Abstract
Background
In 2023, Burkina Faso experienced the largest dengue epidemic ever in Africa. This study aimed to estimate the prevalence of symptomatic, subclinical, and asymptomatic dengue and determine the associated factors among adult contacts of dengue in the Central Region, Burkina Faso.
Methods
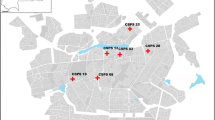
This cross-sectional study included contacts of dengue probable cases through cluster sampling in 2022–2023. These suspected cases that tested positive were identified from the five health facilities (Pissy CMA, Saaba CM, Kossodo CMA, Samandin CM, and Marcoussis CSPS) that reported the highest number of cases in 2021 per district. All participants underwent dengue and malaria rapid diagnostic tests (RDT). Samples positive for non-structural 1 protein antigen (AgNS1) and/or immunoglobulin M (IgM) were tested for serotype detection by reverse transcription polymerase chain reaction (RT-PCR). Binary logistic regression was done to identify the determinants of asymptomatic, subclinical, and symptomatic dengue among contacts of probable dengue cases.
Results
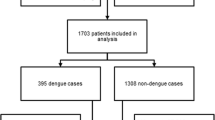
A total of 484 contacts were included, mostly in 2023 (75.2%). Most participants were females (58.6%), residing (24.3%) and passing their daytime (23.1%) in Saaba. The overall prevalence of dengue was estimated at 15.1% [95% confidence interval (CI): 12.0–18.6%], representing cases not seeking care in hospitals. Asymptomatic cases represented 2.9% (95% CI: 1.6–4.8%). Subclinical and symptomatic cases accounted for 6.0% (95% CI: 4.1–8.5%) and 6.2% (95% CI: 4.2–8.7%), respectively. Of the 58 samples tested by RT-PCR, 10 were confirmed for serotype 3 in 2023. Malaria cases were estimated at 5.6% (95% CI: 3.7–8.0%). After adjustment, participants claiming that a virus transmits dengue were likelier to have asymptomatic dengue [adjusted odds ratio (aOR) = 7.1, 95% CI: 2.4–21.0]. From the multivariable analysis, subclinical dengue was statistically associated with being included in the study in 2023 (aOR = 30.2, 95% CI: 2.0–455.5) and spending the daytime at Arrondissement 4 (aOR = 11.5, 95% CI: 1.0–131.0). After adjustment, symptomatic dengue was associated with living less than 50 m away from cultivated land (aOR = 2.8, 95% CI: 1.1–6.9) and living less than 50 m from a stretch of water (aOR = 0.1, 95% CI: 0.0–0.6).
Conclusions
The overall burden of dengue among populations not seeking care in hospitals was quite high, with few asymptomatic cases. Efforts to manage dengue cases should also target non-hospital cases and raise population awareness. The 2023 epidemic could be due to dengue virus (DENV)-3.
Graphical Abstract

Similar content being viewed by others
Background
Dengue is one of the most widespread arthropod-borne viral diseases, menacing about half of the world's population [1]. Global estimates show 390 million cases annually, with good evidence of dengue in at least 128 countries [2]. The World Health Organization (WHO) regions of America, Asia, and Africa are the most affected by dengue [1]. Estimates give a similar burden in Africa than in America, with 16 (11–22) million versus 13 (9–18) million [3]. However, due to poor surveillance, dengue cases have been underreported in Africa [4, 5].
At least 58% of African countries reported dengue already [2]. East Africa has been most affected, followed by West Africa [2, 6]. Since 2014, studies have highlighted that West Africa is becoming a new front for dengue fever [7]. In Burkina Faso, dengue fever was first reported in 1925, and cases were later reported in 1982 [8, 9]. The subsequent studies confirmed that it is endemic-epidemic in the country, with the circulation of all 4 serotypes [5, 10,11,12]. According to data from passive surveillance, health districts in the Central region are the most affected by dengue in the country [13]. In 2023, Burkina Faso experienced the largest epidemic in Africa, with 154,867 suspected cases, 70,433 probable cases and 709 deaths [14]. As of March 3, 2024, the country reported a total of 164,848 suspected cases, including 73,497 probable and confirmed cases, with a case fatality rate of 0.45% (over suspected cases) [15].
