Abstract
Background
Motion detecting archival data loggers such as accelerometers have become increasingly important in animal biotelemetry and offer unique insights into animal behavior, energetics, and kinematics. However, challenges remain for successful deployment and interpretation of data from captive and wild animals. Accelerometer sensors require being packaged in an archival tag that has a firm attachment in a fixed (known) orientation to accurately measure the relevant motion of the animal. This requirement can lead to handling stress and attachment techniques that can affect the tagged animal’s natural behavior and welfare, and lead to behavioral artifacts in the data. Accelerometer data also require careful interpretation to correctly identify behavioral events of interest such as foraging. For endothermic species, changes in stomach temperature can produce temperature signatures indicative of foraging events. In this paper, we present a novel method for recording foraging events in free-swimming white sharks.
Methods
We used a combination of accelerometer loggers and pop-up archival transmitting (PAT) tags (MK10, Wildlife Computers) to examine the feeding and kinematics of white sharks (Carcharodon carcharias) in the wild. We validated feeding results using a captive juvenile white shark where controlled feeding experiments could be conducted in an aquarium setting at the Monterey Bay Aquarium. We fed data logger instrument packages to eight free-swimming white sharks. Deployment durations ended naturally when the package was regurgitated and ranged from 2 to 12 days. While inside the stomach, the orientation of the data logger package was arbitrary and resulted in slow shifting over time, a challenge for normal analysis routines. We present one of these datasets to illustrate a novel methodology for calibrating accelerometer orientation, and evaluate the utility of resulting data.
Results
We obtained accurate accelerometer measurements including surge, heave, and sway from data loggers with shifting orientation through data post-processing. We measured consistent dips in stomach temperature followed by a steady prolonged heating during controlled feeding events in a captive white shark. Similar thermal signatures identified in wild white shark records confirmed feeding events while acceleration data characterized the associated prey capture behavior.
Conclusions
We provided proof of concept for a novel and non-invasive technique for accelerometer data logger deployment extending the possibilities for their use in bio-logging. The placement of data-logging tags in the stomachs of endothermic white sharks produced high quality tri-axial acceleration data in addition to stomach temperature data capable of detecting feeding events. The technique has the potential for distinguishing between attempted and successful foraging events. This approach to accelerometer coordinate correction could be applied in other systems where logger orientation is unknown or changes after deployment.
Similar content being viewed by others
Background
Animals spend the vast majority of their lives outside of our view, historically making the acquisition of data on their behavior, ecology and physiology challenging [1, 2]. This is especially true for marine animals. The advent of biotelemetry and more recently biologging has opened the doors to gathering data on many aspects of the biology of these animals. Initially, such data included simple locational telemetry (e.g., via radio or acoustic signals) and largely focused on reconstructing the geographical movement of the tagged individuals. Over the last decade, advances in data-logging capacity have resulted in a proliferation in the sensors available to measure behavioral, as well as physiological parameters [3–6]. Tags containing these new sensors collect vast amounts of data that are not easily remotely transferable due to data transmission limitations. As a result, data are usually archived in on-board memory and have to be physically recovered to be accessed. While many of these sensors have been increasingly used in air-breathing endotherms [7, 8], their incorporation into research studying fish has been slower [9–11]. Whereas most birds or seals predictably roost or haul-out on land, permitting their capture and recapture with minimal stress, fish can often only be captured with hook and line, with attendant risks to the animal’s health [12–14]. Another significant hurdle is represented by the ability to recapture a tagged individual, which in most cases is unlikely using conventional fishing. Although, this can be a viable method to reacquire archival tags in a heavily fished commercial species [15]. One way to tag individuals without capture stress is to deploy tags on free-swimming animals, which is usually performed using some form of darting system [16, 17]. Because such systems are usually based on tethers, they are unlikely to have much application in motion-sensitive tags [18].
A number of methodological solutions have been adopted to facilitate recovery of data-logging tags. The most common rely on a timed release mechanism (either of galvanic nature or electronic), a buoyant housing, and a VHF or Argos transmitter [10, 18]. This allows tags to be located after they have released from the fish. Although VHF transmitters have substantial ranges, fish can swim long distances in a matter of days. This limits the technique, in general to short deployments (<5 days) to ensure that tags are recovered [10, 19, 20]. Additionally, stressful capture and release can disrupt the natural behavior of tagged fish for periods ranging from a few hours to days [14, 21, 22], reducing the amount of natural behavior recorded from individuals, and risking the animal’s survival and well-being, which might be especially important in threatened species [23].
Accelerometer data also require careful interpretation to correctly identify behavioral events of interest such as foraging. For endothermic species, changes in stomach temperature can produce temperature signatures indicative of foraging. Heat increment of feeding has been recorded in endothermic sharks and tunas with stomach tags [24–26]. Although many factors influence the thermal record of digestion events (e.g., body size, meal size, and ambient temperature), the capacity to detect feeding events in stomach or peritoneal tag thermal records has been well established. The heat increment is associated with the metabolic processes of digestion. Once ingested, food is subject to mechanical and enzymatic digestion and the process of this specific dynamic action (SDA) results in a metabolic increment of oxygen consumption with a heat by-product [26]. Furthermore, in many cases ingestion results in stomach temperature reductions attributable to the potential cooler temperature of the prey, or food item, and associated influx of ambient water. These reductions in temperature or dips can be used to mark the initiation of the feeding event [27, 28].
