Abstract
Background
Fish movements are often studied using radio or acoustic tags assuming the handling and tagging procedures have little effect on the behavior of the animal. Indeed, many studies provide guidelines for acceptable methods. However, these studies generally assume the fish are otherwise healthy but this may not always be the case. One example is the infection of juvenile salmon in the western USA by the naturally-occurring parasitic copepod Salmincola californiensis, for which little is known about the effects on results from tagged animals. We report on observational data from juvenile Chinook salmon (Oncorhynchus tshawytscha) surgically implanted with telemetry tags relative to the numbers of S. californiensis within their branchial cavities and on their bodies to determine if the intensity of infection resulted in differences in mortality shortly after tagging or post-release activity in a reservoir over a period of about 4 months.
Results
The data indicate a negative effect of copepods in the branchial cavities on short-term mortality (within 24 h of tagging) and suggest negative effects on movements after release into the reservoir. Short-term mortalities were infrequent and, due to the observational nature of the data, few tagged fish had more than three copepods in their branchial cavities, although surveys of fish in the reservoir indicate much greater infection intensities are common. Copepod numbers on the body did not appear to be associated with short-term mortality or movements after release. The number of copepods on the body was unrelated to the number within the branchial cavities, indicating site-specific counts are needed to assess the infection.
Conclusion
Infection with Salmincola californiensis is common in juvenile Chinook salmon in western USA reservoirs and may affect the viability of fish used in studies of telemetered animals. Our limited assessment suggests infection by Salmincola californiensis affects the short-term morality of tagged fish and may affect long-term viability of tagged fish after release; however, the intensity of infection in the sample population did not represent the source population due to the observational nature of the data. We suggest these results warrant further study into the effects of infection by Salmincola californiensis on the results obtained through active telemetry and perhaps other methods requiring handling of infected fish.
Similar content being viewed by others
Background
The topic of selecting subjects for use in studies using telemetry is one each researcher must address based on the goals of the study. In some cases subjects are randomly or opportunistically selected from the population [1, 2], but more often they are selected for particular traits, such as being large enough to carry the transmitter [3] or being disease- or injury-free [4, 5]. Selection of disease- or injury-free subjects may not be warranted or possible in all cases. One example is the study of juvenile Chinook salmon (Oncorhynchus tshawytscha) inhabiting some rivers and reservoirs in western USA, where the parasitic copepod Salmincola californiensis is ubiquitous.
The number and types of studies of juvenile Chinook salmon in western Oregon USA has increased sharply in recent years due to a 2008 finding that the existing system of dams for flood control and hydropower jeopardizes certain salmonid stocks [6]. Many of these studies use cultured fish raised in conventional or experimental hatcheries in lieu of naturally-produced fish, primarily due to the difficulty in capturing fish from deep flood-control reservoirs; however, data from naturally-produced fish are preferred when possible. Active telemetry is a common tool in these studies and requires that the tagged population be carefully selected from the available untagged population to ensure the results represent untagged fish as closely as possible [7]. There is currently little information about the effects of S. californiensis on the health or behavior of juvenile Chinook salmon, particularly when telemetry tags are used.
Copepods of the genus Salmincola are common ectoparasites of salmonid fishes [8–10]. These freshwater animals have several life stages culminating in a parasitic female that attaches to the host, usually prior to copepod egg fertilization and incubation [11]. Kabata and Cousens [11] described the adult female of S. californiensis as about 4 mm long and the adult male about one quarter of the length of the female, making the adult female (often with egg sacs) the most likely life stage observed during macroscopic examination. The parasites attach externally and have been observed on nearly all external surfaces of the body, but most commonly on the gills, opercula, and fin bases [12]. An attachment site is excavated by the parasite and it feeds on epithelial cells it scrapes from the nearby tissue [11, 12]. Damage to gill filaments of the host due to the presence of the parasite has been associated with loss of fitness due to impairment of gas exchange from a reduction in functional gill tissue and has been described affecting fecundity and temperature tolerance [8, 12, 13]. Several control measures have been described for cultured populations [14–16].
