Abstract
Background
Soft-bodied marine invertebrates comprise a keystone component of ocean ecosystems; however, we know little of their behaviors and physiological responses within their natural habitat. Quantifying ocean conditions and measuring organismal responses to the physical environment is vital to understanding the species or ecosystem-level influences of a changing ocean.
Results
Here we describe a novel, soft-bodied invertebrate eco-sensor tag (the ITAG), its trial attachments to squid and jellyfish, and the fine-scale behavioral measurements recorded on captive animals. Tags were deployed on five jellyfish (Aurelia aurita) and eight squid (Loligo forbesi) in laboratory conditions for up to 24 h. Using concurrent video and tag data, movement signatures for specific behaviors were identified. These behaviors included straight swimming (for jellyfish), and finning, jetting, direction reversal and turning (for squid). Overall activity levels were quantified using the root-mean-squared magnitude of acceleration, and finning was found to be the dominant squid swimming gait during captive squid experiments. External light sensors on the ITAG were used to compare squid swimming activity relative to ambient light across a ca. 20-h trial. The deployments revealed that while swimming was continuous for captive squid, energetically costly swimming behaviors (i.e., jetting and rapid direction reversals) occurred infrequently. These data reflect the usefulness of the ITAG to study trade-offs between behavior and energy expenditure in captive and wild animals.
Conclusions
These data demonstrate that eco-sensors with sufficiently high sampling rates can be applied to quantify behavior of soft-bodied taxa and changes in behavior due to interactions with the surrounding environment. The methods and tool described here open the door for substantial lab and field-based measurements of fine-scale behavior, physiology, and concurrent environmental parameters that will inform fisheries management, and elucidate the ecology of these important keystone taxa.
Similar content being viewed by others
Background
Soft-bodied invertebrates such as squid and jellyfish play critical roles in many ocean ecosystems. They are broadly distributed and often centrally located within food webs as predators and prey to a wide array of taxa across multiple trophic levels [1, 2]. Occasionally cited as keystone taxa, some squid and jellyfish species provide a crucial link between apex predators (e.g., marine mammals, turtles, sharks, tunas, billfish) and the invertebrates’ smaller pelagic, mesopelagic and planktonic prey [3–6]. Additionally, both taxa are an increasingly important fisheries resource. Jellyfish fisheries in 15 different countries yield 425,000 tons per year based on catches reported between 1996 and 2005 [7]. Globally, cephalopods are a vital fishery, comprising about 20 % of global total fisheries landings and landing values [1, 8, 9].
There is increasing evidence that changing environmental conditions may have a variety of influences on squid and jellyfish behavior, physiology and ecology. Several species of squid and jellyfish are undergoing poorly understood range expansions [10–12], and their abundance and voracious foraging are altering ecosystems through predation of available nekton and fishes [13–15]. Additional responses to changing environmental conditions may consist of more nuanced behavioral and physiological changes. For example, decreased temperatures or increased pCO2 levels can induce delays in squid embryo development [16, 17]. Vertical movements and respiratory patterns of the Humboldt (jumbo) squid Dosidicus gigas appear tied to both dissolved oxygen and local environmental temperatures [18–20]. Some medusae may undergo reverse development when environmental conditions are reproductively unfavorable, enabling “temporal persistence” and longer foraging periods [21]. Understanding these changes is vital to properly manage soft-bodied invertebrates fisheries (i.e., ecosystemically), as well as other fisheries and systems where these species play a key ecological role [22, 23].
Yet, quantifying these organismal changes relative to the local environment has been a challenge. Jellyfish and squid are incredibly diverse, and they inhabit a range of environmental and physical regimes. Biologging tags can offer a way to track animals and environmental conditions in situ. Unfortunately commercially available tools have often been limited by low sampling rates or bottlenecked in tag-to-satellite transmission capabilities. As a result, many fine-scale behaviors (i.e., swimming, turning, accelerations, respiration rates) are not discernible in these data records [24]. Measurements of such responses would elucidate the natural swimming modes, predation behaviors, and metabolic rates of squid and jellyfish. Further, environmental measures are often made in the broad area but not at immediate surroundings of the animal [20], potentially obscuring vital fine-scale animal–environment interactions with conditions such as thin layers, thermoclines or oxygen minimum zone boundaries. Even with state-of-the-art oceanographic equipment such as CTDs and Argo Floats, we often lack fine-scale habitat measures, particularly in coastal areas, at scales relevant to soft-bodied organisms.
Finally, attachment to these animals has been challenging due to their innate body design and relative fragility. A few invertebrate tagging efforts have shown promise by successfully attaching off-the-shelf tags to collect novel behavior such as jellyfish vertical excursions [25–27]. While low sampling rates (every 1–5 min) limit any assessment of fine-scale behaviors associated with habitat structure, higher sampling rates will allow new insights into the behavior of jellyfish and other taxa [28]. In addition, tags were typically affixed to jellyfish by attaching plastic cable-ties and lines to oral arms; this attachment technique cannot be applied across many jellyfish taxa where these morphological features are absent. Large Humboldt squid (D. gigas) were tagged using relatively large depth-temperature satellite transmitting tags (with ~1 Hz resolution) and “Crittercam” video packages [20]. Reducing the size of the tagging package will enhance the range of available species and age/size classes available for study, and measurements at higher sampling frequencies (>60 Hz) will help to enable the characterization of animal swimming modes, predation behaviors, and metabolic rates of squid and jellyfish.