Here we describe a novel method to deploy accelerometers in the stomachs of large free-swimming sharks and how, with the addition of stomach sensors, unique insights into feeding behavior can be achieved [28]. The system is tested on white sharks (Carcharodon carcharias) during their annual coastal residency off the California coast. Stomach temperature data interpretation was validated in a captive specimen in a controlled ocean pen and aquarium setting [29].
Methods
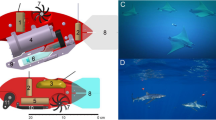
We instrumented free-ranging adult and sub-adult white sharks (C. carcharias) with multisensor data loggers (“Daily Diary” tags), described in detail in Wilson et al. (2008). The tags recorded (5 Hz) tri-axial acceleration, pressure, and temperature and enabled the measurement of locomotory activity, diving depth, and stomach temperatures in the wild. We used wire clamps (Clamp Tite) and stainless steel wire to attach the data loggers to a satellite-linked pop-up archival transmitting tag (MK10, Wildlife Computers). Additional buoyancy (syntactic foam, 3 M) was used to compensate for the increased specific gravity of the acceleration data logger (Fig. 1a). Data logger packages were concealed in marine mammal blubber obtained with permits from stranding mortalities and ingested by free-swimming sharks attracted to a research vessel with a seal decoy. Within 15 days loggers were usually regurgitated along with, in some cases, other undigested stomach contents. The approximate location of the floating tags was determined as they reported to the ARGOS satellite system. The tags were then recovered at sea using an ultra high frequency directional antenna.
Stomach tag package and desired orientation. The data logging stomach tag (a) consists of a pop-up archival transmitting (PAT) tag (MK10, Wildlife computers) attached with stainless wire to a tri-axial accelerometer data logger. Additional syntactic foam was attached to achieve positive buoyancy and maintain the antenna above the sea surface. The orientation of the data logger once inside the stomach was unknown; therefore, a primary goal was to calibrate the acceleration data through post-processing to align with the shark’s body (b) as shown
While inside the shark’s stomach, the orientation of the acceleration data logger was arbitrary and resulted in slow shifting over time, a challenge for acceleration data analysis. To address this problem, we developed a procedure using the acceleration data in relation to the recorded changes in depth and tail motion to solve the orientation of the logger relative to the shark’s body iteratively over the course of the record (Fig. 1b).
Acceleration data analysis
We developed a post-processing procedure (described below) for determining the logger orientation at regular time intervals, and then transformed the data coordinate system to align with a prone orientation of the sharks body (Fig. 1b). A calibrated tri-axial accelerometer that is held static should measure −1.0 g in the direction of the earth’s center of mass. Re-setting the coordinate space orientation to a static accelerometer would require only a simple coordinate transformation minimizing acceleration along the Z axis; however, the dynamic motion of the shark must be accounted for (e.g., ascending, descending, rolling, turning or surging). Over long time periods it is accurate to assume that the average orientation of a continually swimming ram-ventilating shark is in the prone position since the duration of ascending and descending periods should tend toward parity. This applies to the frequency of other motions such as left and right tail beats. We sought to determine the minimum time window (non-overlapping) over which this assumption holds. We calculated the mean change in depth between successive measurements within time windows ranging from 1 to 20 min. We then calculated the mean and variance among the window means and plotted these as a function of window size. We selected the minimum window threshold at a point when the mean tended toward zero and variance also approached a minimum asymptote. We then developed routines for coordinate space correction within each time window. We assumed that over short time periods (>time window) the tag orientation inside the shark’s stomach remained constant, and tested this assumption post hoc. The orientation solution has three steps, corresponding with the three dimensions. We developed Matlab (Mathworks) routines to execute these steps (Additional files 1, 2, 3, 4, 5, 6, 7).
Step 1: solve the vertical orientation of the Z axis
The mean acceleration vector (integrating X, Y, and Z) over each time window was used to determine the orientation of the logger in relation to a prone shark body orientation. We re-set the coordinate space orientation minimizing this integrated mean acceleration vector along the Z axis. This was done by solving for a mean acceleration of zero on the X and Y axis, respectively. We used the following standard transformation matrix equations:
to rotate about the X axis so that
where β is the angle of rotation, and X 1, Y 1, and Z 1 are the initial acceleration values. Then repeated for the Y axis, respectively.
Once the vertical (Z axis) orientation of the logger has been solved relative to the shark’s average prone position, the remaining solution must lie along a single rotational plain about the Z axis (Fig. 1b) and can be accomplished in two additional steps.