Salmincola californiensis has been found on several species of Pacific salmonids, including those found in western Oregon reservoirs [9, 10, 17–19]. Monzyk et al. [18, 19] described the copepod on naturally-produced juvenile Chinook salmon in reservoirs on two tributaries of the Willamette River in Oregon with prevalence nearing 90% and noted intensities ranging to over 20 copepods per fish. Effects of these parasites on their hosts have been described in laboratory and hatchery environments, but descriptions of the effects on free-ranging fishes are lacking. Here we describe observational data on the short-term mortality and long-term activity of telemetered naturally-produced juvenile Chinook salmon in a reservoir on a Willamette River tributary relative to infection with S. californiensis to provide information on the potential effects of infection on data from tagged fish.
Methods
The data were collected from fish at Cougar Reservoir, a flood-control reservoir on a tributary of the Willamette River in western Oregon. This 8 km long reservoir in the west slope of the Cascade mountain range was created in 1963 by the construction of Cougar Dam on the South Fork of the McKenzie River about 74 km east of Eugene, Oregon. During 2011 and 2012, we macroscopically examined 281 juvenile Chinook salmon of natural origin when selecting candidates for surgical implantation of transmitters for a study of in-reservoir behavior and dam passage; details of the source study are described by Beeman et al. [20–22]. The fish we report on were captured from within Cougar Reservoir using a 91.7 m-long Lampara seine that fished up to 7.6 m deep. We also examined 1,800 hatchery-reared fish collected from two nearby hatcheries, but only one copepod was found during macroscopic examinations and we do not describe them further. The natural-origin fish were age 0 + (fall samples) and 1 + (spring samples) based on their length and had likely been in the reservoir for 7–20 months prior to tagging.
Fish were collected, tagged, and released during spring (March, April and May) of 2011 and in the fall (October and November) of 2011 and 2012. Fish were considered suitable for tagging if they were between 95 and 180 mm in fork length, were free of major injuries, had no external signs of gas bubble trauma or fungus, were less than or equal to 20% descaled, and were not previously tagged with telemetry tags. In the fall of 2011, fish with large numbers of copepods in the branchial cavities, including the gill arches, gill filaments, and medial sides of the operculum of each side, were often rejected from tagging due to the poor expected outcome from the handling and surgical procedures. In 2012, an explicit selection criterion was adopted by which fish with more than a total of five copepods in the branchial cavities were omitted from tagging due to regional concerns about the potential effects of the copepods on fish health (recognizing that no data were available for setting such a criterion). We therefore describe prevalence and intensity data from both years but only use data from 2011 in statistical analyses due to the copepod number restriction in 2012.
Tag implantation and fish recovery were completed at the Cougar Dam adult fish facility slightly downstream from the dam. The procedures are described briefly here and in detail by Surgical Protocols Steering Committee [23]. To implant the transmitter, fish were anesthetized using buffered tricane methanesulfonate (MS-222, Argent Chemical Laboratories, Redmond, WA, USA) at a concentration of 75–80 mg/L in water with a temperature ranging from 6.4 to 14.4°C and averaging 8.3°C. Water temperatures at the tagging location often differed from those of the reservoir upstream, requiring tempering at a rate of 0.5°C per 15 min until they were within 2°C prior to transfer to pre-tag holding containers. All weighing, measuring, and containment equipment was treated with a 0.25 mL/L concentration of Stress Coat® (Aquarium Pharmaceuticals, Inc., Chalfont, PA, USA) to reduce electrolyte-related handling stress. Fish weight and length and incidental observations such any injuries, descaling, and the location and number of copepods on the body and in the branchial cavities were recorded from anesthetized fish prior to surgery, except that copepod location was not recorded in the spring of 2011. Tags were inserted through a 3–5 mm long incision along the linea alba anterior to the pelvic girdle. The incision was closed with two simple interrupted sutures using 5-0 monofilament (Ethicon Monocryl©). Up to three fish were placed in 7 L of river water within a 19 L partially-perforated bucket following surgery. Buckets were then fitted with lids and placed in a raceway provided with flowing river water, where fish were held 18–36 h. Each bucket was floated in the raceway using a bicycle rubber inner tube around its top to allow fish access to air to adjust their buoyancy. Fish were released from the buckets at the water surface after transport to a site near the head of the reservoir. The time from first exposure to anesthetic to the end of the surgery averaged 6.9 min (range 5.4–8.4 min) and the time from the beginning of the external examination to the end of surgery averaged 2.6 min (range 1.4–3.5 min). Fish were divided into Reject, Mortality, and Released groups to denote fish rejected from tagging, tagged but dying prior to release, and tagged and released alive. Fish handling, including anesthesia and surgery, was performed by skilled trained staff.