The purpose of this work was threefold: first, design and develop a modular, relatively small tag platform applicable to a potentially diverse array of soft-bodied invertebrates. Second, use this platform to integrate high-resolution movement data with fine-scale environmental measurements. Third, test the tool on representative species of the target taxa of jellyfish and squid. To this end, a novel soft-bodied invertebrate tag (ITAG) was developed to record fine-scale animal movement, orientation, dive profiles, and external environmental conditions (light and temperature). The attachment methods and initial data from captive deployments on both jellyfish (Aurelia aurita) and squid (Loligo forbesi) are described here. These initial measurements were paired with video recordings to characterize specific behavioral signatures recorded by the tag. The results reveal a suite of behaviors and validate the ability of this new tool to elucidate energetic trade-offs between recorded swimming behaviors. The goal of the paper was to describe these steps in initial development of the tag and some preliminary outcomes.
Methods
ITAG design overview
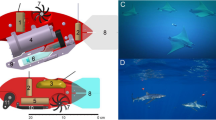
The tag design leverages modularity to enable the ITAG to be deployed across taxa. Specifically, the ITAG consists of two components (Fig. 1): (a) a buoyant waterproof eco-sensor package that records behavioral and environmental data, and (b) a base that serves as the point of attachment to the animal. The tag electronics are a universal component, while the base and attachment method are animal specific. This modular format allows the same tag electronics to be used with a range of animals with varying morphologies.
The tag was designed with the shape, behavior, and propulsive modes of squid and jellyfish in mind. Squids swim utilizing high-acceleration jet propulsion or by rowing of their fins [29], and their hydrodynamically shaped bodies maximize propulsive efficiency by reducing drag. Large jellyfish predominantly utilize rowing propulsion [30], which is characterized by lower acceleration rates when compared to jet propulsion [31]. In addition, large jellyfish possess bluff, less hydrodynamically streamlined body shapes that are more optimized for efficient swimming [30, 31]. On squid, the ITAG is attached to the dorsal surface of the squid rostrum, and positioned in line with the squid body to reduce drag. On jellyfish, the tag is attached to the dorsal surface of the bell. In this configuration, the low height and minimal aspect ratio of the tag reduces flow separation along the surface of the bell (Fig. 1a, b).
ITAG electronics module
The ITAG electronics are based on the marine mammal acoustic behavior DTAG [32]. The governing constraints for the ITAG design were that it should be small, lightweight, neutrally buoyant in seawater, pressure tolerant, and capable of ca. 24 h deployments, with the consideration that deployment durations could be increased in the future. The result is a modular 108.4 mm × 64.0 mm × 28.7 mm package (Fig. 1), with a Li-ion rechargeable battery, external sensors, and a radio antenna embedded in a syntactic foam frame that is sealed in urethane rubber. To avoid a bulky and heavy pressure housing, the ITAG circuit boards are oil-filled with a flexible bladder. The electronics are designed around a low-power programmable digital signal processor (DSP), which combines data from the sensors and stores that information on a memory array. Use of a DSP enables real-time filtering and loss-less compression of signal streams when required. The ITAG currently has 3-axis accelerometer (KXSC7-1050, Kionix, NY, USA), 3-axis magnetometer (HMC1043LMC1021 Honeywell, MA, USA), pressure (Series 1 TAB, Keller, VA, USA) temperature (USP10975, U.S. Sensor Corp, CA, USA), and light sensors (PDV-P8104, Advanced Photonix, Inc, MI, USA). Orientation, which is parameterized by the Euler angles pitch, roll, and heading, requires two sets of sensors; pitch and roll are calculated using data from the accelerometers, and heading is calculated from the magnetometer. To improve the heading estimate, the three magnetometer signals are corrected for pitch and roll. This process, called gimballing, effectively transforms the magnetic-field measurement to that which would be made on a horizontal surface with the same heading. ITAG power consumption is approximately 50 mW while recording, and the operating duration is currently limited by battery size. As configured, the tag can collect high-resolution behavior and environmental data for deployments greater than 30 h. An embedded VHF beacon is then used to locate the tag after release and surfacing and can operate for over 7 days following the end of the recording period (Table 1).
ITAG base module
Squid musculature lends itself to relatively invasive tag attachment mechanisms using needle pins to puncture the mantle or fin [33]. If a tag is mounted internally, there is high risk of damaging internal organs; certain external attachment may impede swimming by minimizing fin motion or cause abrasion to the skin [34, 35]. These mounting issues are exacerbated by a large tag package. Using the first generation ITAG and VEMCO dummy tags (V16, 98 mm, 16 g, NS, Canada), the locations and attachment mechanisms for captive squid Loligo forbesi were evaluated during preliminary trials conducted in March 2013 (VEMCO) and April 2014 (ITAG) at the University of the Azores in Horta, Portugal (Fig. 2). Mounting a hydrodynamic tag externally close to the posterior tip of the mantle reduced internal-damage and swim-impedance issues. The presence of dense muscle and cartilage at the mantle ensured secure suture attachment and reduced external tissue damage. VEMCO tags were attached for up to 1 week on three large squid (mantle lengths 45, 39, and 47 cm) without any impairment of swimming movement or causing any obvious serious tissue injury in the short term. The ITAG squid base utilizes a silicone pad, ca. 2 mm in thickness, as a compliant interface between the tag electronics and the animal to minimize tissue abrasion. A rigid plastic spine is embedded in the silicone to provide the point of attachment to the tag electronics module. Additionally, the silicone pad has four pairs of 1 mm diameter holes at each end that allow for easy attachment via absorbable sutures (catgut, 3:0) to the dorsal surface of the animal near the rostrum (Fig. 1a).