According to the WHO, dengue cases are more asymptomatic or mild than symptomatic [1]. In a systematic review, asymptomatic dengue prevalence was pooled at 59.3% (95% confidence interval (CI): 43.8–74.8%), higher during outbreaks than outside outbreak periods [16]. However, most studies included in that review were done in Asia and South America. On the contrary, a systematic review that included one study from Africa claimed a prevalence of 0.2% among healthy populations [17]. Using studies published in Africa between 2000 and 2019, another systematic review found a lower prevalence of all markers among asymptomatic cases than symptomatic dengue [18]. Thus, the pooled prevalence of asymptomatic dengue was 0.0% (95% CI: 0.0–0.5%), 3.5% (95% CI: 0.8–7.8%) and 15.6% (95% CI: 9.9–22.2%), respectively for ribonucleic acid (RNA), immunoglobulins (Ig)M and IgG [18]. Among febrile populations, dengue prevalence reached 24.8% (95% CI: 13.8–37.8%), 10.8% (95% CI: 3.8–20.6%) and 8.4% (95% CI: 3.7–14.4%), respectively, for IgG, IgM, and RNA [18].
Data on the actual extent of the different types of dengue in Africa is therefore scarce. In Burkina Faso, most studies included data of febrile participants from hospitals or laboratories [10, 19,20,21]. However, these data do not give the true burden of dengue as not all symptomatic cases would seek care at health facilities, nor the asymptomatic cases. To break the transmission and control of dengue in Burkina Faso, a big challenge is to estimate the actual burden of dengue in the area through household-based data.
This study aimed to estimate the prevalence of symptomatic, subclinical, and asymptomatic dengue fever and determine the associated factors among adult contacts of probable cases through a cluster sampling in the Central Region, Burkina Faso.
Methods
Study design
This study is part of an overall research aiming to estimate the prevalence of symptomatic and asymptomatic dengue among adults in Burkina Faso in 2022 and 2023 [22]. The overall study included two populations, dengue suspected cases from selected health facilities and the contacts of those turning probable cases. Within this research, we conducted cluster sampling through household-based data collection, systematically targeting contacts of dengue probable index cases. Data were collected from September to November 2022 and September to November 2023, at the end of the rainy season when malaria and dengue cases peak.
Study setting
This study was conducted in Burkina Faso. It targeted the Central Region with the Capitale City of Ouagadougou surrounded by the six rural municipalities of Koubri, Saaba, Pabré, Komsilga, Komki-Ipala, and Tanghin-Dassouri [23]. Ouagadougou is structured into 12 arrondissements and 55 sectors, with 2,415,266 inhabitants in 2019 [24]. According to the same population and housing census, the Central Region had a population of 3,032,668 inhabitants, with 62.4% of adults [25].
The regional health system includes 5 health districts, which are Bogodogo, Baskuy, Boulmiougou, Sig-Nonghin and Nongr Massom health districts.
Sample size estimation
We determined the minimum sample size using the formula for estimating a single proportion [26]:
-p: anticipated prevalence of asymptomatic dengue fever;
-Z1-α/2: percentage standard deviation corresponding to the two-sided significance level. For α = 5%, Z1-α/2 = 1.96.
-e: precision of 1.75% (half of 3.5%, the prevalence of immunoglobulin M in Africa).
We assumed that the contacts would be surveyed late, so the prevalence of immunoglobulin M was used to calculate the sample size. It was pooled at 3.5% (95% CI: 0.8–7.8%) among apparently healthy people in Africa [18]. The required sample size was estimated at 424, then adjusted to 472, considering an adjustment of 10% non-response rate with the formula (n = \(\frac{{n}_{0}}{1-{n}_{r}}\)).
Participants characteristics
The study considered individuals aged 16 years and older, family contacts of dengue probable cases, regardless of the presence of fever or other symptoms. Mentally debilitated individuals were excluded from the study. Participants were further classified as follows based on clinical and serological characteristics:
Dengue fever suspected case: fever (≥ 38.5 °C) in the last five days with at least 2 of the following symptoms (headache, retro-orbital pain, myalgias, arthralgias, skin rash, bleeding manifestations, or shock syndrome) [27].
Probable dengue fever: a suspected case with a positive rapid diagnostic test (RDT) for dengue non-structural 1 protein antigen (AgNS1) and/or immunoglobulins (Ig)M and/or IgG. Probable cases identified from the health facilities were the index cases. Probable cases identified among the contacts of index cases were referred to as symptomatic cases. These symptomatic cases are undiagnosed infection [17] that did not seek care for the ongoing episode in a health facility. Without antibody titers, we did not consider distinguishing primary and secondary infections.
Subclinical dengue case: presenting a fever and/or other symptoms but not fitting the definition of a suspected case. It is sometimes referred to as mild infection, as considered in some studies.
Asymptomatic dengue case: participants turning positive for dengue RDTs with positive AgNS1 and/or IgM markers without any fever or symptom, fitting clinically undetectable infections [17].
Dengue contacts of an index case: participant sharing the same household, house, or compound (Celibateriums in French) with an index case.