Each tagged fish received an acoustic tag and a passive integrated transponder tag. The dimensions of the acoustic tag (Juvenile Salmon Acoustic Telemetry System [24]) varied between years. In 2011 the acoustic tag dimensions (mm) were 12 high × 5 wide × 4 deep, with an average mass of 0.43 g in air (0.42 g in spring, 0.44 g in fall). In 2012 the acoustic tag dimensions (mm) were 11 long × 5 wide × 3 deep with a mass of 0.31 g in air. The 90th percentile of acoustic tag life, empirically determined from a subsample of 50 tags in each year and season, ranged from 66.0 to 129.0 days depending on the battery type and pulse rate. The full-duplex passive integrated transponder tag was 12.5-mm long, had a diameter of 2 mm, and weighed 0.10 g.
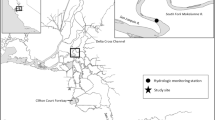
Fish movements were determined from detections at hydrophones placed throughout the reservoir. Sixteen hydrophones were spaced among six arrays in the main body of the reservoir and 12–16 hydrophones, depending on year, were placed within about 100 m of the dam outlet (Figure 1). The hydrophones within arrays in the main body of the reservoir were spaced about 100 m from shore and 200 m apart based on empirical data collected in situ indicating 82% of expected transmissions were detected at range of 105 m from a hydrophone (JWB, unpublished data). Hydrophones near the dam outlet were spaced closer together to enable estimation of fish positions, but only fish presence is reported here. The detection probabilities of the arrays were empirically estimated to be at or near 1.0 during each year of study. Further details of the telemetry detection equipment can be found in Beeman et al. [20–22].
The prevalence and intensity of infection by adult female copepods, post-tag mortality, and time from release until the last known fish movement were summarized. Prevalence was calculated as the percent of fish infected with adult female copepods. Intensity, as the sum of copepods on both sides of the fish, was divided into areas of the branchial cavities, body, and total (branchial cavities + body) for fish bearing copepods. Pearson product-moment correlation was used to determine the association between intensity, fork length, and the number of copepods in the branchial cavities versus the rest of the body. Post-tag mortality, the mortality of tagged fish prior to release on the four dates with mortalities, was statistically compared among fish with and without copepods in either the branchial cavities or on the body using Fischer’s Exact test and an α = 0.10. Post-tag mortality data from fish with three or more copepods in the branchial cavities or two or more on the body were pooled prior to analysis due to small sample sizes of fish with the highest copepod intensities. The time of the last known fish movement was assigned as the time of the first detection at the last array of detection and was presumed a surrogate for mortality after adjusting for empirically-determined tag lives and dam passage; other potential factors such as tag loss were assumed to be independent from infection. The distributions of the times from release to this event were estimated using the Kaplan–Meier survivorship function with subjects censored at the time of dam passage or at the estimated 90th percentile of their tag life. The sparseness of the data required pooling fish with four or more copepods in the branchial cavities as well as pooling fish with more than one copepod on the body. Data from 2012 were omitted from analyses of post-tag mortality and time to the last known movement due to the restrictive selection criterion applied that year. In addition, the event times were censored at 129.0 days to coincide with the empirically-determined 90th percentile of tag life in 2011. Statistical significance was assessed using the Log-Rank test and an α of 0.10 [25]. Analyses were completed using SAS/STAT® software, version 9.3, of the SAS System for Windows Copyright © 2002–2010 SAS Institute, Inc.