Step 2: solve the lateral orientation of the Y axis
The metronomic tailbeat of a swimming shark provides a consistent acceleration signal in frequency and alternating direction. We assumed that accelerating force induced by the tailbeat peaked laterally (side to side; parallel to the Y axis) with respect to the shark’s body (see Fig. 2). We identified the tailbeat frequency and peak power using a fast Fourier transform (FFT) algorithm (FFT function, Matlab, Mathworks) applied over each time window. We then re-aligned the coordinate space orientation by rotating about the Z axis until the tailbeat signal strength was maximized along the Y axis. We used the following matrix transformation equation:
Time window selection criteria. The mean change between successive depth measurements over increasing time windows should approach zero as the average orientation of the shark approaches a ‘prone’ position. The mean (a) of the window means tended toward zero and variance (b) also approached a minimum asymptote, allowing for a visual determination of time window selection in which the assumption of a mean ‘prone’ position could be confidently assumed
While this step aligns the coordinate space laterally, it does not discriminate between left and right; therefore the final remaining step, determining front and back, has two possible solutions.
Step 3: solve the horizontal orientation of the X axis
After solving for the lateral axis, the adjusted coordinate space should be either already correctly oriented (front-back) or else remains to be reversed 180° with respect to the horizontal X axis (rotating about the Z axis). Assuming a clear positive relationship between vertical displacement and pitch over the time window, we tested the slope of this relationship (standard linear regression) and, if a negative slope was found, the coordinate space orientation was re-set by multiplying vectors X i and Y i by negative one; effectively rotating 180° about the Z axis.
Overall dynamic body acceleration (ODBA) was calculated from the X, Y, and Z acceleration as their sum minus static acceleration determined from a 6 s running mean of the same as per [30]. To visually evaluate relative sustained ODBA over a longer time period (300 min), ODBA was further smoothed by applying a 40-s running mean.
Controlled feeding validation
For a controlled validation experiment of stomach temperature as a measure of prey ingestion, we instrumented a captive white shark with an acoustic sensor tag (V16TP, Vemco) measuring temperature and pressure. The tag was concealed in a salmon fillet and freely ingested by the shark. Stomach temperature data transmitted from the tag were recorded via acoustic receiver (VR2, VEMCO) and ambient temperature was logged using a temperature logger (Tidbit, ONSET). The time, mass, and caloric content of meals fed to the captive white shark were recorded in relation to changes in stomach temperature.
Results
Using a novel method for determining accelerometer logger orientation inside a swimming animal, we corrected tri-axial acceleration data coordinate space to produce accurate measurements of sway, surge, and heave after post-processing. Signature dips in stomach temperature followed by steady and prolonged heating occurred during controlled feeding events in a captive white shark.
Similar signatures identified in a wild white shark’s record confirmed feeding while acceleration data characterized the associated prey capture behavior. On eight occasions white sharks in the wild were instrumented with the archival accelerometer and temperature sensor packages (Table 1). Following the initial consumption, additional ingestion events were detected in two of the eight individuals. We present detailed results from deployment 4.
Accelerometer data correction
The mean change in depth tended toward a zero asymptote as a function of increasing time window. Similarly standard deviation was minimized with increasing time window (Fig. 2) allowing for a visual assessment of a time window over which mean position of the shark could be assumed to be prone. With the time window selected, transforming the coordinate system while minimizing acceleration force along the Z axis (step 1) resulted in a mean of −1 and a mean of zero along the X and Y axes confirming correct transformation. At all window sizes the tailbeat could be clearly identified as a power peak in the X and Y axes around 4 Hz (Fig. 3a). Transformation of the coordinate system about the Z axis resulted in very clear maximum peaks as the X and Y axes, respectively came in and out of alignment with the direction of maximum tailbeat acceleration power (Fig. 3b). This allowed for unambiguous determination of tag orientation with regard to the shark body and subsequent re-setting of the axis (step 2). After completing the final step to determine if the tag was facing anterior or posterior (step 3) the positive relationship between vertical displacement and pitch was consistently positive in all time windows. The Matlab code used for data correction can be found in the supplement material (Additional files 1, 2, 3, 4, 5, 6, 7).