Data collection
We conducted face-to-face interviews with a structured questionnaire and RDTs, using the Kobo Toolbox. The questionnaire included participants-level factors (sociodemographic characteristics and knowledge of dengue) and household-level factors (environmental characteristics).
Index cases were identified through a three-stage sampling [22]. These symptomatic participants who were at least positive for AgNS1 and/or IgM were followed home. Then, all people residing in the same household or compound as the dengue probable case were asked to participate in the study. Participants were tested for malaria and dengue using RDTs. The SD Bioline Malaria Ag P.f kit (SD Standard Diagnostics, INC., Republic of Korea) and the Standards Q Malaria P.f Ag kit (SD Biosensor, Inc., Republic of Korea) were used to test for malaria by Plasmodium falciparum. The WONDFO Dengue NS1/IgG/IgM kit (Guangzhou Wondfo Biotech Co., Ltd, China) was used to test for the infection with the dengue virus. The tests followed the manufacturers procedures. Participants at least positive for AgNS1 and/or IgM were sampled for serotyping.
Participants positive for dengue RDT without presenting any symptom or sign were contacted up to the following 10 days to confirm they were not presymptomatic.
Characterization of dengue viruses serotypes
Extraction of dengue viral RNA
Dengue virus RNA extraction was performed using the QIAamp Viral RNA Mini Kit (Qiagen, Germany), according to the manufacturer protocol. The extract was stored at -80 °C for molecular testing.
Serotyping of dengue viruses
Identification of the dengue virus genotypes was performed using Sacace PCR kit for detection (Sacace Biotechnologies, Italy). Differentiation of Dengue virus genotypes 1, 2, 3 and 4 in clinical material Dengue Real-™ Genotype (Sacace, Como, Italy) and the PCR steps followed the manufacturer instructions. This resulted in a total reaction volume of 25 µl for each PCR. To guarantee the quality of the results, three controls, which are negative control of extraction (NCE), positive control of amplification (C +) and negative control of amplification (NCA) were used.
The PCR reaction mixtures contained in sterile 0.2 ml microtubes were introduced onto the SaCycler-96 Real-Time PCR v.7.3 plate (Sacace Biotechnology, Italy) for amplification. The amplification program consisted of one cycle of 50 °C for 30 min, 95 ˚C for 15 min, followed by five cycles of 95 ˚C for 10 s, 56 ˚C for 40 s, and 72 ˚C for 20 s, and finally 40 cycles of 95 ˚C for 10 s, 54 ˚C for 40 s, and 72 ˚C for 20 s.
The results were interpreted using the RealTime_PCR software v7.9 (Dna -Technology LLC, Moscow, Russia) by the crossing or not crossing of the threshold line by the fluorescence curve. The genotypes were then identified following the manufacturer’s protocol.
Statistical analyses
The software STATA/IC 16.1 (StataCorp LLC, College Station, Texas 77,845 USA) was used for analyses.
The prevalence of asymptomatic, subclinical, and symptomatic dengue fever was estimated with 95% confidence intervals. They were presented for the overall sample, symptomatic cases, subclinical cases, and asymptomatic cases. Independent variables were presented with mean ± standard deviation (SD) or median [interquartile range (IQR)] for the quantitative variables. Qualitative variables were presented with frequency and percentage.
Further, a binary logistic regression was done to identify the determinants of asymptomatic, subclinical, and symptomatic dengue among contacts of probable dengue cases. A univariate binary logistic regression was first done to identify the sociodemographic and environmental factors associated with asymptomatic, subclinical, and symptomatic dengue. Then, factors significant at 20% and those pertinent (like age and sex) even not significant in the univariate logistic regression were included in the multivariable analysis. The model with the lowest Akaike Information Criteria (AIC) and the narrowest intervals was retained for each dengue type.
Results
Overall dengue prevalence
Details of dengue prevalence among the 484 included participants are presented in Table 1.
The overall prevalence of dengue was estimated at 15.1% (95% CI: 12.0% –18.6%). Isolated or associated immunoglobulin M predominated among dengue cases. All cases with symptoms (subclinical and symptomatic) accounted for 12.6% (9.7% –15.9%). The ratio of asymptomatic to symptomatic was 0.5:1 (14/30). The ratio of asymptomatic to all cases with symptoms was 0.2:1.
The RT-PCR tests performed on 58 samples positive to AgNS1 and/or IgM in 2022–2023 confirmed 10 participants positive for serotype 3 in 2023. They were either symptomatic (4 positive for AgNS1 + IgM, 2 for IgM and 1 for AgNS1) or subclinical (2 positive for AgNS1 and 1 for IgM).
Among the dengue-negative participants, 34.1% were positive for IgG but could be past primary dengue or secondary early cases.