Results
The prevalence of infection was similar between years and varied slightly among Reject, Release, and Mortality groups. The prevalence across years was 86.5% (Table 1). The prevalence of the groups ranged from 87.8 to 100.0% in 2011 and from 75.0 to 100.0% in 2012. Copepods were noted in the branchial cavities of 74.7% of the fish and on the bodies of 38.1% of the fish.
The intensity of infection was generally similar between years but varied among groups and location. At a mean intensity of 2.6 and a range of 0–19, the branchial cavities had more copepods than the body (mean 0.8, range 0–7, Table 1). The maximum intensity of 19 copepods in the brachial cavities was from a fish in the fall 2011 Reject group that had 3 copepods on the body. Total intensity (branchial cavities plus body) averaged 3.4 copepods per infected fish, ranged from 1 to 22 per individual, and was primarily a reflection of the numbers in the branchial cavities. The intensities of infection of the Release group in 2011 (used for statistical analyses) were low relative to the Reject group, but similar to those of the Mortality group in that year. There was a weak positive correlation between fish length and number of copepods (including zero) in the branchial cavities (r = 0.2249, P = 0.0007, N = 222), but no statistically significant relation between length and the number on the body (r = 0.0386, P = 0.5671, N = 222). Moreover, the number on the body was uncorrelated with the number within the branchial cavities (r = 0.0941, P = 0.1294, N = 261), indicating one had no predictive value for the other.
Post-tag mortalities were infrequent (2 of 6 tag dates in each year) and 11 of the 16 mortalities occurred in fish tagged on one date in 2011. The sample dates with mortalities were the first two of the fall season in each year (October 18 and 19, 2011 and October 24 and 25, 2012), when surface water temperatures were an average of 13.6°C in 2011 and 10.9°C in 2012 compared to 6.9–10.5°C (2011) and 6.4–7.8°C (2012) for the later dates (ranging from November 2 to 29).
In most cases, the post-tag mortality of fish with even one copepod was greater than uninfected fish, but the data were quite variable and fish with more than three copepods in their branchial cavities or two on their bodies were rare (Figure 2). There was a statistically significant effect of post-tag mortality associated with intensity in the branchial cavities (df = 3, P = 0.0273), but not on the body (df = 2, P = 0.8374).
The data suggest a negative effect on the long-term movements of infected fish, but the sparseness of the observational data precludes meaningful conclusions. The range of times from release to the last known movement was smallest for fish with four or more copepods in the branchial cavities but did not appear to be affected by the number on the body. Event times of fish tagged in 2011 with four or more copepods in their branchial cavities were much shorter than those of fish with a lesser intensity, but there were few fish in this category (Figure 3). Event times of fish with no more than three copepods in the branchial cavities were up to 129.0 days. The longest event time of a fish with four or more copepods in the branchial cavities (N = 11) was 96.6 days and all but three of the fish had event times of 19.9 days or less. Despite these trends, the data does not support significant differences among the event time distributions of fish with 0, 1, 2, 3, or four or more copepods in the branchial cavities (df = 4, χ 2 = 0.9451, P = 0.9180) nor for fish with 0, 1, or 2 or more copepods on the body (df = 2, χ 2 = 0.9831, P = 0.6256).
Time at large (release to last known movement) versus number of S. californiensis in the branchial cavities (left panel) or on the body (right panel). Open symbols indicate observations censored at dam passage or the 90th percentile of tag life, solid symbols indicate the last known observation of fish with unknown passage.