The delicate nature of jellyfish tissue is particularly challenging for tag attachment. Consequently, tags are limited in size and capability [36]. Jellyfish tagging has had significant success in very large jellyfish with oral arms where tags can be attached using plastic cable-ties [25, 26]. Unfortunately, tag placement on the oral arms yields noisy acceleration data due to fluid-induced motion of the tag when interacting with feeding currents and propulsive jets, and cannot be used for many species where such morphological features are absent. Attachment of tags on the dorsal surface of the jellyfish bell can facilitate acceleration measurements that yield information about swimming cycles and energetics. However, pins and sutures easily tear gelatinous tissue, and result in short attachment periods. As such, the jellyfish ITAG base is a solid urethane platform with a rigid plastic spine and four recessed suction cups (Fig. 1b). The gentle suction provided by the cups on the apex of the bell allows for secure attachment, and the plastic spine connects the tag to the base.
In both the squid and jellyfish configurations, two corrodible, nickel–chromium wires are used to couple the electronics module to the base module. The tag can be programmed to send an electric current through these wires to activate fast corrosion and release after a specified recording duration (e.g., 1.5, 8, 24 h). When the tag and base are coupled, the ITAG is neutrally buoyant. Once the tag releases from the base, the positively buoyant ITAG floats to the surface holding a vertical position, allowing the VHF radio antenna to break the surface of the water and transmit its location.
Jellyfish trials
Laboratory investigations of attachment methods and deployments of the ITAG on jellyfish were conducted between August and November 2013 at the Marine Biological Laboratory and the Environmental Systems Laboratory in Woods Hole, MA. Initial tests of attachment methods were conducted on captive Cyanea capillata and Chrysaora quinquecirrha (collected in Vineyard Sound). ITAG deployments were conducted in a 182.8 cm diameter tank (1 m depth) on five Aurelia aurita (provided by the New England Aquarium) of approximately 17–22 cm bell diameter (Fig. 2a). Due to the limited bell size, neutrally buoyant ITAGs were affixed to five jellyfish in water using two of the four suction cups on the dorsal surface of the jellyfish bell near the apex, similar to an approach used in box jellyfish (Chironex fleckeri) [37]. Using the tags and simultaneous video recordings (Canon 7D, Japan) of attachment, release, and swimming behavior, observations of jellyfish swimming behavior were correlated with the accelerometer outputs. Data were later analyzed using custom Matlab algorithms.
Squid trials
Squid ITAG trials were conducted between March 15 and April 1, 2014 at the Porto Pim Aquarium, a public facility run by Flying Sharks in Faial Island, Azores, Portugal (Fig. 2b). Squid (L. forbesi) were fished at the island slope (200 m) by hand jigging. Animals were delivered to the facility via boat in coolers filled with running seawater. After capture and transport, animals were placed in a 4 m × 8 m oval holding tank of 4 m depth containing 47 tons of filtered seawater, where they rested between 2 and 24 h before being recaptured (using soft hand-nets) for tag attachment. Water in the facility was pumped in from the adjacent Atlantic and maintained at 17.7 ± 0.9 °C. A series of initial mechanical (centrifugal), pressurized sand filters, tower protein skimmers and UV filtration were used to filter ca. both the input and tank water which aerated the water and allowed for ca 77.7 % of water to be filtered per hour. Salinity and pH were maintained at 35.55 ppt and 7.86, respectively. ITAGs were deployed on eight individual squid with an average mantle length of 58 cm (52 cm min; 69 cm max). Animals were taken out of the holding tank, placed on a v-shaped padded tagging table, and their gills were ventilated with flowing seawater during tagging. The tag base was sutured near (5–10 cm from the end) the posterior tip of the squid dorsal mantle with the tag affixed, and animals were given a 15 min acclimatization period in a small soft and black plastic raft before being released back into the holding tank. Animals were out of the water for a mean 08:49 mm:ss (06:15 mm:ss min; to 11:00 ms:ss max). There were no obvious changes in swimming behavior due to tag attachment.
After tag attachment and a subsequent acclimatization period, animals were released back into the holding tank with conspecifics, tope sharks (Galeorhinus galeus), and smaller “prey” fish. Immediately after release into the holding tank, tagged squid schooled with conspecifics, exhibiting normal coloration patterns and body positioning. Other conspecifics did not show aggression or additional interest toward the tagged animals. Mean durations of ITAG recordings were 20:43:51 (hh:mm:ss) but up to 24:28:49. Tags separated from the base only as result of the timed release mechanism (i.e., no tags released prematurely) and thus attachments could have been extended.
In addition to tag data, three overhead, high-definition video cameras (GoPro, CA, USA) and two sideways high-definition video cameras (Sony HDR-XR550, Tokyo, Japan) located at the ends of the holding tank concurrently recorded specific behaviors of tagged and untagged animals. Video cameras were synchronized with each other using successive flashes of an external camera flash (Canon, NY, USA), and tag data were synchronized by recording the arming of the tag with one of the five video cameras. Using the tags and simultaneous video recordings of tag attachment, animal release and swimming behavior, it was possible to coordinate camera-observed behaviors such as forward and backward finning, direction reversal, jetting, and gradual, lateral turns with ITAG sensor data. Finning was defined as movement where fins were predominantly moving and strong mantle contractions were not obvious. During jetting, fins were typically held near the ventral surface of the mantle or not extensively used. Camera recordings were limited to ca. 90 min due to the memory limitations of the cameras. Data were later analyzed using custom Matlab algorithms.