White shark Tail beat signal. The peak frequency of the tailbeat (a) should be consistently detected in the horizontal plane (swaying acceleration) measured by the X and Y axes (blue and red, respectively; see Fig. 1a) near 0.4 Hz or 2.5 s. Rotating the coordinate system about the Z axis (b) the tail beat frequency power is maximized along the X and Y axes at perpendicular angles. The desired coordinate orientation was reached where power peaked along the Y axis
Stomach temperature and feeding signature
The temperature sensor acoustic tag fed to a captive juvenile white shark remained inside the stomach for 31 days before expulsion. After ingesting the tag, the shark remained in an ocean pen (6 days) and was later transported to a large (3.8 million liters) display tank at the Monterey Bay Aquarium where the shark retained the tag within the stomach for another 25 days (Fig. 4). Comparing 6 days in the variable temperature pen phase and the subsequent 6 days in the continuous temperature aquarium environment provided a “natural experiment” to control for environmental temperature variability, a factor that could potentially effect stomach temperature. The acoustic stomach tag reported temperature readings at pseudo-random intervals between 45 and 120 s and these were recorded on passive receivers at a mean interval of 100.2 s (±30.96 SD). Few, if any transmissions were missed by the receivers. During the 6-day pen phase the shark ingested fish fillets on four separate days in addition to the initial tag ingestion and the time of each feeding was recorded. Ambient temperature in the pen was variable ranging between 18.35 and 21.28 °C (mean = 19.60 °C). Despite the variable ambient temperature, all five recorded feeding events were clearly evident as local minima in the stomach temperature time series characterized by a sudden drop in temperature, presumably from the ingestion of relatively colder food or ambient seawater. This temperature dip was typically followed by a steady and prolonged increase consistent with the heat increment of digestion. During the control period (the following 6 days in the Aquarium) ambient temperature remained relatively constant (mean 20.04 ± 0.089 °C). Each of the four controlled feedings in the Aquarium was clearly discernible by the same distinctive sudden drop in stomach temperature followed by steady heat increment associated with digestion.
Stomach temperature of captive white shark. The stomach temperature of a juvenile white shark (black line) reflected controlled feedings (arrows), in both a variable and controlled ambient temperature (blue line) environment (ocean pen and Monterey Bay Aquarium display, respectively). Feeding events were associated with a sudden temperature drop, followed by a steady and sustained rise in both environments
Some additional stomach temperature dips were recorded at times other than when food was intentionally offered both in the pen and inside the Aquarium (for instance on day eight; Fig. 4) and may be associated with ad lib feeding events. Subsequent steady rises in the stomach temperature were consistent with caloric intake, whereas a temperature dip without a subsequent increment would suggest water influx without food. At both locations, schooling fishes such as Pacific sardine (Sardinops sagax) were available for potential predation by the instrumented white shark; therefore, the occurrence of additional uncontrolled feeding events could not be ruled out.
In the wild shark instrumented with the archival accelerometer and temperature sensor package (Table 1; Deployment 4), a signature reduction of body temperature followed by warming suggested that foraging was also being recorded (Fig. 5a). In addition to the initial feeding accompanying the tag ingestion, a single feeding event was recorded on day five of the data record. The thermal record of this event resembled both the initial tag feeding in this and seven additional deployments, as well as the controlled feeding events in the captive white shark. In this instance a series of sudden temperature drops over a 30- to 40-min period (Fig. 5b) was followed by a steady rise in the stomach temperature from 22.4 to 25.6 °C over the subsequent day. In this case, the corrected accelerometer data provided additional support for identifying the feeding event.
Feeding event shown in white shark stomach tag data. The stomach temperature of a wild white shark (a) showing a feeding signature consistent with those found in the controlled feeding experiment. A more detailed (300 min window; outlined in box in a) look at this feeding event revealed a series of 5 stomach temperature dips (b) consistent with multiple ingestions. The series of ‘ingestion’ occurred after a period (c) of near-surface swimming, following a series of three vertical approaches to the surface. The first ascent was the longest and steepest and was associated with a large peak in sustained active acceleration (c) shown by the overall body acceleration (ODBA) smoothed to distinguish high and sustained energy exertion through accelerating motion
Approximately 60 min before the series of stomach temperature dips, there was a large burst in activity evident from the peak in smoothed ODBA (minute 30; Fig. 5d). This burst of acceleration was associated with a sudden and rapid ascent from a depth of ~28 m toward the surface (Fig. 5c). Two additional ascents toward the surface followed the initial rush, then a relatively constant depth (3–5 m) was maintained for the next 45 min until the stomach temperature dips occurred, after which point a long period of depth oscillations between the surface and 10–45 m ensued. A closer look at the burst ascent is presented in a 220 s window in Fig. 6. The coordinate correction enabled a detailed analysis of the surge, sway and heave (blue, red and green lines, respectively; Fig. 6b). In the minutes preceding the burst ascent the shark maintained a relatively constant depth of ~28 m. At second 70 the shark initiated the steep burst ascent and tailbeat amplitude increased substantially (red line; Fig. 6b). At the peak of the ascent the heaving acceleration approached 0 while surge approached 1, consistent with a near vertical body orientation. The ascent leveled off just below the surface at second 90 and a few seconds later there was a large and sudden dip in the surge and sway. Between this point and second 130, there were numerous erratic changes in orientation along with sustained high levels of ODBA. At second 130 the shark descended gradually to 20 m and once again ascended toward the surface. On the second ascent, the increased amplitude in tailbeat was again evident, but less pronounced than the first ascent.
