Abstract
Background
Brucellosis is a zoonotic affliction instigated by bacteria belonging to the genus Brucella and is characterized by a diverse range of pervasiveness, multiple transmission routes, and serious hazards. It is imperative to amalgamate the current knowledge and identify gaps pertaining to the role of ticks in brucellosis transmission.
Methods
We systematically searched China National Knowledge Infrastructure (CNKI), WanFang, Google Scholar, and PubMed on the topic published until April 23, 2022. The procedure was performed in accordance with the Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) guidelines. The selected articles were categorized across three major topic areas, and the potential data was extracted to describe evidence-practice gaps by two reviewers.
Results
The search identified 83 eligible studies for the final analyses. The results highlighted the potential capacity of ticks in brucellosis transmission as evidenced by the detection of Brucella in 16 different tick species. The pooled overall prevalence of Brucella in ticks was 33.87% (range: 0.00–87.80%). The review also revealed the capability of Brucella to circulate in parasitic ticks' different developmental stages, thus posing a potential threat to animal and human health. Empirical evidence from in vitro rodent infection experiments has revealed that ticks possess the capability to transmit Brucella to uninfected animals (range: 45.00–80.00%). Moreover, significant epidemiological associations have been found between the occurrence of brucellosis in animals and tick control in rangelands, which further suggests that ticks may serve as potential vectors for brucellosis transmission in ruminants. Notably, a mere three cases of human brucellosis resulting from potential tick bites were identified in search of global clinical case reports from 1963 to 2019.
Conclusions
It is imperative to improve the techniques used to identify Brucella in ticks, particularly by developing a novel, efficient, precise approach that can be applied in a field setting. Furthermore, due to the lack of adequate evidence of tick-borne brucellosis, it is essential to integrate various disciplines, including experimental animal science, epidemiology, molecular genetics, and others, to better understand the efficacy of tick-borne brucellosis. By amalgamating multiple disciplines, we can enhance our comprehension and proficiency in tackling tick-borne brucellosis.
Similar content being viewed by others
Background
Brucellosis, a zoonotic disease caused by bacteria belonging to the genus Brucella, is a widespread affliction with a global presence in over 170 countries across all five continents [1]. The genus Brucella is composed of 12 species, including B. abortus, B. ovis, B. melitensi, B. suis, B. canis, B. neotomae, B. ceti, B. pinripedialis, B. mieroti, B. vulpis, B. inopinata, and B. papionis [2]. B. melitensi is the most significant pathogen responsible for human brucellosis worldwide, followed by B. abortus and B. suis [3]. Although Europe, Australia, and Canada have successfully eradicated brucellosis, brucellosis continues to be a significant concern in highly endemic areas such as Africa, parts of Asia, and Latin America [4]. Like other zoonotic diseases, brucellosis causes significant economic losses to animal husbandry due to decreased fertility, abortions, and lowered milk production. This poses a significant threat to the livestock, meat, and dairy industries [5]. Human health is also at risk, as brucellosis infections may result in long-term clinical symptoms, including sweating, joint pain, and fatigue [6]. Moreover, brucellosis profoundly impacts human social development, particularly among impoverished populations, impeding the creation of a healthy society and the sustainable development of human societies [7].
The prevention and control of brucellosis is an intricate undertaking due to its diverse transmission routes, including respiratory, gastrointestinal, contact, biological, and sexual transmission (Fig. 1). Moreover, the disease's high susceptibility to relapse poses an additional challenge to managing and containing it. Therefore, it is crucial to implement robust measures to curb the spread of brucellosis and minimize the risk of relapse [8]. The prevention of brucellosis spread among farmers is often hindered by their insufficient knowledge and resources in effectively controlling outbreaks. Such limitations are primarily attributed to the inadequate regulatory procedures and compensation mechanisms implemented by regional administrations, coupled with the government’s inattention to monitoring and addressing the disease’s potential spread [9].
Common routes of transmission of brucellosis. (1) Respiratory transmission: respiratory inhalation of aerosols from Brucella contamination of the environment. (2) Gastrointestinal transmission: ingestion of raw unpasteurized dairy products and undercooked meat. (3) Contact transmission: contact with body fluids of infected animals. (4) Biological transmission: bite from infected ticks and other arthropods. (5) Sexual transmission. Note: the solid lines on the diagram denote confirmed transmission routes, while the dotted lines indicate potential transmission routes
Although vaccines have been essential in preventing brucellosis, it's crucial to acknowledge their potential downsides. Toxic residues found in vaccines can be harmful to animals and humans, and their use can interfere with routine disease detection tests [10, 11]. It is noteworthy that brucellosis can present with a range of diverse symptoms, posing a diagnostic challenge for healthcare professionals. Consequently, misdiagnosis and delayed treatment are prevalent, ultimately jeopardizing patient safety by increasing the likelihood of complications [12]. Additionally, despite the annual registration of 500,000 cases of brucellosis, the World Health Organization (WHO) estimates that around a quarter of patients still go unreported and unrecorded. Consequently, the prevention and control of brucellosis continue to pose significant challenges [13, 14].