Discussion
The criteria for selecting research subjects in studies using tagged animals must be carefully chosen. Animals must certainly be capable of carrying the transmitter and surviving in a normal fashion with respect to the behaviors of interest, but the set of criteria chosen to achieve that can be quite variable. Descriptions of the effects of telemetry tags and tagging on fish swimming performance, stress response, predator avoidance, buoyancy and other measures are widespread in the literature; however, such studies often assume the subjects are otherwise healthy. Of course, the goal is to ensure that inferences made from tagged animals represent the larger population of untagged animals. From that standpoint, some may argue that all animals in the sample should be tagged. After all, if we are to represent untagged fish, should not there be similar proportions of healthy, sick, and moribund fish in both populations? While noble, that premise is often unrealistic and may be unethical. There are certainly fish condition thresholds above which the behaviors exhibited by the tagged fish are affected by the handling and tagging procedures themselves and resulting inferences are not representative of untagged fish. Additionally, there are ethical issues to consider when using animals for research and these issues may be heightened in studies of populations already at risk [26, 27]. In some cases, it may not be possible for tagged fish to represent all untagged fish and when so, it needs to be known and explicitly stated in the results.
The data we examined supported statistically significant effects of infection by S. californiensis on post-tag mortality of juvenile Chinook salmon surgically implanted with telemetry tags, but did not support statistically significant effects on post-release migration behavior. The data also suggest that post-tag mortality may be affected temporally, or by water temperature, or some combination of these factors with copepod intensity, given that mortality only occurred on the two dates with the highest water temperatures in each year. We also found that counting the number on the body, which is faster for the observer and less intrusive for the fish than examining under each operculum, is not useful for predicting the number within the branchial cavities. The branchial cavities must therefore be examined to accurately describe the intensity of infection, whether fish are examined during selection for tagging or to more generally describe the infection of a fish population.
The prevalence of infection from our data was similar to more complete surveys of the reservoir, but fish with high intensities known to occur in the reservoir were absent from our sample. This was likely a result of the limited sampling required to meet our needs for fish to tag and potentially an expression of the selectivity of the sample locations or method we used. Monzyk et al. [18] examined over 2,500 juvenile Chinook salmon from Cougar Reservoir in 2012 using a variety of sampling methods and found 29% of the infected fish had more than four copepods in their branchial cavities and some fish had over 20 (in 2011 they examined over 1,300 fish and measured prevalence but not intensity [19]). Our data included only six fish with more than four copepods in their branchial cavities, highlighting one of the pitfalls of observational data.
Conclusion
Fish may have naturally-occurring diseases that can affect their response to handling or tagging. We found evidence that infection with the parasitic copepod S. californiensis affected short-term mortality of acoustic-tagged naturally-produced juvenile Chinook salmon, but the data did not support effects on the long-term viability of the tagged fish after release. We also found the effects were related to the intensity of infection in the branchial cavities and not on the body, and that one was not a suitable index for the other. The data we examined provided information to make a preliminary assessment of the effects of S. californiensis on the fate of tagged juvenile Chinook salmon, but included the pitfalls of observational data including poor representation of the study population (intensity level) and lack of control of potentially-important factors such as water temperature and temporal and spatial sampling strata. We suggest the results from our evaluation warrant more rigorous study of the effects of S. californiensis on juvenile Chinook salmon in waters where they coexist. In addition, the results highlight the need for careful selection of study animals and the importance of explicitly stating how the study fish sample represents the untagged fish population.
