins at our field site was between 0.5 m and 3 m in 2 days, which is within the range of previous estimates. However, movement on this scale, combined with site and array characteristics that introduced error into positioning estimates, meant that urchins would have had to display a net displacement of at least 30–40 m to clearly distinguish a change in position from error in this array. This means that a minimum of 80 days would be necessary to detect urchin movement, and that our field test of 72 days was not long enough to allow clear detection of urchin movement behaviour. TB urchins occupied less space overall than FL1 tagged urchins. Our reference tag on the bottom, however, shows an estimated area of occupation which overlaps with both tagging methods, indicating that positioning error of the array is large compared to urchin movement and complicating the separation of lost tags from still-attached tags on slowly moving individuals. It will be critical for studies that tag organisms with slow movement or sedentary behaviours to control array error as much as possible and develop methods of separating lost tags from slow-moving or immobile tagged individuals. There are, however, many ways to reduce array error, both through maximizing array performance and through application of newer acoustic technology such as the HR2 High Residency Acoustic Receivers newly available from Innovasea, which will allow finer analysis of the movement behaviour of slow and sedentary marine invertebrates. These concerns make the choice of tagging method especially critical, as insuring high tag retention and minimal effects on tagged individuals will increase confidence in the analysis of fine-scale movement behaviour.
Initially, both tagging methods were successful in the field and diving observations confirmed individuals moved. In the lab, it was only after a considerable amount of time had passed that the persistent injuries caused by the T-bar tags began to have deleterious effects. Likewise, in the field, TB urchins appear to have lost tags through time and showed long-term altered behaviour relative to FL urchins. Combined, these tests provide a proof of concept for using acoustic telemetry to examine not only urchin, but also other marine invertebrate behaviour over larger spatial and temporal scales than has previously been possible.
Conclusions
The information to be gained by using acoustic telemetry to examine marine invertebrate movement behaviour has enormous implications. Little is known about many economically and ecologically important invertebrate species’ movement behaviours, particularly over large spatial and temporal scales. Questions and hypotheses that could be addressed through the application of acoustic telemetry to echinoids are abundant, particularly questions that rely on individual movement data such as the allometric relationship between body size and movement and temporal patterns of individual movement such as periods of sedentary behaviour followed by periods of fast or active movement. In addition, hypotheses about the influence of environmental parameters (e.g., tidal water movement, substratum, season or habitat) on individual movement behaviour are ideally suited to being answered using acoustic telemetry. Having a reliable tagging method and understanding the sub-lethal impacts of the tagging procedure are key to moving forward and beginning to address such questions.
Availability of data and materials
Additional Supporting Information may be found in the online version of this article. The datasets generated and/or analysed during the current study will be available in the Open Data repository, the Government of Canada Open Data portal (http://open.canada.ca).
Abbreviations
- HPE:
-
Position Error Sensitivity values
- FL1:
-
Dyneema® fishing line
- FL2:
-
Nickel titanium fishing line
- TB:
-
T-bar tags
- NS:
-
Nylon screws
- MLI:
-
Maurice-Lamontagne Institute
References
Turchin P. Translating foraging movements in heterogeneous environments into the spatial distribution of foragers. Ecology. 1991;72:1253–66. https://doi.org/10.2307/1941099.
Nathan R, Getz WM, Revilla E, Holyoak M, Kadmon R, Saltz D, et al. A movement ecology paradigm for unifying organismal movement research. Proc Natl Acad Sci USA. 2008;105:19052–9. https://doi.org/10.1073/pnas.0800375105.
Stephens DW, Krebs JR. Foraging Theory. Princeton: Princeton University Press; 1986.
Dingle H. Migration: the biology of life on the move. Oxford: Oxford University Press; 1996. https://doi.org/10.1016/S0160-9327(97)84881-8.
Deakin Z, Hamer K, Sherley R, Bearhop S, Bodey T, Clark B, et al. Sex differences in migration and demography of a wide-ranging seabird, the northern gannet. Mar Ecol Prog Ser. 2019;622:191–201. https://doi.org/10.3354/meps12986.
Dewar H, Prince ED, Musyl MK, Brill RW, Sepulveda C, Luo J, et al. Movements and behaviors of swordfish in the Atlantic and Pacific Oceans examined using pop-up satellite archival tags. Fish Oceanogr. 2011;20:219–41. https://doi.org/10.1111/j.1365-2419.2011.00581.x.