Tick-borne diseases (TBDs) are of great concern due to the potential for ticks to harbor a wide range of diseases that can be transmitted to humans, livestock, and wildlife [15, 16]. In recent years, changes in both microclimatic and macroclimatic conditions, as well as human behavior, have resulted in an expansion of the potential suitability areas, further increasing the likelihood of human exposure to these vectors [17]. This scoping review attempts to provide a deeper insight into the role of ticks in the transmission of brucellosis and identify gaps in the existing literature. To achieve this, we analyze the prevalence of Brucella in ticks, the detection of brucellosis in ticks, and the potential risk of tick-borne transmission. This thorough analysis will provide a more profound understanding of the relationship between ticks and brucellosis, and lay the groundwork for future research in this area.
Methods
Search strategy
The Systematic Reviews and Meta-Analyses extension for Scoping Reviews (the PRISMA-ScR) guidelines for conducting a scoping review were followed [18]. A literature search was conducted for publications up to April 2022. The review was undertaken to inform the role of ticks in brucellosis transmission. We listed all keywords related to the six key concepts of our research question: tick, Brucella, the prevalence of brucellosis in ticks, the detection methods of brucellosis, and the risk of tick-borne brucellosis. We used the search strategies presented in Additional file 1 to search the databases. We identified published studies based on combinations of our keywords in four bibliographic databases (two in Chinese and two in English): China National Knowledge Infrastructure (CNKI, https://www.cnki.net/), WanFang (www.wanfangdata.com.cn), Google Scholar (https://scholar.google.com), and PubMed (https://pubmed.ncbi.nlm.nih.gov). In conducting our research, we thoroughly examined the reference lists of all relevant studies in order to identify any additional research that may not have been initially detected through our electronic database searches. We did not try to obtain any unpublished studies, and there were no limitations on language.
Inclusion and exclusion criteria
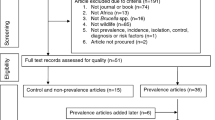
The present study employed a rigorous set of inclusion and exclusion criteria to ensure the quality and relevance of the literature reviewed. As depicted in Fig. 2, a systematic search of various online databases was conducted to identify relevant references. Studies were considered eligible when they met the following eligibility criteria: (i) keywords related to Brucella detection; (ii) prevalence of brucellosis in ticks; (iii) experiments on brucellosis infection by ticks. After the articles were identified, they underwent a rigorous primary screening process. This step involved carefully reviewing the titles and abstracts of each article to determine its relevance to our study objectives. During the full-text screening, any articles that were found to be duplications of previously included studies were excluded. Furthermore, any articles that had significant overlap in terms of data with other studies were also excluded. Additionally, review articles were excluded during the full-text screening process. Lastly, articles that lacked sufficient data to address the research question were also excluded. This process helped to streamline the selection of articles for further review and analysis, thereby enhancing the overall validity and reliability of the study.
Data extraction and analysis
The detailed characteristics of each study were extracted using a pre-designed data-collection Excel form. Information was recorded as follows: study information (the first author, year of publication, location); methodology (experimental method, in detail); characteristics of ticks (species, developmental stage, feeding status); sample size and reported prevalence of brucellosis in ticks. The prevalence data on brucellosis in ticks from all studies were collated, where possible.
Quality assessment of included literature
We assessed the quality of the included articles with reference to Phyllis Munyiva Isaiah et al., using the Joanna Briggs Institute Prevalence Critical Appraisal Tool. All selected studies were scored using the 10 quality control items suggested by the tool. A score of one was awarded for each item fulfilled while a zero score was awarded for each unmet item. Score aggregates were generated and studies were classified as either low (0–3), moderate (4–6), or high (7–10) quality (see Additional file 2).
Gap analysis
After extensive research, identified gaps were categorized into four distinct types: insufficient or imprecise information, biased information, inconsistency or unknown consistency, and a lack of pertinent information. To address these gaps in an effective manner, we assigned priority ratings to each item. This pragmatic and objective approach served as a valuable guide for future improvement strategies, allowing for a more focused and efficient implementation.
Results
Selection of evidence
As depicted in the illustration provided in Fig. 2, we thoroughly searched electronic databases, which yielded 843 papers. After carefully screening each document based on title and abstract relevance, 753 articles were excluded, while 90 articles met the criteria for full-text screening. Eventually, 83 articles were deemed eligible for inclusion in our research. Among the 83 studies analyzed, a total of 23 studies focused on prevalence, 44 examined detection methods, and 16 evaluated risk factors associated with the tick-borne brucellosis.
Methods for detection of brucellosis in ticks
Ticks are known to be potential vectors for transmitting brucellosis. Detecting brucellosis in ticks is crucial for understanding the epidemiology of the disease and implementing appropriate control measures. The present study evaluated the current diagnostic methods for brucellosis, including pathogenic, serological, and molecular biology techniques in Table 1. We also summarize the currently available methods for the detection of brucellosis in ticks.
One of the most widely employed techniques for identifying Brucella in ticks is through culture-based methods [19]. This entails the isolation of the bacteria from tick samples, followed by cultivation in specialized culture media. The ticks are subjected to surface sterilization prior to the aseptic removal of their internal organs or tissues for culture. The cultured samples are then incubated under precise conditions to encourage bacterial growth. Upon the emergence of colonies, they can be subjected to additional identification processes utilizing either biochemical tests or molecular techniques.