Additionally, we used the acceleration data to quantify the animal’s overall activity level using one data set for jellyfish and one data set for squid to describe initial outcomes and sensor analyses. Because the goal of the paper was describing the tag design, development and initial outcomes, sensor analyses are presented primarily from one animal. A biological assessment of behavioral trends of captive organisms compiled from all eight tags deployments is currently underway and not included here. Data were first separated into periods of light and dark using light sensor data. Next root mean square (RMS) values for the absolute value of the acceleration magnitude were calculated on an hourly basis. These hourly values of acceleration RMS were averaged to produce a mean and standard deviation to represent the overall activity of the squid during periods of light and dark. Only data when the squid was free of experimental manipulation was used in this overall activity analysis. The mean, standard deviation, and hourly rate of movements that resulted in peak acceleration rates exceeding 0.15 g were also used to compare the relative activity of the animal during the tagging. This threshold was selected after reviewing the tag and video data.
Results and discussion
Jellyfish trials
Initial laboratory investigations of attachment methods on the captive Cyanea capillata and Chrysaora quinquecirrha found that soft, thin-walled silicone suction cups can be attached to the central, relatively firm dorsal surface of a jellyfish bell up to 4 h at a time. Attachment duration was enhanced with 3M Vetbond, a veterinary-grade cyanoacrylate that cures upon contact with water. By employing both suction cups and 3M Vetbond, cups remained attached without any slippage to the dorsal surface of jellyfish bells for over 24 h, eventually sloughing off in approximately 1 week (n = 5).
The subsequent ITAG deployments on A. aurita captured specific jellyfish behaviors: remaining stationary, swimming in a straight line, and turning. The accelerometer data for an entire recording period of a single deployment of 160 s, which includes arming of the tag, attachment, animal release, and recovery of the animal, is shown in Fig. 3a. While the total acceleration does not reveal a clear swimming pattern (Fig. 3b), isolating the separate acceleration and orientation components during straight line swimming for six consecutive swimming cycles reveals the periodic nature of this mode of swimming (Fig. 3c, d). Separating the accelerometer and magnetometer data into individual components (i.e., x-, y-, and z-component acceleration; pitch, roll, and heading) reveals how the output was dependent on the tag attachment location on the jellyfish (Fig. 3c, d). For reference, the acceleration and orientation axes are shown on the diagram above Fig. 3d. Due to the axisymmetric body plan and swimming actuation of jellyfish, it might be expected that one component of acceleration (z-component) would sufficiently characterize the total acceleration of the animal if the tag is fixed exactly on the apex of the dorsal surface and the jellyfish is swimming perfectly straight. However, values changed for all directional components, indicating that the position of the sensor was offset from the animal’s axis of symmetry. Similarly, pitch should be invariant during straight swimming if the tag was affixed on the apex of the bell; yet our observations reflect this offset. The tag was affixed towards one side using two suction cups instead of four because of relatively small size of the jellyfish. Future deployments will be conducted on larger jellyfish (>30 cm) that allow for tag attachment using all four suction cups to verify this possibility.
Accelerometer output from a single ITAG deployment on a captive Aurelia aurita. a The accelerometer output for the entire duration of the trial. b The total acceleration during a period of straight line swimming (indicated by a red star in a, b). c The x- (red), y- (blue), and z- (black) components of acceleration during the specific event. d The pitch (red), roll (blue), and heading (black) in degrees of the tagged jellyfish during the specific event. The rotational and translational axes are indicated in the jellyfish diagram above (d). The corresponding video for c and d can be found in Additional file 2
The acceleration amplitude of ±0.003 g during straight swimming is consistent with earlier work using particle visualizations and video with captive A. aurita (Katija, unpublished data; see Additional file 1: Figure S1; see also methods from [38]). For a 10 cm bell diameter individual swimming unimpeded in a tank, the acceleration amplitude ranges between ±0.002 g at a frequency greater than 1 Hz after two swimming cycles. For larger A. aurita, the duration of a swimming cycle (i.e. the inverse of pulse frequency) increases with increasing bell diameter (McHenry, 2003), and is consistent with swimming frequencies of 2 Hz.
Squid trials
Examining an entire tag deployment offers insight into squid activity levels over the course of a day (Fig. 4). The light sensor captures both the natural lighting coming through skylights above the tank, as well as the building’s incandescent lights (Fig. 4a). High light levels near the start of the deployment occurred when the tag was in air during attachment. The light levels then decreased slightly when the animal was placed in a dark, aerated acclimation raft. Upon release into the tank, light levels increased again and then decreased to essentially zero as the sun set outside the aquarium and overhead lights were turned off. Light levels then increased again as natural daylight began to seep back into the building from ceiling skylights.
ITAG output across a single deployment on a captive Loligo forbesi. a Ambient light within the experimental facility detected by the ITAG light sensor, reflecting both natural patterns and the electric lights being turned on and off. b Total acceleration of the tagged squid during daytime and nighttime conditions. c Orientation of the tagged squid during daytime and nighttime conditions
The general diel squid activity patterns were evaluated using the RMS magnitude of total acceleration (Fig. 4b). The elevated region of acceleration that occurred at the start of recording corresponds to handling the tag in air before attachment to the squid. The higher total acceleration towards the end of the trial (around 17 h) reflects manipulation of the tagged squid that resulted in increased activity, notable as peaks in acceleration (Figs. 4b, 5a, 6a). This manipulation involved introducing a PVC pipe into the field of vision of the squid, which would encourage the squid to jet or accelerate rapidly away from the pipe. Similar events of increased acceleration (spikes in same figures) seemed to have occurred at several points during the night (e.g., ca hour 7 several occur in a row), and these behaviors were not induced by manual manipulation. Thus, excluding the times of artificially elevated activity levels (due to manual manipulation), the data indicate a higher number of increased activity events at night when compared to the day (Fig. 6b, c).