Prey capture shown in white shark stomach tag data detail. Data detail showing the rapid ascent (a) from 28 m to the near-surface associated with elevated ODBA. The corrected X, Y, and Z accelerometer traces (b) show the surge (blue), sway (red), and heave (green), respectively, enabling detailed reconstruction of the event. Oscillations in the swaying acceleration (red) reflect the tail beat frequency and amplitude. The location of the three X and Y traces relative to 0 g and the Z trace relative to −1 g reflect the body orientation. For example, during the peak of the ascent the shark was nearly vertical. The ODBA trace (c) reflects an integrated measure of active acceleration. Note that ODBA was elevated during the burst ascent, and sustained at a higher level for an additional 30 s after the peak of the ascent, consistent with a struggle with a relatively large prey
Discussion
The field of biologging has increasingly relied on the use of accelerometers and other motion-based sensors as vital new tools to advance physiological, kinematic, and behavioral aspects of animal ecology [6, 30–32]. However, key challenges remain for increasing deployment feasibility and reducing deployment related stress that can introduce behavioral artifacts and animal health risks [18]. One key challenge is to further develop tag placement options that can reduce stress and additionally provide secondary validation for identifying specific biological activities such as foraging. Here we demonstrated the effective use of motion-sensitive data loggers inside the stomach of a large swimming endotherm, the white shark. By introducing the tag wrapped in appropriate food material, the instruments were voluntarily consumed and stress was minimized. The instrument packages were naturally retained in the sharks’ stomach for time periods appropriate to the memory and power capacity of contemporary instruments (2–29 days). We also overcame the requirement to firmly mount the instruments using potentially invasive techniques, and instead solved for the tag’s orientation inside the shark’s stomach and then reoriented the coordinate space in post-process.
Stomach tags have been used to measure and identify signature temperature changes and quantify feeding in a variety of endothermic marine taxa including fishes [24], birds [33], and mammals [34]. In combination, acceleration and stomach temperature data have the capacity to distinguish between successful and attempted prey capture, while stomach temperature alone cannot detect unsuccessful foraging attempts, and acceleration alone cannot confirm successful ingestion of prey. Distinguishing foraging attempts and successful ingestion can help better inform energy budgets and foraging strategies which can be particularly relevant for species such as large predatory sharks that may forage only occasionally [35]. This approach could also be extended to ectothermic species by recording additional measurements such as impedance [28] that can reflect digestive activity when temperature alone cannot.
Acceleration data can also provide invaluable data on hunting behavior. In this record presented, after coordinate space correction, we were able to recreate the motion and locomotion of the shark during a successful predation event. The captive feeding experiment provided an opportunity to evaluate stomach temperature responses to feeding in both constant and variable ambient temperature environments, validating the interpretation of the wild shark record. For instance, the captive shark was offered single bite-size meals, and the stomach temperature dips corresponded to each feeding event. In the wild shark record there was a series of temperature dips over approximately 45 min. A series of temperature dips, rather than a single dip, could result from multiple feedings on small prey, or consumption of a large prey requiring multiple bites to consume. Capturing multiple small prey should elicit a corresponding number of locomotory bursts from the predator, whereas capturing one large prey could result from only a single relatively large burst of acceleration. The single large burst in ODBA in the wild shark prior to the stomach temperature dips (Fig. 6c) was therefore more consistent with pursuit and capture of a single large prey item, which was then consumed in a series of bites. The corrected heave, surge, and sway traces together with the depth and temperature data enable further reconstruction of the predation sequence.
During coastal foraging in California white sharks commonly patrol at depths between 5 and 50 m [36, 37], presumably facilitating visual perception of pinniped prey silhouetted against the diffuse lighting of the ocean surface [38, 39]. Visually oriented vertical approaches to experimental decoys are typically initiated from depths ≥17 m [40]. The constant depth (~28 m) swimming in the minutes before the burst assent in our wild shark data was consistent with this scenario (Fig. 6a, b). Contact with the prey likely occurred at the apex of the ascent just after second 90 (Fig. 6). At this point, there was a sudden decrease in surge and sway, while heave remained relatively constant, indicative of sudden deceleration or rapid turning. Such sudden deceleration is consistent with impacting a large prey item. Following this purported initial ‘strike,’ a sustained period of relatively high overall acceleration and body movement followed, lasting about 30 s. Together, the burst ascent and period of elevated body movement near the surface lasted 40–50 s and accounted for the sustained elevated ODBA period prior to ingestion (Figs. 5d, 6c).
Interestingly, the series of stomach temperature dips indicating ingestion only began some 60 min after the burst ascent. Following initial strikes, white sharks often release their prey and return to it after minutes or hours [38, 41]. Occasionally, they also seize and transport pinniped prey tens or hundreds of meters away [41] (unpublished observations). Relocating a large prey outside the initial blood plume before partitioning into bites may reduce competition from opportunistic conspecifics attracted by the scent. After the initial burst ascent, the shark made two more ascents to a similar shallow depth over a period of 10 min (Fig. 6a). Following the third ascent, there was period of near-surface swimming (Fig. 6b) characterized by low sustained ODBA (Fig. 6c) and gradually decreasing stomach temperature (Fig. 6b). This period lasted almost an hour and ended with the onset of the stomach temperature dips (~minute 100). This discrete pattern of relatively constant depth swimming differed substantially from the subsequent depth oscillations after minute 120 when the stomach temperature rose smoothly indicating feeding was complete. Between the purported initial ‘strike’ and the ingestion of the prey, the stomach temperature decreased gradually but unevenly (Fig. 6; minutes 30–90). This variable decrease likely reflected small intermittent influxes of ambient seawater, which might be expected if the shark swam with mouth opened wider than usual while holding and transporting the prey.