Molecular techniques have revolutionized the detection of various pathogens, including brucellosis in ticks [46]. Polymerase chain reaction (PCR) has emerged as a highly effective molecular method for amplifying specific DNA sequences of Brucella bacteria present in tick samples. PCR is capable of detecting even low levels of bacterial DNA, providing an unparalleled level of sensitivity and specificity. Several PCR-based assays, including conventional PCR, real-time PCR, nested PCR, and multiplex PCR [33, 47], have been developed to facilitate rapid and accurate detection of Brucella in tick samples based on different molecular markers (16S rRNA, Bscp31, IS711, Omp22) [48]. Bscp31 and Omp22 have been highlighted as reliable and frequently used markers for brucellosis detection in ticks. These cutting-edge molecular techniques offer a promising alternative to conventional, culture-based methods and have opened up new avenues for tick-borne Brucella detection and research.
Recently, rapid Brucella detection techniques such as loop-mediated isothermal amplification (LAMP) and recombinase polymerase amplification (RPA) have been developed [49, 50]. These techniques could operate under constant temperature conditions, do not require expensive equipment, and provide rapid results, demonstrating the great potential in improving the diagnosis of brucellosis. Furthermore, the advent of metagenomic next-generation sequencing (mNGS) technologies has scaled up the possibilities for detecting and characterizing microbial pathogens. mNGS enables the simultaneous sequencing of millions of DNA fragments, providing a comprehensive view of the microbial community present in tick samples. Through the analysis of the sequence data, it is possible to identify the presence of Brucella DNA in ticks and determine its genetic characteristics. Notably, mNGS provides a more detailed view of the microbial composition and functional potential of tick-associated bacteria, including Brucella [51]. Overall, several methods can currently be employed to detect brucellosis in ticks, providing valuable insights into the epidemiology and transmission dynamics of this zoonotic disease. However, there is no consensus on which approach is more sensitive to detecting brucellosis in ticks. Further research and comparative studies are required to determine the sensitivity of different molecular markers for brucellosis detection in ticks.
Presence and prevalence of brucellosis in ticks
Several studies have shown that ticks can carry Brucella. According to the earliest survey conducted in 1937, ticks were found to be capable of carrying Brucella for an extended period during experimental conditions. Additionally, the live bacteria were identified in the feces of the ticks [52]. Since the initial study, there have been ongoing studies on the detection of Brucella in different tick species. Through conducting a thorough review of literature on the presence or prevalence of brucellosis in ticks, we revealed that brucellosis could be present among 16 different tick species, and the overall prevalence of brucellosis in ticks was about 33.87% (2524/7452) ranging from 0.00% to 87.80%, as highlighted in Table 2.
In addition, Brucella has been observed at various developmental stages of ticks. For example, Gudoshnik and Wang et al. [57, 63] found that Brucella could be present in different developmental stages of D. marginatus by animal experiments. Moreover, the prevalence rate of the Brucella was 40.9% in larvae 4.6% in female ticks, which developed from the same batch of Brucella-positive eggs. Researchers from Mexico, China, and other countries have confirmed that Brucella can be transmitted via vertical route [55, 56, 58].
Moreover, recent research has revealed that the presence of Brucella can be identified in various tissues and organs of ticks (Fig. 3). According to Huang et al. [62], they were able to use a fluorescent quantitative PCR technique to detect the copy number of the Bcsp31 gene of Brucella in the salivary gland and midgut of D. nuttalli. The study was successful in detecting the BCSP31 protein of Brucella at the protein level as well. Furthermore, the ability of Brucella to adapt to the intracellular environment of ticks' primary cells has been demonstrated in a study where primary cells from the salivary glands and midgut tissue of D. nuttalli were isolated and cultured in vitro. This finding suggests that Brucella has the capability to survive and replicate within tick cells, which may play a role in its transmission and persistence within tick populations [71].
Risk evaluation of tick-borne brucellosis transmission. (1) After adult ticks become infected with Brucella, there is the potential for vertical transmission to occur, leading to the infection of different developmental stages of ticks through eggs. (2) Infected ticks have the capability to transmit Brucella to humans. (3) Ticks may also transmit Brucella after biting healthy animals. (4) There is a possibility of “co-feeding transmission” where infected, healthy ticks feed on the same host. This complex mode of transmission highlights the intricate interactions between ticks, hosts, and pathogens in natural ecosystems. Note: the solid lines on the diagram denote confirmed transmission routes, while the dotted lines indicate potential transmission routes
Risk assessment of tick-borne brucellosis transmission
A series of experiments were conducted to investigate the potential role of ticks in the transmission of Brucella and their ability to infect healthy animals. One set of experiments involved engorged female ticks obtained from guinea pigs infected with Brucella [56, 57]. These ticks were cultured, and a homogenate was injected into healthy guinea pigs. The results showed positive test results for Brucella in the injected guinea pigs, indicating that transmission may had occurred. Another similar experiment involved ticks obtained from sheep infected with brucellosis. These ticks were placed on healthy guinea pigs, allowing them to feed and potentially transmit the bacteria. After the ticks were fed, detection was conducted on the guinea pigs to detect any signs of infection. The serological tests were positive, further supporting the potential ability of ticks in transmitting Brucella [53, 54]. These experiments shed light on the potential role of ticks as vectors for transmitting Brucella. The ability of ticks to communicate Brucella has been experimentally demonstrated in some studies. For example, a study published in 1979 showed that the soft tick (Ornithodoros moubata) could acquire and transmit B. abortus, the causative agent of bovine brucellosis [72]. Several studies have investigated the role of ticks in the transmission of Brucella species. One study in Spain found that ticks collected from livestock were positive for Brucella DNA, suggesting their potential role as vectors for brucellosis transmission [73]. Similarly, another study conducted in Iran detected Brucella DNA in ticks collected from sheep and goats [74]. While further research is needed to fully understand the mechanisms and significance of tick-borne transmission in brucellosis, these findings highlight the importance of considering ticks as potential sources of infection.