Massé A, Côté SD. Linking habitat heterogeneity to space use by large herbivores at multiple scales: from habitat mosaics to forest canopy openings. For Ecol Manage. 2012;285:67–76. https://doi.org/10.1016/j.foreco.2012.07.039.
Godfrey JD, Bryant DM. Effects of radio transmitters: Review of recent radio-tracking studies. Sci Conserv. 2003;214:83–95.
Bodey TW, Cleasby IR, Bell F, Parr N, Schultz A, Votier SC, et al. A phylogenetically controlled meta-analysis of biologging device effects on birds: Deleterious effects and a call for more standardized reporting of study data. Methods Ecol Evol. 2018;9:946–55. https://doi.org/10.1111/2041-210X.12934.
Wilson RP, McMahon CR. Measuring devices on wild animals: What constitutes acceptable practice? Front Ecol Environ. 2006;4:147–54. https://doi.org/10.1890/1540-9295(2006)004[0147:MDOWAW]2.0.CO;2.
Sun A, Whelan S, Hatch S, Elliott K. Tags below three percent of body mass increase nest abandonment by rhinoceros auklets, but handling impacts decline as breeding progresses. Mar Ecol Prog Ser. 2020;643:173–81. https://doi.org/10.3354/meps13341.
Rasiulis AL, Festa-Bianchet M, Couturier S, Côté SD. The effect of radio-collar weight on survival of migratory caribou. J Wildl Manage. 2014;78:953–6. https://doi.org/10.1002/jwmg.722.
Kissling WD, Pattemore DE, Hagen M. Challenges and prospects in the telemetry of insects. Biol Rev. 2014;89:511–30. https://doi.org/10.1111/brv.12065.
Batsleer F, Bonte D, Dekeukeleire D, Goossens S, Poelmans W, Van der Cruyssen E, et al. The neglected impact of tracking devices on terrestrial arthropods. Methods Ecol Evol. 2020;11:350–61. https://doi.org/10.1111/2041-210x.13356.
Lennox RJ, Cooke SJ, Davis CR, Gargan P, Hawkins LA, Havn TB, et al. Pan-Holarctic assessment of post-release mortality of angled Atlantic salmon Salmo salar. Biol Conserv. 2017;209:150–8. https://doi.org/10.1016/j.biocon.2017.01.022.
Hussey NE, Kessel ST, Aarestrup K, Cooke SJ, Cowley PD, Fisk AT, et al. Aquatic animal telemetry: a panoramic window into the underwater world. Science. 2015;348:1255642. https://doi.org/10.1126/science.1255642.
Florko K, Davidson E, Lees K, Hammer L, Lavoie M, Lennox R, et al. Tracking movements of decapod crustaceans: a review of a half-century of telemetry-based studies. Mar Ecol Prog Ser. 2021;679:219–39. https://doi.org/10.3354/meps13904.
Donaldson MR, Hinch SG, Suski CD, Fisk AT, Heupel MR, Cooke SJ. Making connections in aquatic ecosystems with acoustic telemetry monitoring. Front Ecol Environ. 2014;12:565–73. https://doi.org/10.1890/130283.
Bruce B, Harasti D, Lee K, Gallen C, Bradford R. Broad-scale movements of juvenile white sharks Carcharodon carcharias in eastern Australia from acoustic and satellite telemetry. Mar Ecol Prog Ser. 2019;619:1–15. https://doi.org/10.3354/meps12969.
Coates JH, Hovel KA, Butler JL, Klimley AP, Morgan SG. Movement and home range of pink abalone Haliotis corrugata: Implications for restoration and population recovery. Mar Ecol Prog Ser. 2013;486:189–201. https://doi.org/10.3354/meps10365.
Dujon AM, Stieglitz TC, Amice E, Webber DM. Snail leaps and bounds: drivers of the diel movement pattern of a large invertebrate, the Caribbean queen conch (Lobatus gigas), in a marginal inshore habitat. Can J Zool. 2019;97:436–45. https://doi.org/10.1139/cjz-2018-0106.
Lees K, Mill A, Skerritt D, Robertson P, Fitzsimmons C. Spatial overlap, proximity, and interaction between lobsters revealed using acoustic telemetry. Mar Ecol Prog Ser. 2020;645:109–24. https://doi.org/10.3354/meps13376.
Miyoshi K, Kuwahara Y, Miyashita K. Tracking the Northern Pacific sea star Asterias amurensis with acoustic transmitters in the scallop mariculture field of Hokkaido. Japan Fish Sci Springer Japan. 2018;84:349–55. https://doi.org/10.1007/s12562-017-1162-5.