This detailed reconstruction provides a proof of concept for stomach deployment of motion-based sensors including accelerometers. It has to be noted that during accelerated motions accelerometers become increasingly unreliable in estimating attitude in sea turtles, and that full inertial sensors, including gyroscopes are needed to more accurately determine pitch and roll [42]. This same technique can extend to magnetometer and gyroscopic sensors as well, and should lead to even better behavior reconstruction.
Successful re-orientation of the coordinate space depends on the diving behavior of the subject. The time window over which mean position of the shark can be assumed to be prone will vary as a function of factors such as habitat depth, and extent and frequency of vertical excursions. For example in a subject that makes only short ascents and descents (narrow depth-range), a relatively small time window will suffice compared to a subject that makes longer or slower vertical excursions. Therefore, the appropriate time window should be calculated independently for each deployment while the same threshold for variance and mean vertical displacement can be used as described here (Fig. 2).
This specific example of successful accelerometer logger deployment and data interpretation illustrates a general approach to deployment options that does not require fastening or a priori knowledge of logger orientation. This approach can be extended to numerous other systems, and to cases where recalibration is necessary following post-deployment shifting of logger orientation. Although approaches to solving logger orientation will likely differ between swimming and other modes of locomotion, the same principles will apply in many cases.
Conclusions
In this example of accelerometer deployment, we provide proof of concept for a novel and non-invasive technique extending the possible uses of motion sensors such as accelerometers, magnetometers and gyroscopes in bio-logging. By placing data logging tags in the stomachs of endothermic white sharks through voluntary consumption, we were able to produce high quality tri-axial acceleration data in addition to stomach temperature data to detect feeding events and behavior. In this application, the technique has the potential for distinguishing between attempted and successful foraging events. Furthermore, this approach to motion sensor deployment and coordinate correction can be applied in other systems where logger orientation is unknown or has changed after deployment.
References
Cooke SJ, Hinch SG, Wikelski M, Andrews RD, Kuchel LJ, Wolcott TG, Butler PJ. Biotelemetry: a mechanistic approach to ecology. Trends Ecol Evol. 2004;19:334–43.
Ropert-Coudert Y, Wilson RP. Trends and perspectives in animal-attached remote sensing. Front Ecol Environ. 2005;3:437–44.
Block BA. Physiological ecology in the 21st century: advancements in biologging science. Integr Comp Biol. 2005;45:305–20.
Kooyman GL. Genesis and evolution of bio-logging devices: 1963–2002. Mem Natl Inst Polar Res. 2004;58:15–22.
Rutz C, Hays GC. New frontiers in biologging science. Biol Lett. 2009;5:289–92.
Wilson RP, Shepard ELC, Liebsch N. Prying into the intimate details of animal lives: use of a daily diary on animals. Endanger Species Res. 2008;4:123–37.
Sato K, Mitani Y, Cameron MF, Siniff DB, Naito Y. Factors affecting stroking patterns and body angle in diving Weddell seals under natural conditions. J Exp Biol. 2003;206:1461–70.
Shepard ELC, Wilson RP, Quintana F, Laich AG, Forman DW. Pushed for time or saving on fuel: fine-scale energy budgets shed light on currencies in a diving bird. Proc R Soc Lond B Biol Sci. 2009:rspb20090683.
Gleiss AC, Wright S, Liebsch N, Wilson RP, Norman B. Contrasting diel patterns in vertical movement and locomotor activity of whale sharks at Ningaloo Reef. Mar Biol. 2013;160:2981–92.
Nakamura I, Watanabe YY, Papastamatiou YP, Sato K, Meyer CG. Yo-yo vertical movements suggest a foraging strategy for tiger sharks Galeocerdo cuvier. Mar Ecol Prog Ser. 2011;424:237–46.
Tanaka H, Takagi Y, Naito Y. Swimming speeds and buoyancy compensation of migrating adult chum salmon Oncorhynchus keta revealed by speed/depth/acceleration data logger. J Exp Biol. 2001;204:3895–904.
Graves JE, Horodysky AZ, Latour RJ. Use of pop-up satellite archival tag technology to study postrelease survival of and habitat use by estuarine and coastal fishes: an application to striped bass (Morone saxatilis). Fish Bull. 2009;107:373–83.
Moyes CD, Fragoso N, Musyl MK, Brill RW. Predicting postrelease survival in large pelagic fish. Trans Am Fish Soc. 2006;135:1389–97.
Skomal GB, Bernal D. Physiological responses to stress in sharks. In: Carrier JC, Musick JA, Heithaus MR, editors. Sharks and their relatives: biodiversity, adaptive physiology and conservation. Boca Raton: CRC Press; 2010. p. 459–90.