Moreover, multivariable analyses have been conducted to investigate the factors contributing to the risk of brucellosis on farms. These analyses have shown that improvements in farm biosecurity and hygiene practices can significantly reduce the risk of brucellosis. Additionally, some studies suggested that tick bites compromise the immune response of infected animals, which may lead to increased susceptibility of cattle to Brucella infection [75]. It is important to note that while ticks may play a role in the transmission of brucellosis, they are not the sole means of transmission.
The transmission of brucellosis to humans by ticks has been discussed scarcely in the literature. Upon reviewing the database, we found only three case reports from 1963 to 2019 regarding brucellosis transmission to humans. One of the cases involved a slaughterhouse worker in the United Kingdom who contracted brucellosis after being bitten by a tick while at work. Initially, it was thought that the worker contracted brucellosis from the slaughtered animal, but a tick was removed from under the worker's shoulder before the onset of the illness [76]. The investigators recommended that attention be given to the possibility of vector brucellosis transmission. In 2011, a study by Simsek and colleagues in Turkey reported the cases of six patients with brucellosis, one of whom had a possible brucellosis infection caused by a tick bite [77]. A decade later, in 2019, researchers identified that one patient had not come into contact with unpasteurized dairy products, and instead, the brucellosis was linked to repeated tick bites [78]. The identification and confirmation of tick-borne brucellosis cases pose a significant challenge due to the diverse range of potential sources for the pathogen, including unpasteurized dairy and meat products [79]. The complexity of establishing epidemiological links necessitates rigorous biosafety protocols and ethical considerations during experimental validation. These limitations make it difficult for researchers to conduct comprehensive investigations into the disease, despite the severe implications for animal and human health [80, 81]. Further research is required to address these challenges and advance our understanding of this elusive pathogen.
It has been traditionally believed that arthropods, specifically ticks, only transmit pathogenic microorganisms by biting their hosts or through vertical propagation. However, recent research has suggested that this view may be flawed. It has been observed that ticks living and feeding in close spatiotemporal proximity can also lead to the transmission of pathogenic microorganisms between ticks, termed co-feeding transmission [82]. This presents new opportunities and avenues for transmission. Although co-feeding transmission has only been documented in some pathogen infection, such as TBE group flaviviruses [83], and Borrelia burgdorferi [84], its possible mechanism in tick-borne Brucella warrants further investigation.
Gap analysis for the role of ticks in the transmission of brucellosis
By undertaking a detailed scoping review, we found that the literature on tick-borne Brucella transmission to animals or humans was scarce, with several factors contributing to the gap in the role of ticks in the transmission of brucellosis. First, research on TBDs is presently limited due to ethical and biosafety factors that prevent Brucella research from being carried out in ordinary laboratories. Second, there is a lack of solid evidence supporting ticks as effective vectors, with insufficient data from animal experiments, clinical trials, and large-scale epidemiological studies. Third, current detection methods for Brucella in ticks lack updates, and more convenient tools are needed. Finally, the intricate process by which ticks transmit Brucella bacteria, as well as the variations in vector capacity among different tick species, remain largely unresolved and require further investigation. This critical analysis seeks to better understand the reasons for this gap and outlines strategies to bridge it. Hopefully, this will provide valuable information for the biological field on how to not simply bridge but also close this gap, thereby creating more substantial evidence for tick-borne Brucella.
Discussion
The increasing prevalence of zoonotic diseases globally has been a source of mounting apprehension. Recent findings have revealed that the majority of zoonotic diseases, approximately 71.80%, originate from wild animals (22.80% are vector-borne diseases) [85]. Ticks have been identified as the primary culprits for transmitting a vast array of diseases to domestic animals compared to other arthropods [16, 86]. Despite many studies on the relationship between brucellosis transmission and ticks, ticks' precise role in transmitting this disease and the associated risks remain poorly understood.
More than 800 species of ticks in 18 genera have been identified in the world, of which about 80 species of ticks are considered to be the vectors of disease transmission in the world. Understanding ticks’ biology, its ecological roles, and vectors for numerous pathogens is crucial for developing effective strategies to prevent and control tick-borne diseases and ensure the health of both humans and animals [87]. This scoping review indicated that Brucella had been detected in 16 species of ticks, although there remain tick species in which Brucella has yet to be identified. It must be noted that there is a lack of evidence to suggest that other tick species carry Brucella bacteria. This could be attributed to the rarity of brucellosis in the area. Regrettably, there is an evident dearth of research and testing in this regard, rendering it challenging to make definitive conclusions on the presence and prevalence of brucellosis in ticks.