Overall, Plasmodium falciparum cases represented 5.6% (95% CI: 3.7% – 8.0%).
Asymptomatic dengue prevalence
Considering all participants positive for dengue RDT, 19.2% (14/73) were asymptomatic. From the overall sample, asymptomatic cases represented 2.9% (95% CI: 1.6% –4.8%). There was no coinfection with malaria.
Subclinical dengue prevalence
Of the 73 dengue cases, subclinical cases accounted for 39.7%. Subclinical dengue was estimated at 6.0% (95% CI: 4.1%–8.5%). Five (5) subclinical cases associated with malaria.
Symptomatic dengue prevalence and markers
Among positive cases, 41.1% were symptomatic. In the overall sample size, symptomatic dengue was estimated at 6.2% (95% CI: 4.2%–8.7%). In addition, 5 cases were also positive for malaria.
Sociodemographic features of the participants
The sociodemographic characteristics are presented below in Table 2. For the different types of dengue, most participants were included from the epidemic year 2023. Most asymptomatic cases came from Saaba Medical Centre (CM) (6/14), and most subclinical (72.4%) and symptomatic (50.0%) cases from Pissy Medical Centre with Surgical Antenna (CMA), respectively. Among the uninfected participants and subclinical cases, half were aged more than 28 years (median = 28 years). Half of the asymptomatic cases were older than 35, while half of symptomatic cases aged more than 23 years. Only asymptomatic cases were dominated by males (57.1%).
While most asymptomatic cases attained tertiary level (6/14), the secondary level of education dominated the other types of dengue. Similarly, most asymptomatic cases were students or private employees (4/14 each), while students predominated among the other types of dengue. Most asymptomatic cases spent their daytime in Saaba (5/14; 35.7%) like participants not infected with dengue (95; 23.1%).
Knowledge and prevention measures for dengue
The distribution of knowledge and prevention measures is shown in Table 3.
The knowledge of dengue reached 71.4% among asymptomatic while most subclinical cases (34.5%) and symptomatic cases (40.0%) did not know about dengue. Similarly, only 10.3% of the subclinical cases and 13.3% of the symptomatic ones knew that dengue is caused by a virus, versus 50.0% of asymptomatic participants. The knowledge of dengue transmission ways was estimated to be 92.9%, 48.3%, and 46.7% among asymptomatic, subclinical, and symptomatic cases, respectively. Participants who knew there was a difference between dengue and malaria represented 44.8% (16/29) and 46.7% (14/30) of subclinical and symptomatic cases, while up to 85.7% of asymptomatic cases knew there was a difference.
Regarding the use of prevention measures, most participants used prevention measures, respectively at 92.2% for non-dengue participants, 85.7% for asymptomatic cases, 93.1% for subclinical cases and 93.3% for symptomatic participants. Except for symptomatic cases (46.7%), most participants (about 55.0%) used vector control measures in the other groups. The use of bed nets reached 70% in the different groups, excluding symptomatic cases (63.3%).
Symptoms of the subclinical cases
As per the definition, subclinical cases did not meet the definition of suspected cases by associating fever with 2 of the relevant symptoms. They mostly experienced fever (28/29) and headaches (23/29) (see Fig. 1).
Factors associated with asymptomatic dengue
Factors associated with asymptomatic dengue are presented in Table 4. From the univariate analysis of the binary logistic regression, the data collection period, the age, education level, knowledge of dengue, its germ, and its transmission ways, being aware that dengue is different from malaria, and cultivated land at less than 50 m from the house, were statistically associated with asymptomatic dengue at the level of 20%. Although not significant at 20%, the sex was input in the multivariable analysis.
After adjustment and considering the lowest Akaike Information Criterion (AIC) and small OR confidence intervals, only the knowledge of the germ transmitting dengue (P = 0.013) was significantly associated with asymptomatic dengue fever at 5%. Thus, participants knowing that dengue is transmitted by a virus had a 5.7-fold increase in the chance of having asymptomatic dengue compared to those not knowing.
Factors associated with subclinical dengue
The factors associated with subclinical dengue are presented in Table 5. From the univariate analysis, subclinical dengue was statistically associated at 5% with data collection period (P = 0.030), residence (other arrondissements vs Saaba, P = 0.004), place during the daytime (other places vs Saaba, P = 0.011), knowledge of dengue transmission ways (P = 0.012), presence of stagnant water in the house (P = 0.009) and the presence of cultivated land less than 50 m from the house (P = 0.027).
After adjustment, the data collection period (P = 0.014) and the place during the daytime were associated with increased odds of subclinical dengue. Participants included in 2023 had 30 times higher odds of being subclinical cases than those surveyed in 2022. Participants spending their daytime in Arrondissement 4 (P = 0.049) vs those spending the daytime in Saaba had about an 11 times increased chance of being subclinical cases.