Block BA, Dewar H, Blackwell SB, Williams TD, Prince ED, Farwell CJ, Boustany A, Teo SL, Seitz A, Walli A, et al. Migratory movements, depth preferences, and thermal biology of Atlantic bluefin tuna. Science. 2001;293:1310–4.
Graham RT, Roberts CM, Smart JCR. Diving behaviour of whale sharks in relation to a predictable food pulse. J R Soc Interface. 2006;3:109–16.
Jorgensen SJ, Reeb CA, Chapple TK, Anderson S, Perle C, Van Sommeran SR, Fritz-Cope C, Brown AC, Klimley AP, Block BA. Philopatry and migration of Pacific white sharks. Proc R Soc B Biol Sci. 2010;277:679–88.
Gleiss AC, Norman B, Liebsch N, Francis C, Wilson RP. A new prospect for tagging large free-swimming sharks with motion-sensitive data-loggers. Fish Res. 2009;97:11–6.
Gleiss AC, Norman B, Wilson RP. Moved by that sinking feeling: variable diving geometry underlies movement strategies in whale sharks. Funct Ecol. 2011;25:595–607.
Whitney NM, Papastamatiou YP, Gleiss AC. Integrative multi-sensor tagging: emerging techniques to link elasmobranch behavior, physiology and ecology. In: Carrier JC, Musick JA, Heithaus MR, editors. Biology of sharks and their relatives. Second. Boca Raton: CRC Press; 2012. p. 265–84.
Skomal GB. Evaluating the physiological and physical consequences of capture on post-release survivorship in large pelagic fishes. Fish Manag Ecol. 2007;14:81–9.
Sundström LF, Gruber SH. Effects of capture and transmitter attachments on the swimming speed of large juvenile lemon sharks in the wild. J Fish Biol. 2002;61:834–8.
Cooke SJ. Biotelemetry and biologging in endangered species research and animal conservation: relevance to regional, national, and IUCN Red List threat assessments. Endanger Species Res. 2008;4:165–85.
Carey FG, Kanwisher JW, Don Stevens E. Bluefin tuna warm their viscera during digestion. J Exp Biol. 1984;109:1–20.
Whitlock RE, Walli A, Cermeño P, Rodriguez LE, Farwell C, Block BA. Quantifying energy intake in Pacific bluefin tuna (Thunnus orientalis) using the heat increment of feeding. J Exp Biol. 2013;216:4109–23.
Clark TD, Brandt WT, Nogueira J, Rodriguez LE, Price M, Farwell CJ, Block BA. Postprandial metabolism of Pacific bluefin tuna (Thunnus orientalis). J Exp Biol. 2010;213:2379–85.
McCosker JE. The white shark, Carcharodon carcharias, has a warm stomach. Copeia. 1987;1987:195–7.
Meyer CG, Holland KN. Autonomous measurement of ingestion and digestion processes in free-swimming sharks. J Exp Biol. 2012;215:3681–4.
Ezcurra J, Lowe C, Mollet H, Ferry L, O’Sullivan J. Captive feeding and growth of young-of-the-year white sharks, Carcharodon carcharias, at the Monterey Bay Aquarium. In: Domeier ML, editor. Global perspectives on the biology and life history of the white shark. Boca Raton: CRC Press; 2012. p. 3–16.
Shepard E, Wilson R, Halsey L, Quintana F, Laich A, Gleiss A, Liebsch N, Myers A, Norman B. Derivation of body motion via appropriate smoothing of acceleration data. Aquat Biol. 2009;4:235–41.
Gleiss AC, Jorgensen SJ, Liebsch N, Sala JE, Norman B, Hays GC, Quintana F, Grundy E, Campagna C, Trites AW, Block BA, Wilson RP. Convergent evolution in locomotory patterns of flying and swimming animals. Nat Commun. 2011;2:352.
Williams TM, Wolfe L, Davis T, Kendall T, Richter B, Wang Y, Bryce C, Elkaim GH, Wilmers CC. Instantaneous energetics of puma kills reveal advantage of felid sneak attacks. Science. 2014;346:81–5.
Wilson R, Püt K, Grémillet D, Culik B, Kierspel M, Regel J, Bost C, Lage J, Cooper J. Reliability of stomach temperature changes in determining feeding characteristics of seabirds. J Exp Biol. 1995;198:1115–35.
Kuhn CE, Costa DP. Identifying and quantifying prey consumption using stomach temperature change in pinnipeds. J Exp Biol. 2006;209:4524–32.
Carey FG, Kanwisher JW, Brazier O, Gabrielson G, Casey JG, Pratt HL. Temperature and activities of a white shark, Carcharodon carcharias. Copeia. 1982;1982:254–60.
Goldman KJ, Anderson SD. Space utilization and swimming depth of white sharks, Carcharodon carcharias, at the South Farallon Islands, central California. Environ Biol Fishes. 1999;56:351–64.
Jorgensen SJ, Arnoldi NS, Estess EE, Chapple TK, Rückert M, Anderson SD, Block BA. Eating or meeting? Cluster analysis reveals intricacies of white shark (Carcharodon carcharias) migration and offshore behavior. PLoS One. 2012;7:e47819.