As we explained above ticks can acquire Brucella bacteria by feeding on infected animals and subsequently transmit the bacteria to other hosts during subsequent blood meals. In addition to ticks, other blood-feeding parasites such as lice and fleas have also been implicated as potential vectors for brucellosis. A study conducted in Ethiopia found that lice collected from cattle were positive for Brucella DNA, suggesting their potential role in transmitting the bacteria [88]. Similarly, a study conducted in Mexico detected Brucella DNA in fleas collected from dogs [55]. It is important to note that while these studies provide evidence for the potential role of ticks and other blood-feeding parasites as vectors for brucellosis, further research is needed to understand the extent of their involvement in disease transmission fully.
As mentioned above, the advancement of Brucella detection methods has been of paramount importance in studying the relationship between ticks and Brucella. The emergence of PCR technology has revolutionized the detection of Brucella in ticks, making it more secure, rapid, and accessible on a large scale. In addition, the development of metagenome sequencing technology has enhanced the probability of detecting brucellosis in ticks. However, it is important to explore and analyze whether the newly discovered and improved technical detection methods for brucellosis can be effectively applied to ticks.
There is little literature on tick-borne brucellosis in humans, and empirical evidence is limited mainly due to various factors such as biosecurity measures and the numerous routes of human brucellosis infection. Humans may infect Brucella through multiple ways, including consuming contaminated raw milk and dairy products, close contact with infected animals, or laboratory exposure [89]. The transmission route of brucellosis can be challenging to determine when patients report both a history of tick bites and contact with infected animals and their products, making it difficult to ascertain whether ticks are an effective vector in transmission. Additional research is needed better to understand the mechanisms of human infection by ticks and to bridge this imperative knowledge gap.
To effectively conduct a risk assessment for tick-borne brucellosis transmission, several factors need to be considered, including the prevalence of Brucella-infected ticks in the area, the behavior and habitat of the ticks, and the potential for human exposure. The prevalence of brucellosis in ticks can be obtained through surveillance programs conducted by public health agencies or research institutions. Tick surveillance data can help identify areas with a higher risk of tick-borne brucellosis transmission. Identifying high-risk areas based on tick behavior and habitat can help guide preventive measures. Certain geographical regions may have a higher prevalence of infected ticks, increasing the risk of transmission. Human exposure, such as outdoor activities, occupation, and geographical location, play a role in determining the likelihood of encountering infected ticks. Individuals who spend a significant amount of time outdoors, such as hikers, campers, or outdoor workers, are at a higher risk of tick bites.
Given the current state of environmental changes, closely monitoring the potential impact on tick distribution and the associated risk of tick-borne brucellosis is of utmost importance. First of all, ticks are highly sensitive to environmental changes, as they spend most of their life cycle in this setting. Their survival is dependent on several climatic variables, including vegetation and the presence of appropriate hosts. Studies have found that climatic changes, such as rising temperatures, can positively affect ticks' survival. This can lead to an increase in number, activity, range of ticks and the potential for these parasites to spread, become established, and persist in new locations [90, 91]. Second, climate change affects both the reproduction hosts and the reservoir hosts involved in the tick lifecycle and spread of TBDs, respectively. Increasing temperatures will expand the distribution range of hosts as well as their abundance and activity. In addition, as a result of climate change, people may resume outdoor activity earlier in the spring and maintain it longer in the fall. With the increase in length of exposure to tick habitat, combined with an extended season of tick activity, the likelihood of encountering ticks carrying unknown pathogens increases, thereby elevating the risk of human infection with brucellosis and other tick-borne illnesses [92]. This fits with another concept, the emergence and spread of zoonosis are heavily influenced by a variety of factors, many of which are related to climate change and environmental factors. Habitat variation and atmospheric and climatic changes are among the most significant of these factors, and they can significantly impact the spread of diseases from animals to humans [93]. Therefore, in the context of adaptation to climate change, we must remain vigilant in our efforts to mitigate these risks and safeguard against potential health hazards posed by ticks.
Conclusions
The present scoping review provides a comprehensive overview of the existing literature about the role of ticks in brucellosis transmission. We have revealed that Brucella is present across a variety of developmental stages in ticks, indicating the potential for widespread transmission and dissemination of this pathogen within and among tick populations. Available detection methods for brucellosis detection were also presented and evaluated. The potential role of ticks as vectors of brucellosis and the risks they may pose suggests the need for further studies. The assessment of the role and specific mechanisms of ticks in the epidemiology and transmission of brucellosis revealed the need for other in-depth studies, as well as the availability of quick and safe research methods to explore Brucella in ticks. The increased risk of tick-borne diseases is highlighted as essential to better understanding the interactions between ticks, Brucella, animal hosts, humans, and the environment.
Availability of data and materials
Not applicable.