Factors associated with symptomatic dengue
In the absence of any symptomatic case in the non-epidemic year (2022), the variable was not included in the analyses. The binary logistic analyses for symptomatic dengue are found in Table 6.
From the univariate binary logistic regression, the residence, the place during the daytime, knowledge of transmission ways, hollow containers or used tyres in the house and cultivated land less than 50 m from the house were statistically associated with symptomatic dengue at 5%. After adjustment, participants who reported cultivated land less than 50 m from their house had a three fold increased chance of being symptomatic for dengue (P = 0.028). On the contrary, those living less than 50 m from a stretch of water had a reduced chance than those not living near (P = 0.013).
Discussion
This study aimed at estimating the burden of asymptomatic, subclinical, and symptomatic dengue and determining the associated factors among household contacts using a cluster sampling around index cases in 2022 and 2023. As the data collection was household-based and included people who did not seek care for ongoing symptoms or signs, the overall prevalence would represent the total burden of misdiagnosed dengue.
The overall prevalence of dengue reached 15.1% (95% CI: 12.0%–18.6%) among the contacts of probable dengue. This prevalence was relatively high and represents the extent of dengue cases escaping the health system. If control measures do not target them, they will constitute reservoirs of dengue virus transmission in the communities. This overall prevalence was higher than what was found in 2013–2014 (8.7%; 33/379) [11] and 2022 (8.2%, 95% CI: 6.2%–10.6%) among suspected cases in the Central Region [22]. A lower prevalence was also spotted from perifocal investigations around 149 index cases, with 4.4% dengue, mostly among children (332/346) [28]. From 11 cluster studies, with recruitment in a predefined radius around dengue index cases, the prevalence ranged from 2.2% to 21.5% (median 7.9%) [17]. On the contrary, it was lower than the overall prevalence (25.3%; 740/2929) of probable and confirmed cases between December 2014 and February 2017 in the same region [10]. Dengue among contacts was dominated by the cases presenting some symptoms (12.6%), like subclinical (6.0%) and symptomatic (06.2%) cases, followed by a few asymptomatic cases (2.9%). Poor knowledge of dengue (34.5% and 40%), a virus causing dengue (10.3% and 13.3%), dengue transmission ways (48.3% and 46.7%), and that dengue is different from malaria (44.8% and 46.7%) was usual among subclinical and symptomatic cases. Particularly, symptomatic cases had the lowest use of bed nets (63.3%) and recurse to vector control measures (46.7%). This poor knowledge and the low use of prevention measures could partially explain the high prevalence of subclinical and symptomatic dengue.
Asymptomatic dengue was estimated at 2.9% (95% CI: 1.6–4.8%), lower than expected from the literature. A similar study found 7.5% of strictly asymptomatic cases after 2 years of follow-up in Cambodia [28]. A systematic review estimated actual asymptomatic cases at 8% [17]. From the same review, the asymptomatic rate from only cluster studies lay between 7.4% and 92% (median 42%) [17]. These prevalences were consistent with what was pooled from a systematic review in Africa, with 0.0% (95% CI: 0.0%–0.5%) for RNA, 3.5% (95% CI: 0.8%–7.8%) of IgM and 15.6% (95% CI: 9.9%–22.2%) of IgG [18]. However, the asymptomatic prevalence was pooled at 59.3% among family members using missing febrile status as the main criteria [16]. The WHO gives asymptomatic dengue the highest burden of dengue [1]. The discrepancies in asymptomatic dengue burden could be due to differences in the study populations. They could also have to do with the unclear and imprecise definitions of the term asymptomatic, which includes inapparent or clinically undetected infections [17], missing febrile status [16], and underdiagnosed or mild infections [17]. Asymptomatic cases predominantly used prevention measures (85.7%), vector control measures (57.1%) and bed nets (71.4%), which could have contributed to lower the prevalence. They also showed high knowledge of dengue transmission ways (92.9%) and the fact that dengue differs from malaria (85.71%). Beyond that, it is necessary to know the actual extent of asymptomatic dengue, as such cases can transmit dengue to mosquitoes despite their lower levels of viremia [29]. By the way, asymptomatic cases are more infectious to mosquitoes than symptomatic ones at a given level of viremia [29].
From this sample, IgM alone or associated with other markers was the most prevalent marker overall (60.2% in total) and among all types of dengue. IgM is a marker of a recent primary infection; coupled with IgG, it is still a recent primary infection or a late secondary infection. On the contrary, a study that included febrile participants found a high proportion of AgNS1 (11%) and a low proportion of IgM (4%), though [10]. Delayed contact with the participants would explain the predominance of IgM markers. Finally, these participants did not seek care and passed the acute stage (AgNS1), when they could be infectious to mosquitoes. Moreover, this health-seeking behavior is risky as complications could quickly arise.