Accelerometer output from a single ITAG deployment on a captive Loligo forbesi. a The accelerometer output for the entire duration of the trial. b The total acceleration during a specific event (indicated by a red star in a, b) corresponding to a directional change, gliding, and finning. (c) The x- (red), y- (blue), and z- (black) components of acceleration during the specific event. d The pitch (red), roll (blue), and heading (black) in degrees of the tagged squid during the specific event. The rotational and translational axes are indicated in the squid diagram above (d). The video corresponding to b, c and d can be found in Additional file 3
Timing and frequencies of accelerometer output from a captive Loligo forbesi. a Accelerations and hourly rms values relative to time of day. b Fast Fourier transform of the total acceleration record indicates that 0.8 Hz was the dominant frequency of squid movement during the duration of the tag deployment. The inset figure provides an example of a single bout of finning during the trial (indicated by a red star in a, b), and has the same periodicity (0.8 Hz) as the dominate frequency identified by the FFT. c Histogram of the peak magnitudes of the total acceleration during the duration of the tag deployment. Lower accelerations with a magnitude of 0.02 g were the most prevalent during the recorded behavior, and are most likely the result of finning. Inset in (c) shows how the number of incidences of higher acceleration behaviors (presumably jetting and rapid maneuvering) is much reduced compare to the lower acceleration behaviors of this animal
The simultaneous video and tag data revealed a number of behaviors exhibited by the tagged squid. For example, a sudden direction change was noted in the video data ca. 16.5 h into recording (indicated by a red star in Fig. 5a, b). During the +15 s noted in Fig. 5b, the tagged squid underwent a series of behaviors that start with a reversal in swimming direction at 3 s followed by backward finning interspersed with gliding (where fins are pulled up against the mantle for 3 s; Fig. 5b). After the glide, finning resumed a repeatable stroke with an average peak acceleration of ±0.2 g and a frequency of 0.85 Hz. The z-component of acceleration (refer to diagram above Fig. 5d for axes directions) was roughly equal to 1 throughout the series of behaviors indicating that the animal swam roughly parallel to the bottom of the tank with relatively minimal fluctuations upward or downward. Supplementary videos showing top and two side views confirms this finding (Additional file 3). However, during direction change and finning, small displacements in the z-direction of the mantle were observed due to the fin motion. Squid movement during finning was predominantly generated by sinusoidal oscillations of the fin surface in the vertical direction gently pitching the animal through the water. The largest differences in acceleration were noted in the x-direction (Fig. 5c, red line) along the longitudinal body axis. The largest increase in acceleration occurred during the direction change, where the squid quickly went from forward to backward swimming using a reversal in fin motion only. Changes in y-direction acceleration were most likely due to asymmetric differences in fin stroke kinematics between the left and right sides or motion of the tag with respect to the animal.
In addition to differences in accelerometer output, the movement signatures from specific behaviors were observed in the orientation of the animal (Fig. 5d). The pitch of the animal (Fig. 5d, red line) increased during the direction reversal, as seen in the simultaneous videos. During the gliding phase, the pitch of the animal remained constant, likely because only the fins remained in one position, tucked against the mantle. Small changes in roll throughout the documented behaviors were also observed. However, the heading data did not appear to match what was recorded in the simultaneous videos. For instance, during direction reversal, the squid maintained the same orientation but reversed swimming direction from forwards to backwards (i.e. a change in 180°). Thus, there was a dramatic change in heading during the direction reversal behavior which was not observed on the magnetometer data. When combined with the jellyfish observations, this suggest that there were significant electromagnetic signal sources in captive environments that served to disrupt the tag’s heading data. Therefore, heading data for captive deployments of tags should be carefully compared with simultaneous video footage to ensure that errors are not introduced to the data set due to the captive environment.
Hourly RMS values (bars) of the total acceleration were presented along with the acceleration data in Fig. 6a. To briefly address potential diel movement patterns, the mean hourly RMS rate was compared for light and no-light conditions. The mean RMS value was 0.011 ± 0.001 g when light was measured by the tag and 0.013 ± 0.001 g when no light was measured. The bars containing sunrise and sunset, and the times we induced movement (around hour 17), were omitted from these analyses. Relatively low levels of acceleration were measured throughout the trial, and the animal most likely spent the majority of its time finning. Evaluating the magnitude of acceleration in frequency space (Fig. 6b) and the occurrence of the magnitude of peak acceleration (Fig. 6c), the most repeated behavior possessed a frequency of 0.8 Hz and amplitude of 0.02 g. These values compared well to the frequency and magnitude of accelerometer measurements during finning. A representative bout of finning was shown in the inset of Fig. 6b. This bout occurs during a period of low total acceleration (indicated by a red star in Fig. 6a, b), and has average frequency and peak acceleration values (0.8 Hz and 0.02 g, respectively) that were similar to measurements from other squid species [29]. Less commonly observed behaviors appear to be higher acceleration events such as rapid direction reversals and jetting. These less common events, defined as peak values above 0.15 g, were larger (0.26 ± 0.11 vs 0.22 ± 0.06 g) and occurred more frequently (13 vs 6 peaks/h) when the tag did not measure any light.