Scheibling RE, Feehan CJ, Hatcher BG. Chapter 31-Strongylocentrotus droebachiensis. In: Lawrence JM, editor. Dev Aquac Fish Sci. Elsevier; 2020. p. 553–91. https://doi.org/10.1016/B978-0-12-819570-3.00031-7.
Filbee-Dexter K, Scheibling RE. Sea urchin barrens as alternative stable states of collapsed kelp ecosystems. Mar Ecol Prog Ser. 2014;495:1–25. https://doi.org/10.3354/meps10573.
Johnson LE, MacGregor KA, Narvaez CA, Suskiewicz TS. Subtidal rocky shores of the northwest Atlantic Ocean: The complex ecology of a simple ecosystem. In: Williams GA, Bohn K, Firth LB, Hawkins SJ, editors. Interact Mar Benthos. Cambridge: Cambridge University Press; 2019.
Ebert TA. A technique for the individual marking of sea urchins. Ecology. 1965;46:193–4.
Clemente S, Hernández JC, Brito A. An external tagging technique for the long-spined sea urchin Diadema aff. antillarum. J Mar Biol Assoc UK. 2007;87:777–9. https://doi.org/10.1017/S0025315407053349.
Duggan RE, Miller RJ. External and internal tags for the green sea urchin. J Exp Mar Bio Ecol. 2001;258:115–22. https://doi.org/10.1016/S0022-0981(01)00213-1.
Kleitman N. The effect of temperature on the righting of echinoderms. Biol Bull. 1941;80:292–8. https://doi.org/10.2307/1537716.
Therneau TM. A package for survival analysis in R. R package version 3.1–12; 2020. https://cran.r-project.org/package=survival.
Venables WN, Ripley BD. Modern Applied Statistics with S. 4th ed. Oxford: Springer; 2002.
R Core Team. R: A language and environment for statistical computing. R Foundation For Statistical Computing, Vienna, Austria; 2020. https://www.R-project.org/.
Lenth R V., Buerkner P, Herve M, Love J, Singmann H. Package ‘emmeans.’ 2022. https://github.com/rvlenth/emmeans.
Ebert TA. Chapter 7-Growth and survival of postsettlement sea urchins. In: Lawrence JM, editor. Developments in aquaculture and fisheries science. 2020;43:95–145. https://doi.org/10.1016/B978-0-12-819570-3.00007-X.
Gianasi BL, Verkaik K, Hamel J-F, Mercier A. Novel use of PIT tags in sea cucumbers: Promising results with the commercial species Cucumaria frondosa. PLoS ONE. 2015;10:127884. https://doi.org/10.1371/journal.pone.0127884.
Rodríguez-Barreras R, Sonnenholzner J. Effect of implanted PIT-tags on growth, survival, and tag retention in the sea urchin Tripneustes ventricosus. Caribb J Sci. 2014;48:132–7. https://doi.org/10.18475/cjos.v48i3.a02.
Lamare MD, Channon T, Cornelisen C, Clarke M. Archival electronic tagging of a predatory sea star—Testing a new technique to study movement at the individual level. J Exp Mar Bio Ecol. 2009;373:1–10. https://doi.org/10.1016/j.jembe.2009.02.010.
Chim CK, Tan KS. A method for the external attachment of acoustic tags on sea stars. J Mar Biol Assoc UK. 2013;93:267–72. https://doi.org/10.1017/S0025315411002128.
Garnick E. Behavioral ecology of Strongylocentrotus droebachiensis (Muller) (Echinodermata: Echinoidea): aggregating behavior and chemotaxis. Oecologia. 1978;37:77–84. https://doi.org/10.1007/BF00349993.
Scheibling RE, Hennigar AW, Balch T. Destructive grazing, epiphytism, and disease: the dynamics of sea urchin-kelp interactions in Nova Scotia. Can J Fish Aquat Sci. 1999;56:2300–14. https://doi.org/10.1139/cjfas-56-12-2300.
Dumont CP, Himmelman JH, Russell MP. Size-specific movement of green sea urchins Strongylocentrotus droebachiensis on urchin barrens in eastern Canada. Mar Ecol Prog Ser. 2004;276:93–101. https://doi.org/10.3354/meps276093.
MacGregor KA, Johnson LE. Seascapes and foraging success: movement and resource discovery by a benthic marine herbivore. Ecol Evol. 2022. https://doi.org/10.1002/ece3.9243.