Studies revealed that dengue does not vary with the types of dengue [29]. In this study, only serotype 3 was confirmed among symptomatic (7/10 confirmed cases) and subclinical (3/10 confirmed cases) cases. Then, it was not possible to determine whether dengue virus serotype 3 was associated with a specific type of dengue. Also, given the small number of confirmed cases by RT-PCR, we cannot conclude if serotype 3 predominated during the 2023 epidemic. However, dengue incidence varies biannually in the country [30], probably due to a shift in dominant serotypes over time [31]. Thus, although all 4 serotypes circulate in the country, the dominant serotypes appear to vary over time [11, 12]. In 2013–2014, if serotypes 2, 3 and 4 were identified to circulate in the Central Region, serotypes 2 and 3 were more frequent, and it seemed serotype 3 predominated [10, 11, 32]. For the 2016–2017 epidemic, despite the co-circulation of serotypes 2 and 3, serotype 2 was identified as dominant [10, 33]. The primary infection of a serotype procures complete immunity for it but not for the other serotypes [34]. With the shift in predominant serotypes, populations may be subject to the phenomenon of antibody-dependent enhancement (ADE) with an immunologic response to the second serotype without neutralizing the virus. Such a phenomenon increases the risk of severe dengue and death.
The factors associated with the different types of dengue were also assessed and were found to differ according to the type of dengue. For the asymptomatic cases, their likelihood of being infected was associated with knowing a virus transmits dengue, which was surprising. Enrolling in the study in 2023 and spending the daytime at Arrondissement 4 increased the odds of being a subclinical case. Similarly, dengue suspected cases residing or staying daytime at Arrondissement 4 were more likely to test positive for dengue RDT in 2022 in the Central Region, Burkina Faso [22, 35]. In effect, Arrondissement 4 is a wet and wooded environment with two dams and the Bangr-Weogo Park, ensuring favorable conditions for mosquito breeding and development. It explains why participants living less than 50 m from cultivated land had increased odds of being symptomatic cases. However, participants living near a stretch of water had reduced odds of being symptomatic cases. In fact, the immature stages of Aedes mosquitoes mostly develop in artificial habitats such as water tanks (45.2%), waste left in the house (24.7%) and tyres (21.6%) [11]. Used tyres were indeed the most common larval breeding sites in urban areas, while in peri-urban and rural areas, drinking troughs and water storage containers were the most frequent sites [36, 37].
This study gives insights into the burden of the different types of dengue from a population-based perspective. However, some limitations can go with the way participants were selected. If that allows epidemiological linkages with the index cases, it will likely overestimate the dengue burden. So, the prevalence of the different types of dengue in the true population could be lower than what was estimated in this study. Despite the cluster sampling, the prevalence of asymptomatic dengue was lower than expected by the WHO. Using RDTs instead of ELISA could also be limiting, as ELISA carries more sensitivity and specificity. This study could be the first to estimate the burden of the different types of dengue in Burkina Faso and will help understand the epidemiology of the disease.
Conclusions
It is crucial for the surveillance system to capture the actual epidemiology of dengue. This study found that several cases are missed by the health system in Burkina Faso, including symptomatic ones. Poor knowledge and low use of preventive measures were found among symptomatic and subclinical cases, explaining probably the high burden. Asymptomatic dengue was rather low, partially due to the fair knowledge of dengue and the high use of preventive measures. In addition, serotype 3 could have originated the 2023 epidemic. To control the disease, interventions should target contacts of dengue cases as well as raise awareness of the disease and the use of preventive measures. Population studies are welcome to provide more accurate estimates of the dengue burden in the country.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AgNS1:
-
Antigen nonstructural protein 1
- CM:
-
Medical centre
- CMA:
-
Medical Centre with Surgical Antenna
- CSPS:
-
Primary healthcare centre
- DENV:
-
Dengue virus
- IgG:
-
Immunoglobulins G
- IgM:
-
Immunoglobulins M
- RDT:
-
Rapid diagnostic test
- WHO:
-
World Health Organization
References
WHO. Dengue and severe dengue [Internet]. World Health Organization. 2023. Available from: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue. Accessed 29 Mar 2023.
Brady OJ, Gething PW, Bhatt S, Messina JP, Brownstein JS, Hoen AG, et al. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis. 2012;6: e1760.
Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496:504–7.
Gubler DJ. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 2002;10:100–3.