References
Egli DP, Babcock RC (2004) Ultrasonic tracking reveals multiple behavioural modes of snapper (Pagrus auratus) in a temperate no-take marine reserve. ICES J Mar Sci 61:1137–1143
Elliott JE, Morrissey CA, Henny CJ, Inzunza ER, Shaw P (2007) Satellite telemetry and pray sampling reveal contaminant sources to Pacific Northwest Ospreys. Ecol Appl 17:1223–1233
Hedin J, Ranius T (2002) Using radio telemetry to study dispersal of the beetle Osmoderma eremita, an inhabitant of tree hollows. Comput Electron Agric 35:171–180
Polovina JJ, Hawn D, Abecassis M (2008) Vertical movement and habitat of opah (Lampris guttatus) in the central North Pacific recorded with pop-up archival tags. Mar Biol 153:257–267
Albert OT, Lambert Y, Vollen T, Freitas C, Heggebakken L (2012) Distinguishing pelagic and demersal swimming of deepwater flatfish by recording of body angles. In: McKenzie JR, Parsons B, Seitz AC, Kopf RK, Mesa M, Phelps Q (eds) Advances in Fish Tagging and Marking Technology. Symposium 76. American Fisheries Society, Maryland, pp 507–527
United States National Oceanic and Atmospheric Administration (NOAA) (1985) Endangered and threatened species: final listing determinations for 16 ESUs of West Coast salmon, and final 4(d) protective regulations for threatened salmonid ESUs. 50 CFR parts 223 and 224. Fed Reg, vol 70, pp 37160–37204. http://www.nmfs.noaa.gov/pr/pdfs/fr/fr70-37160.pdf
Liedtke TL, Rub AMW (2012) Techniques for telemetry transmitter attachment and evaluation of transmitter effects on fish performance. In: Adams NS, Beeman JW, Eiler JH (eds) Telemetry techniques: a user guide for fishery research. American Fisheries Society, Bethesda, pp 45–87
Vaughan GE, Coble DW (1975) Sublethal effects of three ectoparasites on fish. J Fish Biol 7:283–294
Bailey RE, Margolis L (1987) Comparison of parasite fauna of juvenile sockeye salmon (Oncorhynchus nerka) from southern British Columbian and Washington State lakes. Can J Zool 65:420–431
Chigbu P (2001) Occurrence and distribution of Salmincola californiensis (Copepoda: Lernaeopodidae) on juvenile sockeye salmon (Oncorhynchus nerka) in Lake Washington. J Freshw Ecol 16:615–620
Kabata Z, Cousens B (1973) Life cycle of Salmincola californiensis (Dana 1825) (Copepoda: Lernaeopodidae). J Fish Res Board Can 30:881–903
Kabata Z, Cousens B (1977) Host-parasite relationships between sockeye salmon, Oncorhynchus nerka, and Salmincola californiensis (Copepoda: Lernaeopodidae). J Fish Res Board Can 34:191–202
Gall GAE, Mcclendon EL, Schafer WE (1972) Evidence on the influence of the copepod (Salmincola californiensis) on the reproductive performance of a domesticated strain of rainbow trout (Salmo gairdneri). Trans Am Fish Soc 101:345–346
Modin JC, Veek TM (2002) Biological control of the parasitic copepod Salmincola californiensis in a commercial trout hatchery on the Lower Merced River, California. N Am J Aquac 64:122–128
Roberts RJ, Johnson KA, Casten MT (2004) Control of Salmincola californiensis (Copepoda: Lernaeapodidae) in rainbow trout, Oncorhynchus mykiss (Walbaum): a clinical and histopathological study. J Fish Dis 27:73–79
Bowker JD, Carty DG, Wandelear N, Schaffer J, Swee W, LaPatra SE (2012) Efficacy of SLICE premix (0.2% Emamectin Benzoate) for reducing infestations of Salmincola spp. on freshwater-reared rainbow trout. N Am J Aquac 74:428–437
Barndt S, Stone J (2003) Infestation of Salmincola californiensis (Copepoda: Lernaeopodidae) in wild coho salmon, steelhead, and coastal cutthroat trout juveniles in a small Columbia River tributary. Trans Am Fish Soc 132:1027–1032