Conclusions
The high-resolution ITAG can be used to record biologically significant behaviors of soft-bodied invertebrates, including both energetically costly escape behaviors (apparent jetting) as well as more typical swimming motions (rowing or finning). Like many animal behaviors, there was often some overlap of the two actions, and such mixed behaviors could be observed on the tag records (e.g., Fig. 5b–d). Importantly, the simultaneous video recordings allowed for the identification of the behavioral signatures present in the tag sensor data. This post hoc analysis method will likely allow the use of similar, tag-only data collected during future field-based behavioral studies. Laboratory deployments on small jellyfish yield clear relationships between swimming behavior and accelerometer output, and reflect the importance of proper, fixed attachment for future deployments. Additionally, future smaller tags will allow smaller jellyfish and cephalopods to be equipped. Similar to previous studies [39, 40], this preliminary work appears to reveal a trade-off between energetically costly escape behavior such as jetting and more energetically efficient behaviors such as finning, that appears to dominate activity levels of squid. These Loliginid squids are slightly negatively buoyant and their buoyancy likely changes with depth. Thus, such information could provide insight into the trade-off between dynamic swimming and more efficient gliding, a trade-off that may affect fitness [41].
Overall, tagged squid did not appear to be impacted by the tags’ presence, exhibiting normal swimming and coloration behaviors compared to conspecifics without ITAGs. Due to limited availability of large jellyfish, captive deployments of the ITAG were conducted on animals roughly 20 cm in diameter, which prevented attachment of all four suction cups to the surface of the bell. However, perhaps due to the neutrally buoyant properties of the tag, preliminary results of jellyfish swimming behavior (i.e., swimming speed, acceleration, and swimming frequency) appeared to have a smaller affect than might have been expected when compared with untagged individuals of the same size range. Based on these findings the tag could be applied to squid and jellyfish as small as ca. 45 cm mantle length and 30 cm bell diameters (assuming the squid are wide enough to hold the tag), respectively, greatly expanding its species applicability.
The ITAG’s current sensor suite for the characterization of the physical environment includes temperature, pressure (to yield depth information), and light (to yield turbidity, light abortion, and phytoplankton concentration, e.g., [42]) sensors. How field sites differ in the relationships of turbidity, light and plankton conditions will need to be accounted for to accurately apply this sensor. The range of sensitivity of the light sensor (400–700 nm) overlaps with that of squid vision [43] and that of substantial oceanic bioluminescence [44]. This combination of sensors was selected to address spatial preference of invertebrates in light/dark environments and track associations with diel migrations. Their small size also allowed for efficient tag integration. Yet, additional sensors will be required to address specific questions such as whether squid and jellyfish preferentially select regions of minimal O2 and pH, how their behavior and physiology changes in low O2 and low pH conditions, what types of behavior occur when crossing or encountering pycnoclines, or the effect of pH/ocean acidification conditions on swimming and feeding behavior.
Although behavior in captivity may not accurately represent in situ behavior, the collected data (i.e., general activity level and potential swimming modes) will provide insight on the overall energy expenditure of jellyfish and squid during vertical and horizontal traversals of the ocean depths. Swimming behavior is especially important to Loliginid squids, which are negatively buoyant and have to actively swim to maintain and adjust their vertical position in the water column. In situ swimming behavior may also play a role in understanding the mechanisms behind jellyfish bloom events, as well as their dispersal across coastal environments [28]. Further lab and future field tests have the potential to reveal the ITAG’s broad value and the methods utilized herein.
Abbreviations
- ITAG:
-
invertebrate tag—novel ecosensor developed initially for soft-bodied invertebrates such as squid and jellyfish, gathering movement (acceleration), orientation (magnetometer), depth (pressure), light and temperature data
- RMS:
-
root mean squared
References
Clarke MR. Cephalopods as prey. III. Cetaceans. Philos T Roy Soc B. 1996;351:1053–6.
Robinson KL, Ruzicka JJ, Decker MB, Brodeur RD, Hernandez FJ, Quiñones J, Acha EM, Uye S-I, Mianzan H, Graham WM. Jellyfish, forage fish, and the world’s major fisheries. Oceanography. 2014;27:104–15.
Field JC, Baltz K, Phillips AJ, Walker WA. Range expansion and trophic interactions of the jumbo squid, Doscidicus gigas, in the California current. Calif Cooperative Ocean Fish Investig. 2007;48:131–46.
Ruiz-Cooley RI, Markaida U, Gendron D, Aguiniga S. Stable isotopes in jumbo squid (Doscidicus gigas) beaks to estimate trophic position: comparison between stomach contents and stable isotopes. J Mar Biol Assoc UK. 2006;86:437–45.
Rosas-Luisa R, Salinas-Zavalaa CA, Kochb V, Lunac PDM, Morales-Záratea MV. Importance of jumbo squid Dosidicus gigas (Orbigny, 1835) in the pelagic ecosystem of the central Gulf of California. Ecol Model. 2008;218:149–61.
Overholtz W, Link JS, Suslowicz LE. The impact and implications of fish predation on pelagic fish and squid on the eastern USA shelf. ICES J Mar Sci. 2000;57:1147–59.
Richardson AJ, Bakun A, Hays GC, Gibbons MJ. The jellyfish joyride: causes, consequences and management responses to a more gelatinous future. Trends Ecol Evol. 2009;24:312–22.
Clarke MR. Beaks, nets and numbers. Symp Zool Soc Lond. 1977;38:89–126.
Hunsicker ME, Essington TE, Watson R, Sumaila UR. The contribution of cephalopods to global marine fisheries: can we have our squid and eat them too? Fish Fish. 2010;11:421–38.