Ouédraogo JCRP. Influence des facteurs climatiques sur la survenue de la dengue et du paludisme dans les villes de Ouagadougou et de Bobo-Dioulasso [Mémoire de master]. [Ouagadougou, Burkina Faso]: Université Joseph KI-ZERBO; 2017. (In French).
Gainor EM, Harris E, LaBeaud AD. Uncovering the burden of dengue in Africa: considerations on magnitude, misdiagnosis, and ancestry. Viruses. 2022;14:233.
Stoler J, al Dashti R, Anto F, Fobil JN, Awandare GA. Deconstructing “malaria”: West Africa as the next front for dengue fever surveillance and control. Acta Trop. 2014;134:58–65.
Gonzalez J, Du Saussay C, Gautun J, McCormick J, Mouchet J. La dengue au burkina faso (ex Haute-Volta): épidémies saisonnières en milieu urbain à Ouagadougou. Bull Soc Pathol Exot Filiales. 1985;78:7–14 (In French).
Amarasinghe A. Dengue virus infection in Africa. Emerg Infect Dis. 2011;17:1349–54.
Lim JK, Seydou Y, Carabali M, Barro A, Dahourou DL, Lee KS, et al. Clinical and epidemiologic characteristics associated with dengue during and outside the 2016 outbreak identified in health facility-based surveillance in Ouagadougou, Burkina Faso. Forshey BM, editor. PLOS Negl Trop Dis. 2019;13:e0007882.
Ridde V, Agier I, Bonnet E, Carabali M, Dabiré KR, Fournet F, et al. Presence of three dengue serotypes in Ouagadougou (Burkina Faso): research and public health implications. Infect Dis Poverty. 2016;5:23.
Letizia AG, Pratt CB, Wiley MR, Fox AT, Mosore M, Agbodzi B, et al. Retrospective genomic characterization of a 2017 dengue virus outbreak. Burkina Faso Emerg Infect Dis. 2022;28:1198–210.
Ouattara CA, Traore S, Sangare I, Traore TI, Meda ZC, Savadogo LGB. Spatiotemporal analysis of dengue fever in Burkina Faso from 2016 to 2019. BMC Public Health. 2022;22:462.
Manigart O, Ouedraogo I, Ouedraogo HS, Sow A, Lokossou VK. Dengue epidemic in Burkina Faso: how can the response improve? The Lancet. 2024;403:434–5.
WHO African Region. Multi-country Outbreak of Dengue. Brazzaville, Republic of Congo: WHO Afro; 2024 Mar p. 1–10. Report No.: # 004. Available from: https://iris.who.int/bitstream/handle/10665/376307/AFRO.Dengue.Sitrep004-20240303.pdf?sequence=1.
Asish PR, Dasgupta S, Rachel G, Bagepally BS, Girish Kumar CP. Global prevalence of asymptomatic dengue infections - a systematic review and meta-analysis. Int J Infect Dis. 2023;134:292–8.
De Santis O, Bouscaren N, Flahault A. Asymptomatic dengue infection rate: A systematic literature review. Heliyon. 2023;9: e20069.
Simo FBN, Bigna JJ, Kenmoe S, Ndangang MS, Temfack E, Moundipa PF, et al. Dengue virus infection in people residing in Africa: a systematic review and meta-analysis of prevalence studies. Sci Rep. 2019;9:13626.
Bello SOT, Houkpevi A, Zackari S, Tapsoba ASA, Zoure AA, Ilboudo PD, et al. Epidemiology of dengue in patients with febrile syndrome at Saint Camille Hospital, Ouagadougou, Burkina Faso from 2020 to 2021. Af J Clin Exp Micro. 2022;23:398–406.
Seogo PH, Bicaba BW, Yameogo I, Moussa G, Charlemangne KJ, Ouadraogo S, et al. Ampleur de la dengue dans la ville de Ouagadougou, Burkina-Faso, 2016. J Interv Epidemiol. 2021;4:13 (In French).
Sondo KA, Ouattara A, Diendéré EA, Diallo I, Zoungrana J, Zémané G, et al. Dengue infection during pregnancy in Burkina Faso: a cross-sectional study. BMC Infect Dis. 2019;19(1):997.
Ouédraogo JCRP, Ilboudo S, Compaoré TR, Bado P, Nitiéma M, Ouédraogo WT, et al. Determinants and prevalence of symptomatic dengue fever among adults in the Central Region of Burkina Faso: a hospital-based cross-sectional study. BMC Infect Dis. 2024;24:22.
Direction Régionale de la Santé du Centre. Plan d’Action 2022. Burkina Faso: Ministère de la santé et de l’hygiène publique; 2022. (In French).
Institut national de la statistique et de la démographie (INSD). Monographie de la Commune de Ouagadougou. Ministère de l’économie, des finances et de la prospective; 2022. (In French).