Monzyk FR, Emig R, Romer JD, Friesen TA (2013) Life-history characteristics of juvenile spring Chinook salmon rearing in Willamette Valley reservoirs 2012. Report by Oregon Department of Fish and Wildlife, Corvallis, Oregon to the U.S Army Corps of Engineers, Portland, Oregon. http://oregonstate.edu/dept/ODFW/willamettesalmonidrme/sites/default/files/reservoir-research/life-history_characteristics_in_reservoirs_annual_report_2012_-final.pdf
Monzyk FR, Romer JD, Emig R, Friesen TA (2012) Life-history characteristics of juvenile spring Chinook salmon rearing in Willamette Valley reservoirs 2011, Report by Oregon Department of Fish and Wildlife, Corvallis, Oregon to the U.S Army Corps of Engineers, Portland, Oregon. http://oregonstate.edu/dept/ODFW/willamettesalmonidrme/sites/default/files/reservoir-research/life-history_characteristics_in_reservoirs_annual_2011.pdf
Beeman JW, Hansel HC, Hansen AC, Haner PV, Sprando JM, Smith CD et al (2013) Behavior and dam passage of juvenile Chinook salmon at Cougar Reservoir and Dam, Oregon, March 2011–February 2012. U.S. Geological Survey Open-File Report 2013-1079. doi:10.3133/ofr20141177
Beeman JW, Hansel HC, Hansen AC, Evans SD, Haner PV, Hatton TW et al (2014) Behavior and dam passage of juvenile Chinook salmon at Cougar Reservoir and Dam, Oregon, March 2012–February 2013. U.S. Geological Survey Open-File Report 2014-1177. doi:10.3133/ofr20141177
Beeman JW, Hansel HC, Hansen AC, Evans SD, Haner PV, Hatton TW et al (2014) Behavior and dam passage of juvenile Chinook salmon and juvenile steelhead at Detroit Reservoir and Dam, Oregon, March 2012–February 2013. U.S. Geological Survey Open-File Report 2014-1144. doi:10.3133/ofr20141144
Axel G, Beeman J, Brown R, Eppard B, Fielding S, Hockersmith E et al (2011) Surgical protocols for implanting JSATS transmitters into juvenile salmonids for studies conducted for the U.S. Army Corps of Engineers. Pacific Northwest National Laboratory Database. http://jsats.pnnl.gov/Publications/Technical/2011/Axel_et_al_2011_USACE_SurgicalProtocols_V1_2011.pdf
McMichael GA, Eppard MB, Carlson TJ, Carter JA, Ebberts BD, Brown RS et al (2010) The juvenile salmon acoustic telemetry system: a new tool. Fisheries 35(1):9–22
Hosmer DW, Lemeshow S (1999) Applied survival analysis regression modeling of time to event data. Wiley, New York
Putman RJ (1995) Ethical consideration and animal welfare in ecological field studies. Biodivers Conserv 4:903–915
Wilson RP, McMahon CR (2006) Measuring devices on wild animals: what constitutes acceptable practice? Front Ecol Environ 4:147–154
Authors’ contributions
JWB drafted the manuscript and performed data analyses. ACH conducted the fieldwork, performed data analyses, created figures, and helped draft the manuscript. JMS conducted the fieldwork and helped draft the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We thank the science and administrative staff of the Columbia River Research Laboratory for conducting the work, and David Griffith of the U.S. Army Corps of Engineers for his commitment to science in the Willamette River Basin. This work was made possible with funding from the Portland District of the U.S. Army Corps of Engineers, Portland, Oregon contracts W66QKZ03023085 and W66QKZ13045979. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Compliance with ethical guidelines
Competing interests The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Beeman, J.W., Hansen, A.C. & Sprando, J.M. Observational data on the effects of infection by the copepod Salmincola californiensis on the short- and long-term viability of juvenile Chinook salmon (Oncorhynchus tshawytscha) implanted with telemetry tags. Anim Biotelemetry 3, 20 (2015). https://doi.org/10.1186/s40317-015-0056-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40317-015-0056-5