Tricas TC, McCosker JE. Predatory behavior of the white shark (Carcharodon carcharias), with notes on its biology. Proc Calif Acad Sci. 1984;43:221–38.
Martin RA, Hammerschlag N. Marine predator–prey contests: ambush and speed versus vigilance and agility. Mar Biol Res. 2012;8:90–4.
Strong Jr WR. Shape discrimination and visual predatory tactics in white sharks. Gt White Sharks Biol Carcharodon Carcharias Acad Press NY. 1996:229–40.
Klimley AP, Pyle P, Anderson SD. The behavior of white sharks and their pinniped prey during predatory attacks. Gt White Sharks Biol Carcharodon Carcharias Ed AP Klimley DG Ainley. 1996:175–91.
Noda T, Okuyama J, Koizumi T, Arai N, Kobayashi M. Monitoring attitude and dynamic acceleration of free-moving aquatic animals using a gyroscope. Aquat Biol. 2012;16:265–76.
Authors’ contributions
Conceived and designed the experiments: SJJ ACG JME BAB. Performed the experiments: SJJ ACG PEK SDAWTB TKC PEK. Analyzed the data: SJJ WTB. Contributed reagents/materials/analysis tools: SJJ ACG BAB. Wrote the paper: SJJ ACG. Edited and improved the manuscript: ACG BAB JME PEK. All authors read and approved the final manuscript.
Acknowledgements
We thank J. Barlow, R. Elliot, A. Carlisle, R. Hamilton, T. O’Leary, C. Logan, J. Cornelius, B. Becker, C. Farwell, C. Harrold, R. Kochevar, K. Lewand, J. O’Sullivan, J. Ganong, L. Rodriguez, J. Welsh, M. Murray, C. Lowe, B. Bettencourt, A. Swithenbank, M. Castleton, A. P. Klimley, and C. Winkler and the entire elasmobranch husbandry team at the Monterey Bay Aquarium for assistance with field work, lab work, data processing, editing and inspiration. We are grateful to the UC Davis boating program, and the crew of the F/V Barbara H for vessel support. Funding was provided by the Monterey Bay Aquarium Foundation.
Competing interests
The authors declare no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional files
40317_2015_71_MOESM1_ESM.m
Additional file 1. Jorgensen2015_AI1_time_window.m. Matlab routine for analyzing depth time series to determine time window over which average body position is prone relative to earth’s gravity. It requires some user inputs for specific values unique to individual datasets and computer pathways. This file can be viewed by an text editor, and run in Matlab.
40317_2015_71_MOESM2_ESM.m
Additional file 2. Jorgensen2015_AI2_find_up.m. Matlab routine for transforming X, Y, Z acceleration data to orient Z axis vertically with respect to earth’s gravity. Requires definition of time window for parsing data based on results in Jorgensen2015_AI1_time_window.m. This file can be viewed by an text editor, and run in Matlab.
40317_2015_71_MOESM3_ESM.m
Additional file 3. Jorgensen2015_AI3_maximize_tailbeat.m. Matlab routine for transforming X, Y, Z acceleration data to orient Y axis perpendicular a swimming shark’s prone body (and perpendicular to earth’s gravity) by aligning Y axis parallel with maximum swaying acceleration. Requires X, Y, Z data transformed from Jorgensen2015_AI2_find_up.m. This file can be viewed by an text editor, and run in Matlab.
40317_2015_71_MOESM4_ESM.m
Additional file 4. fft_rot.m. Matlab function for fast fourier analysis called out from Jorgensen2015_AI3_maximize_tailbeat.m. This file can be viewed by an text editor, and run in Matlab.
40317_2015_71_MOESM5_ESM.m
Additional file 5. roty3.m. Matlab function for three dimensional coordinate transformation called out from Jorgensen2015_AI3_maximize_tailbeat.m. This file can be viewed by an text editor, and run in Matlab.
40317_2015_71_MOESM6_ESM.m
Additional file 6. rotx3.m. Matlab function for three dimensional coordinate transformation called out from Jorgensen2015_AI3_maximize_tailbeat.m. This file can be viewed by an text editor, and run in Matlab. ***Additional file 7 – rotz3.m. Matlab function for three dimensional coordinate transformation called out from Jorgensen2015_AI3_maximize_tailbeat.m. This file can be viewed by an text editor, and run in Matlab.
40317_2015_71_MOESM7_ESM.m
Additional file 7. rotz3.m. Matlab function for three dimensional coordinate transformation called out from Jorgensen2015_AI3_maximize_tailbeat.m. This file can be viewed by an text editor, and run in Matlab.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Jorgensen, S.J., Gleiss, A.C., Kanive, P.E. et al. In the belly of the beast: resolving stomach tag data to link temperature, acceleration and feeding in white sharks (Carcharodon carcharias). Anim Biotelemetry 3, 52 (2015). https://doi.org/10.1186/s40317-015-0071-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40317-015-0071-6