INSD. Résultats préliminaires du Cinquieme Recensement Général de la Population et de l’Habitation du Burkina Faso. Ministere de l’Economie, des Finances et du Développement; 2020. (In French).
Charan J, Biswas T. How to calculate sample size for different study designs in medical research? Indian J Psychol Med. 2013;35:121–6.
Programme national de lutte contre les maladies tropicales négligées. Directives nationales de prise en charge des cas de dengue au Burkina Faso. Ministère de la santé et de l’hygiène publique, Burkina Faso; 2023. (In French).
Ly S, Fortas C, Duong V, Benmarhnia T, Sakuntabhai A, Paul R, et al. Asymptomatic dengue virus infections, Cambodia, 2012–2013. Emerg Infect Dis. 2019;25:1354–62.
Duong V, Lambrechts L, Paul RE, Ly S, Lay RS, Long KC, et al. Asymptomatic humans transmit dengue virus to mosquitoes. Proc Natl Acad Sci USA. 2015;112:14688–93.
Ouédraogo JCRP, Ilboudo S, Ouédraogo E, Ouédraogo WT, Ouédraogo S, Samadoulougou BC, et al. Setting dengue fever epidemic thresholds between 2016 and 2021 in the Central Health Region, Burkina Faso: An ecological study. J Epidemiol Glob Health. 2023;13:557–65.
Rajarethinam J, Ang L-W, Ong J, Ycasas J, Hapuarachchi HC, Yap G, et al. Dengue in Singapore from 2004 to 2016: Cyclical epidemic patterns dominated by serotypes 1 and 2. Am J Trop Med Hyg. 2018;99:204–10.
Tarnagda Z, Congo M, Sagna T, Ouédraogo C, Nikiéma V, Cissé A, et al. Outbreak of dengue fever in Ouagadougou, Burkina Faso, 2013. Int J Microbiol Res. 2014;2:101–8.
Tarnagda Z, Cissé A, Bicaba BW, Diagbouga S, Sagna T, Ilboudo AK, et al. Dengue fever in Burkina Faso, 2016. Emerg Infect Dis. 2018;24:170–2.
Adams B, Holmes EC, Zhang C, Mammen MP, Nimmannitya S, Kalayanarooj S, et al. Cross-protective immunity can account for the alternating epidemic pattern of dengue virus serotypes circulating in Bangkok. Proc Natl Acad Sci U S A. 2006;103:14234–9.
Alix T, Jean Claude Romaric Pingdwindé O, Mohamadi Z, Sylvain I. Analyse de la répartition spatiale des cas de dengue dans la Région du Centre (Burkina Faso). Les Cahiers de l’ACAREF. 2023;5:10–26. (In French).
Badolo A, Sombié A, Yaméogo F, Wangrawa DW, Sanon A, Pignatelli PM, et al. First comprehensive analysis of Aedes aegypti bionomics during an arbovirus outbreak in west Africa: Dengue in Ouagadougou, Burkina Faso, 2016–2017. PLoS Negl Trop Dis. 2022;16: e0010059.
Ouédraogo WM, Toé KH, Sombié A, Viana M, Bougouma C, Sanon A, et al. Impact of physicochemical parameters of Aedes aegypti breeding habitats on mosquito productivity and the size of emerged adult mosquitoes in Ouagadougou City. Burkina Faso Parasites Vectors. 2022;15:478.
Acknowledgements
Not applicable.
Funding
The Programme d’Appui à l’Enseignement Supérieur (PAES) and the Fonds National de la Recherche et de l’Innovation pour le Développement (FONRID), Burkina Faso funded the study. The funders had no role in the study design, nor the decision to write this manuscript.
Author information
Authors and Affiliations
Contributions
All the authors conceived the study. JCRPO, SI and BP collected, analyzed, and interpreted the data, under the supervision of LGBS. JCRPO drafted the manuscript. All authors read and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Comité d’Ethique Institutionel pour la Recherche en Santé (CEIRES) of the Institut de Recherche en Sciences de la Santé (IRSS) [number 032–2022/CEIRES] and the Comité d’Ethique national pour la Recherche en Santé (CERS) [N° 2022–12-257].
Signed informed consent was obtained from each participant. Participants aged 16 to 18 years had to sign an informed assent in addition to their parents or caregivers consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ouédraogo, J.C.R.P., Ilboudo, S., Bado, P. et al. Estimating dengue burden among family contacts through cluster investigation around probable cases in 2022 and 2023 in the Central Region, Burkina Faso. Infect Dis Poverty 13, 45 (2024). https://doi.org/10.1186/s40249-024-01212-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40249-024-01212-5