Liu H, Ciannelli L, Decker MB, Ladd C, Chan K-S. Nonparametric threshold model of zero-inflated spatio-temporal data with application to shifts in jellyfish distribution. J Agric Biol Environ Stat. 2011;16:185–201.
Malacic V, Petelin B, Malej A. Advection of the jellyfish Pelagia noctiluca (Scyphozoa) studied by the Lagrangian tracking of water mass in the climatic circulation of the Adriatic Sea. Geophys Res Abstr. 2007;9:02802.
Zeidberg LD, Robison BH. Invasive range expansion by the Humboldt squid, Dosidicus gigas, in the eastern North Pacific. Proc Natl Acad Sci. 2007;104:12948–50.
Lynam C, Gibbons M, Axelsen B, Sparks C, Coetzee J, Heywood B, Brierley A. Jellyfish overtake fish in a heavily fished ecosystem. Curr Biol. 2006;16:R492–3.
Lynam CP, Lilley MKS, Bastian T, Doyle TK, Beggs SE, Hays GC. Have jellyfish in the Irish Sea benefited from climate change and overfishing? Glob Change Biol. 2011;17:767–82.
Purcell JE, Arai MN. Interactions of pelagic cnidarians and ctenophores with fish: a review. Hydrobiologia. 2001;451:27–44.
Zeidberg LD, Isaac G, Widmer CL, Neumeister H, Gilly WF. Egg capsule hatch rate and incubation duration of the California market squid, Doryteuthis (=Loligo) opalescens: insights from laboratory manipulations. Mar Ecol. 2011;32:468–79.
Kaplan MB, Mooney TA, McCorkle DM, Cohen A. Adverse effects of ocean acidification on early development of squid (Doryteuthis pealeii). PLoS One. 2013;8:e63714.
Trübenbach K, Pegado MR, Seibel BA, Rosa R. Ventilation rates and activity levels of juvenile jumbo squid under metabolic suppression in the oxygen minimum zone. J Exp Biol. 2013;216:359–68.
Rosa R, Seibel BA. Synergistic effects of climate-related variables suggest future physiological impairment in a top ocean predator. Proc Natl Acad Sci. 2008;105:20777–80.
Gilly WF, Zeidberg LD, Booth JAT, Stewart JS, Marshall G, Abernathy K, Bell LE. Locomotion and behavior of Humboldt squid, Dosidicus gigas, in relation to natural hypoxia in the Gulf of California, Mexico. J Exp Biol. 2012;215:3175–90.
Piraino S, Vito DD, Schmich J, Bouillon J, Boero F. Reverse development in Cnidaria. Can J Zool. 2004;82:1748–54.
Rodhouse P. Managing and forecasting squid fisheries in variable environments. Fish Res. 2001;54:3–8.
Xavier JC, Croxall JP, Reid K. Interannual variation in the diets of two albatross species breeding at South Georgia: implications for breeding performance. Ibis. 2003;145:593–610.
Broell F, Noda T, Wright S, Domenici P, Steffensen JF, Auclair J-P, Taggart CT. Accelerometer tags: detecting and identifying activities in fish and the effect of sampling frequency. J Exp Biol. 2013;216:1255–64.
Hays G, Bastian T, Doyle T, Fossette S, Gleiss A, Gravenor M, Hobson V, Humphries N, Lilley M, Pade N, Sims D. High activity and Levy searches: jellyfish can search the water column like fish. Proc R Soc Lond Ser B Biol Sci. 2011;279:465–73.
Honda N, Watanabe T, Matsushita Y. Swimming depths of the giant jellyfish Nemopilema nomurai investigated using pop-up archival transmitting tags and ultrasonic pingers. Fish Sci. 2009;75:947–56.
Hays GD, Doyle T, Houghton J, Lilley MKS, Metcalfe JD, Righton D. Diving behaviour of jellyfish equipped with electronic tags. J Plankton Res. 2008;30:325–31.
Fossette S, Gleiss AC, Chalumeau J, Bastian T, Armstrong CD, Vandenabeele S, Karpytchev M, Hays GC. Current-oriented swimming by jellyfish and its role in bloom maintenance. Curr Biol. 2015;25:342–7.
Bartol IK, Krueger PS, Stewart WJ, Thompson JT. Hydrodynamics of pulsed jetting in juvenile and adult brief squid Lolliguncula brevis: evidence of multiple jet ‘modes’ and their implications for propulsive efficiency. J Exp Biol. 2009;212:1889–903.
Costello JH, Colin SP, Dabiri JO. Medusan morphospace: phylogenetic constraints, biomechanical solutions, and ecological consequences. Invertebr Biol. 2008;127:265–90.
Dabiri JO, Colin SP, Katija K, Costello JH. A wake-based correlate of swimming performance and foraging behavior in seven co-occurring jellyfish species. J Exp Biol. 2010;213:1217–25.
Johnson M, Tyack PL. A digital acoustic recording tag for measuring the response of wild marine mammals to sound. IEEE J Ocean Eng. 2003;28:3–12.
Downey N, Webber D, Roberts M, Smale M, Sauer W, Singh L. The use of acoustic telemetry in South African squid research (2003–2010). In: Krejcar O, editor. Modern Telemetry. Rijeka, Croatia: InTech; 2011. doi:10.5772/23936
Gilly WF, Markaida U, Baxter CH, Block BA, Boustany A, Zeidberg LD, Reisenbichler K, Robinson B, Bassino G, Salinas C. Vertical and horizontal migrations by the jumbo squid Dosidicus gigas revealed by electronic tagging. Mar Ecol Prog Ser. 2006;324:1–17.