Acknowledgements
We thank our colleagues at the St. Andrews Biological Station for logistical support during the field test, particularly Andrew Cooper and Jonny Day. A top-notch dive team made fieldwork in challenging conditions possible; particular thanks to Frédéric Hartog, Anne-Sara Sean, and Jean-Daniel Tourangeau-Larivière. For invaluable help in the lab, we thank Fany Sardenne and Charlotte Carrier-Belleau. We thank Cooke Aquaculture for access to field sites. All co-authors declare no conflicts of interest. We acknowledge that gains in contemporary knowledge invariably build on a history of race, gender, and sexual orientation discrimination.
Funding
Funding for this research was provided by Fisheries and Oceans Canada.
Author information
Authors and Affiliations
Contributions
KM, CM, ES and SR conceived the ideas and designed methodology; KM, ES and M-FL collected the data; KM and M-FL analysed the data; KM led the writing of the manuscript and prepared all figures. All authors contributed critically to drafts and gave final approval for publication. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shawn Robinson has retired from Biological Station, Fisheries and Oceans Canada.
Supplementary Information
Additional file 1:
Details of tagging methods. Figure S1. Number of nylon screw tagged urchins (green dashed line) and number of hole urchins (orange solid line) through time (days post-tagging), showing that hole urchins are almost entirely composed of nylon screw tagged urchins that have lost their tags (sum of the two groups is always equal or close to the 80 individuals initially tagged with nylon screws). Figure S2. The effect of including more points (increasing the percentage included) on minimum convex polygon area. Estimated areas for 10% to 90% of points are shown, coloured by month of observation. Vertical dashed line indicates the chosen cut-off point (75%) where the small percentage of points very far from the majority cause a steep increase in estimated area. This should ensure that large-error points are excluded from these estimates, while retaining points which represent actual movement and space use by individual urchins. Figure S3. Larger urchins take more time to right themselves than smaller urchins (significant effect of diameter), although there is significant variability between individuals and measures; points and predictions are from Control urchins only. Table S1. Parameter estimates (beta coefficients) from models, with 95% confidence intervals. Figure S4. Test diameter (mm) of urchins before tagging and at the end of the experiment after 3.5 months. Because of mortality and tag loss, the number of individuals in each group after 3.5 months is less than at the beginning, except for the Hole treatment. Significant differences between tagging treatments from post-hoc comparisons are shown with different letters. Figure S5. Gonad weight as a function of tagging treatment. There were no significant differences between tagging treatments. Figure S6. Relationship between gonad wet weight and test diameter for all tagging treatments. Model results (predicted values and 95% Confidence Intervals) are shown. Figure S7. Injuries observed through time as a function of tagging method. No mortality and only two injuries were observed in control urchins. Because injuries could only be followed and definitively assigned to a tagging method as long as the tag was attached, the number of urchins removed from these observations at each time period is shown as the category “Tag lost or urchin dead”. For example, the small number of injuries assigned to the Nylon screw tagging method is a function of the high mortality and high tag loss seen in this treatment; these urchins are also shown in the Hole tag treatment here, as urchins with Very apparent injuries. Figure S8. Net displacement of tagged individuals after 2 days in the field calculated from diver observations. Figure S9. Number of observations per tagged individual. The two tagging methods are shown as different shapes and colours, while release point ‘a’ and ‘b’ are the top and bottom panels, respectively. Points are shown for the total number of observations filtered by the positioning error (HPE). All urchins have at least one point (not filtered: largest and palest point) although for certain individuals that were never detected by the array (ID 10 and 11) this is zero. Successively darker and smaller points are filtered by lower HPE values. Size of the points is scaled by the HPE filter (points filtered by larger values, are larger). Note the different y-axis scales for the two panels. Figure S10. Minimum convex polygons (MCPs) per month for each individual. Points are all detections (HPE < 200), polygons are monthly 75% MCPs and numbers in the upper right of each panel are the MCP area per month in m2. a) Fishing line tagged urchins and b) T-bar-tagged urchins. The detached reference tag is shown in both a and b (id #31) for comparison.
Additional file 2:
Select bibliography of echinoderm tagging references.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
MacGregor, K.A., Lavoie, MF., Robinson, S.M.C. et al. Lab and field evaluation of tagging methods for the use of acoustic telemetry to observe sea urchin movement behaviour at ecologically relevant spatio-temporal scales. Anim Biotelemetry 11, 3 (2023). https://doi.org/10.1186/s40317-022-00309-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40317-022-00309-8