Abbreviations
- TBD:
-
Tick-borne disease
- RBT:
-
Rose Bengal Test
- MRT:
-
Milk Ring Test
- ELISA:
-
Enzyme-linked immunosorbent assay
- FPA:
-
Fluorescence polarisation assay
- CFT:
-
Complement fixation test
- SAT:
-
Standard tube agglutination test
- LAMP:
-
Loop-mediated isothermal amplification
- RPA:
-
Recombinase polymerase amplification
- mNGS:
-
Metagenomic next-generation sequencing
- PCR:
-
Polymerase chain reaction
References
Zheng R, Xie S, Lu X, Sun L, Zhou Y, Zhang Y, et al. A Systematic review and meta-analysis of epidemiology and clinical manifestations of human brucellosis in China. Biomed Res Int. 2018;2018:5712920.
Gumaa MM, Cao X, Li Z, Lou Z, Zhang N, Zhang Z, et al. Establishment of recombinase polymerase amplification for diagnosis of Brucella spp. Mol Cell Probes. 2019. https://doi.org/10.1016/j.mcp.2019.101434.
Pappas G, Papadimitriou P, Akritidis N. The new global map of human brucellosis. Lancet Infect Dis. 2006;6(2):91–9.
Gul SK, Khan A. Epidemiology and epizootology of brucellosis: a review. Pak Vet J. 2007;27(3):145.
Franc KA, Krecek RC, Häsler BN, Arenas-Gamboa AM. Brucellosis remains a neglected disease in the developing world: a call for interdisciplinary action. BMC Public Health. 2018;18(1):1–9.
Dadar M, Shahali Y, Whatmore AM. Human brucellosis caused by raw dairy products: a review on the occurrence, major risk factors and prevention. Int J Food Microbiol. 2019;292:39–47.
Dean AS, Crump L, Greter H, Schelling E, Zinsstag J. Global burden of human brucellosis: a systematic review of disease frequency. PLoS Negl Trop Dis. 2012;6(10): e1865.
Hao H, Wang B, Zhang X, Liu Z, et al. Research progress on the prevention and treatment of brucellosis. Chin J Hyg Insect Equip. 2021;27(04):366–70 (In Chinese).
Hf Ai. Preliminary study on problems and solutions in the prevention and treatment of brucellosis in cattle and sheep. Chin Anim Health. 2022;24(02):32–3 (In Chinese).
Hou H, Liu X, Peng Q. The advances in brucellosis vaccines. Vaccine. 2019;37(30):3981–8.
Wang C. Preliminary study on current status of brucellosis vaccine immunity in sheep. Haikou: Hainan University; 2020.
Afshar S, Mamishi S. Brucellosis: review of clinical and laboratory features and therapeutic regimens in 44 children. Acta Med Iran. 2008;46(6):489–94.
Nicoletti P. Brucellosis: past, present and future. Prilozi. 2010;31(1):21–32.
Ghanbari MK, Gorji HA, Behzadifar M, Sanee N, Mehedi N, Bragazzi NL. One health approach to tackle brucellosis: a systematic review. Trop Med Health. 2020;48(1):1–10.
Zhao GP, Wang YX, Fan ZW, Ji Y, Liu MJ, Zhang WH, et al. Mapping ticks and tick-borne pathogens in China. Nat Commun. 2021;12(1):1–13.
Aktas M. A survey of ixodid tick species and molecular identification of tick-borne pathogens. Vet Parasitol. 2014;200(3–4):276–83.
Madison-Antenucci S, Kramer LD, Gebhardt LL, Kauffman E. Emerging tick-borne diseases. Clin Microbiol Rev. 2020;33(2):e00083-e118.
Moher D, Liberati A, Tetzlaff J. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Checklist of items to include when reporting. PLoS Med. 2009;6: e1000097.
Franco MP, Mulder M, Gilman RH, Smits HL. Human brucellosis. Lancet Infect Dis. 2007;7(12):775–86.
Yagupsky P, Morata P, Colmenero JD. Laboratory diagnosis of human brucellosis. Clin Microbiol Rev. 2019;33(1):e00073-e119.
Gusi AM, Bertu WJ, Jesús de Miguel M, Dieste-Pérez L, Smits HL, Ocholi RA, et al. Comparative performance of lateral flow immunochromatography, iELISA and Rose Bengal tests for the diagnosis of cattle, sheep, goat and swine brucellosis. PLoS Negl Trop Dis. 2019;13(6): e0007509.
Wang G, Fan W, Liu K, Tian L, Wen J. Comparison of advantages and disadvantages of brucellosis serological detection methods. Chin Anim Health Inspect. 2021;38(05):72–5 (In Chinese).
Gupta VK, Nayakwadi S, Kumar A, Gururaj K, Kumar A, Pawaiya RS. Markers for the molecular diagnosis of brucellosis in animals. Adv Anim Vet Sci. 2014;2(3):31–9.
Akhtar R, Chaudhry ZI, Shakoori AR, Ahmad M, Aslamet A. Comparative efficacy of conventional diagnostic methods and evaluation of polymerase chain reaction for the diagnosis of bovine brucellosis. Vet World. 2010;3(2):53–6.
Rahman AKMA, Smit S, Devleesschauwer B, Kostoulas P, Abatih E, Saegerman C, et al. Bayesian evaluation of three serological tests for the diagnosis of bovine brucellosis in Bangladesh. Epidemiol Infect. 2019;147: e73.
Nimri LF. Diagnosis of recent and relapsed cases of human brucellosis by PCR assay. BMC Infect Dis. 2003;3(1):1–7.