Semmens JM, Pecl GT, Gillanders BM, Waluda CM, Shea EK, Jouffre D, Ichii T, Zumholz K, Katugin ON, Leporati SC, Shaw PW. Approaches to resolving cephalopod movement and migration patterns. Rev Fish Biol Fish. 2007;17:401–23.
Moriarty PE, Andrews KS, Harvey CJ, Kawase M. Vertical and horizontal movement patterns of scyphozoan jellyfish in a fjord-like estuary. Mar Ecol Prog Ser. 2012;455:1–12.
Seymour JE, Carrette TJ, Sutherland PA. Do box jellyfish sleep at night. Med J Aust. 2004;181:707.
Katija K, Jiang H. Swimming by medusae Sarsia tubulosa in the viscous vortex ring limit. Limnol Oceanogr Fluids Environ. 2013;3:103–18.
O’Dor RK, Webber DM. The constraints on cephalopods: why squid aren’t fish. Can J Zool. 1986;64:1591–605.
O’Dor R, Webber DM. Invertebrate athletes: trade-offs between transport efficiency and power density in cephalopod evolution. J Exp Biol. 1991;160:93–112.
O’Dor RK, Hoar JA, Webber DM, Carey FG, Tanaka S, Martins H, Porteiro FM. Squid (Loligo forbesi) performance and metabolic rates in nature. Mar Freshw Behav Phy. 1994;25:163–77.
Jaud T, Dragon A-CC, Garcia JV, Guinet C. Relationship between chlorophyll a concentration, light attenuation and diving depth of the southern elephant seal Mirounga leonina. PLoS One. 2012;7:e47444.
Michinomae M, Masuda H, Seidou M, Kito Y. Structural basis for wavelength discrimination in the banked retina of the firefly squid Watasenia scintillans. J Exp Biol. 1994;193:1–12.
Haddock SHD, Case JF. Bioluminescence spectra of shallow and deep-sea gelatinous zooplankton: ctenophores, medusae and siphonophores. Mar Biol. 1999;133:571–82.
Authors’ contributions
TAM, KK, TH and KAS conceptualized the tag. TH and KAS constructed it. TAM and KK conducted the jellyfish trials. TAM, KK, PA and JF conducted the squid trials. TAM led the writing, KK and KAS led the analyses. All authors edited the manuscript. All authors read and approved the final manuscript.
Authors’ information
TAM is a sensory biologist specializing in animal responses to biophysical cues and signals in their environment. KK is a bioengineer, and specializes in biological propulsion, swimming mechanics, and fluid mechanics of a range of gelatinous organisms that include jellyfish. KAS is a mechanical engineer specializing in biomechanics, biologging, and persistent monitoring for both people and animals. TH is an electrical engineer with extensive experience in tag designs, miniature sensors, and tag formats. JF is a marine ecologist using larval recruitment and other life stages to address the movement of fishes and the dynamics of their communities. PA is a behavioral ecologist using telemetry techniques to address movements and habitat use of commercial fishes and apex predators across different habitats for the management and conservation of marine resources.
Acknowledgements
The authors thank the scientific and technical staff at IMAR/DOP and FlyingSharks for their support to the experiments in the Azores, including A. Filipa, G. Graca, R. Prieto, J. Rodeia, N. Serpa, I. Martins, T. Morato, R. Guedes, A. Godinho, L. Silva, G. Graca, P. Martins and V. Rosa. The authors greatly appreciate the generous efforts and support of FlyingSharks and the Faial Nature Park for the use of the Porto Pim Aquarium and their assistance and patience during the trials. Steve Spina at New England Aquarium provided the Aurelia aurita. Dave Remsen at Marine Biological Laboratories and J.H. Costello at Providence College assisted in the collection of Chrysaora quinquecirrha and Cyanea capillata, and R. Galat supported the WHOI facilities. This work was supported by WHOI’s Ocean Life Institute and the Innovative Technology Program, Hopkins Marine Station’s Marine Life Observatory (to KK), as well as the National Science Foundation’s Ocean Acidification Program (to TAM) and NSF’s Program for Innovative Development of Biological Research (to TAM, KK and KAS).
Compliance with ethical guidelines
Competing interests The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding authors
Additional information
T. Aran Mooney, Kakani Katija and K. Alex Shorter contributed equally to this work and share first authorship
Additional files
40317_2015_76_MOESM1_ESM.docx
Additional file 1: Figure S1. Motion details of captive Aurelia aurita. (A) Consecutive still images of a 4.5 cm diameter Aurelia aurita swimming through a laser sheet in the laboratory used to identify the body centroid location over two swimming cycles. Stars and diamonds indicate frames that capture peak contraction and relaxation of the bell during swimming, respectively. The body centroid position was then used to determine the swimming speed (B, black line) and the swimming acceleration (B, gray line) during consecutive swimming cycles. These methods are described in more detail in Katija and Jiang (2013).
40317_2015_76_MOESM2_ESM.wmv
Additional file 2. ITAGged jellyfish, Aurelia aurita (top view).
40317_2015_76_MOESM3_ESM.wmv
Additional file 3. Swimming, ITAGged squid, Loligo forbesi shown from multiple views.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Mooney, T.A., Katija, K., Shorter, K.A. et al. ITAG: an eco-sensor for fine-scale behavioral measurements of soft-bodied marine invertebrates. Anim Biotelemetry 3, 31 (2015). https://doi.org/10.1186/s40317-015-0076-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40317-015-0076-1