Matrone M, Keid LB, Rocha VCM, Vejarano M, Ikuta CY, Rodriguez CA, et al. Evaluation of DNA extraction protocols for Brucella abortus PCR detection in aborted fetuses or calves born from cows experimentally infected with strain 2308. Braz J Microbiol. 2009;40(3):480–9.
Batinga MCA, de Lima JTR, Gregori F, Diniz JA, Muner K, Oliveira TMFS, et al. Comparative application of IS711-based polymerase chain reaction (PCR) and loop-mediated isothermal amplification (LAMP) for canine brucellosis diagnosis. Mol Cell Probes. 2018;39:1–6.
Mukherjee F, Jain J, Patel V, Nair M. Multiple genus-specific markers in PCR assays improve the specificity and sensitivity of diagnosis of brucellosis in field animals. J Med Microbiol. 2007;56(10):1309–16.
Saytekin AM, Ak S. Direct diagnosis of Brucella species through multiplex PCR formed by a new method. J Microbiol Methods. 2018;154:86–94.
Si C. The establishment of multiplex PCR method and detection kit for Brucella abortus, Brucella melitensis, Brucella suis and Brucella canis. Shanghai: Academy of Military Medical Sciences; 2014.
Saini S, Gupta VK, Gururaj K, Singh DD, Pawaiya RVS, Gangwar NK, et al. Comparative diagnostic evaluation of OMP31 gene based TaqMan® real-time PCR assay with visual LAMP assay and indirect ELISA for caprine brucellosis. Trop Anim Health Prod. 2017;49(6):1253–64.
Du Q. Establishment of real-time fluorescence quantitative PCR for rapid detection of Brucella and its preliminary application. Dali City: Dali University; 2019.
Queipo-Ortuño MI, Colmenero JD, Reguera JM, García-Ordoñez MA, Pachón ME, Gonzalez M, et al. Rapid diagnosis of human brucellosis by SYBR green I-based real-time PCR assay and melting curve analysis in serum samples. Clin Microbiol Infect. 2005;11(9):713–8.
Kaden R, Ferrari S, Alm E, Wahab T. A novel real-time PCR assay for specific detection of Brucella melitensis. BMC Infect Dis. 2017;17(1):1–6.
Falcão MVD, Santana VLA, Corrêa FN, Tenório JAB, Mota RA. Development and standardization of a western blotting test for detection of antibodies against B. abortus. Arquiv Bras Med Vet Zootecn. 2019;71:160–6.
Kim JY, Sung SR, Lee K, Lee HK, Kang SI, Lee JJ, et al. Immunoproteomics of Brucella abortus RB51 as candidate antigens in serological diagnosis of brucellosis. Vet Immunol Immunopathol. 2014;160(3–4):218–24.
Wareth G, Melzer F, Weise C, Neubauer H, Roesler U, Murugaiyan J. Proteomics-based identification of immunodominant proteins of Brucellae using sera from infected hosts points towards enhanced pathogen survival during the infection. Biochem Biophys Res Commun. 2015;456(1):202–6.
Han D, Li Z, Li R, Tan P, Zhang R, Li J. mNGS in clinical microbiology laboratories: on the road to maturity. Crit Rev Microbiol. 2019;45(5–6):668–85.
Rodino KG, Pritt BS. Novel applications of metagenomics for detection of tickborne pathogens. Clin Chem. 2021;68(1):69–74.
Mu J. Establishment of LAMP assay for Brucella spp. differential diagnosis. Hohhot: Inner Mongolia Agricultural University; 2021.
Moehling TJ, Choi G, Dugan LC, Salit M, Meagher RJ. LAMP diagnostics at the point-of-care: emerging trends and perspectives for the developer community. Expert Rev Mol Diagn. 2021;21(1):43–61.
Qin L, Nan W, Wang Y, Zhang Y, Tan P, Chen Y, et al. A novel approach for detection of Brucella using a real-time recombinase polymerase amplification assay. Mol Cell Probes. 2019;48: 101451.
Zhang S. Development of rapid nucleic acid detection methods for Brucella. Changchun: Jilin University; 2020.
Ren H. Development and application of recombinase polvmerase amplification assay for three important infectious pathogens. Changchun: Academy of Military Medical Sciences; 2016.
Padilla PF, Nielsen K, Ernesto SL, Yu W. Diagnosis of brucellosis. Open Vet Sci J. 2010;4(1):46.
Ouyang Z, Huang T, Wen T. Research progress of laboratory detection methods for brucellosis. Chin J Anim Husb Vet Med. 2021;48(9):11–2 (In Chinese).
Hosseini-Chegeni A, Tavakoli M, Telmadarraiy Z, Sedaghat MM, Faghihi F. Detection of a Brucella-like (Alphaproteobacteria) bacterium in Boophilus spp. (Acari: Ixodidae) from Iran. J Med Microbiol. 2017;5(3):66–8.
Mayer-Scholl A, Draeger A, Göllner C, Scholz HC, Nöckler K. Advancement of a multiplex PCR for the differentiation of all currently described Brucella species. J Microbiol Methods. 2010;80(1):112–4.
Dao TNT, Lee EY, Koo B, Jin CE, Lee TY, Shin YA. A microfluidic enrichment platform with a recombinase polymerase amplification sensor for pathogen diagnosis. Anal Biochem. 2018;544:87–92.
Xu X, Han A, Ye S, Guan W, Lou Y, et al. Metagenomic analysis of microbial community structure, antibiotic resistance genes and virulence factors in ticks intercepted at Wenzhou port. Chin J Vector Biol Control. 2021;32(6):763–71 (In Chinese).
Wang Y, Iminjian P, Chen C. Focuses on the role of parasites in the transmission of brucellosis. Chin J Endemiol. 2016;35(4):235–7 (In Chinese).
Zotova A, Bolditzina K. An experiment in infecting ticks with brucellosis under laboratory conditions. Izv kazakh Fil Akad Nauk (Ser Zool). 1943;2:48–9.
Galuzo I, Kaïtmazova E. Ixodid ticks-the possible vectors of brucellosis. lzvestiya Kazakhskogo Filiala Akademii Nauk SSSR. 1944; (3): 123–137.
Tovar RM. Infection and transmission of Brucella by ectoparasites. Am J Vet Res. 1947;8(26):138–40.
Pritulin P. On the transmission of brucellosis by the pasture ticks Dermacentor nuttallia and Hyalomma marginatum. Veterinariya. 1954;31(7):31.
Gudoshnik AN. Study of the rôle of ixodid ticks in the circulation of the causal agent of brucellosis. 1955.
Li Z. The role of ticks in the transmission of brucellosis in cattle and sheep. Chin J Vet Med. 1958;04:132–7 (In Chinese).
Khrustcheva NF. Experimental observations of brucellosis carriage in bloodsucking ticks. Alma-Ata: 1969.
Peres J. Isolation of Brucella canis from ticks (Rhipicephalus sanguineus). Muhammad Ali Society; 1986.
Khan MN, Hayat CS, Iqbal Z. Role of ixodid ticks in transmission of bacterial diseases in cattle and buffaloes in Pakistan. Pak J Agri Sci. 1997;34(0–4):86–8.
Huang T, Zhang J, Sun C, Liu Z, He H, Wu J, Geriletu. A novel arthropod host of brucellosis in the arid steppe ecosystem. Front Vet Sci. 2020;7:566253.
Wang Q, Zhao S, Wureli H, Xie S, Chen C, Wei Q, et al. Brucella melitensis and B. abortus in eggs, larvae and engorged females of Dermacentor marginatus. Ticks Tick Borne Dis. 2018;9(4):1045–8.
Huang F. Genetic diversity of dominant ticks and detection of specific genes of Brucella in Xilingol League, Inner Mongolia. Hohhot: Inner Mongolia Agricultural University; 2019.
Jiang M, Yang M, Song R, Wang Y, Sheng J, et al. Molecular epidemiological analysis of Brucella in ticks in Xinjiang Uygur autonomous region. Chin J Endemiol. 2019;12:947–50 (In Chinese).
Li Y, Wen X, Li M, Moumouni PFA, Galon EM, Guo Q, et al. Molecular detection of tick-borne pathogens harbored by ticks collected from livestock in the Xinjiang Uygur autonomous region, China. Ticks Tick Borne Dis. 2020;11(5): 101478.
Zhang K, Li A, Wang Y, Zhang J, Chen Y, Wang H, et al. Investigation of the presence of Ochrobactrum spp. and Brucella spp. in Haemaphysalis longicornis. Ticks Tick Borne Dis. 2021;12(1):101588.
Song S, Guo J, Yang Y, Sang C, Yang M, Wang Y. Species identification of pet dog ticks in Shihezi area and detection of Brucella carriers. Chin Anim Husb Vet Med. 2021;48(05):1717–24 (In Chinese).
Carpi G, Cagnacci F, Wittekindt NE, Zhao F, Qi J, Tomsho L, et al. Metagenomic profile of the bacterial communities associated with Ixodes ricinus ticks. PLoS ONE. 2011;6(10): e25604.
Song C, Hu Y, Tang L, Li Q, Guo X, Bai H, et al. Analysis of the diversity of microorganisms carried by leather tick by next-generation sequencing technology. Heilongjiang Anim Sci Vet. 2018; (21): 5–9–254 (In Chinese).
Huang T. Study on the traceability of Brucella melitensis from Inner Mongolia and the characteristics of its new arthropod host. Hohhot: Inner Mongolia Agricultural University; 2020.
Grimsdell J, Appendix A. Changes in populations of resident ungulates. Serengeti: dynamics of an ecosystem. 1979: 353–9.
Asencio MA, Herraez O, Tenias JM, Garduño E, Huertas M, Carranza R, Ramos JM. Seroprevalence survey of zoonoses in Extremadura, southwestern Spain, 2002–2003. Jpn J Infect Dis. 2015;68(2):106–12.
Sabzevari S, Shoraka H, Seyyedin M. Seroepidemiological survey of brucellosis and Q fever among high-risk occupations in northeast of Iran for first time. Iran J Microbiol. 2021;13(3):325.
Miller R, Nakavuma JL, Ssajjakambwe P, Vudriko P, Musisi N, Kaneene JB. The prevalence of brucellosis in cattle, goats and humans in rural Uganda: a comparative study. Transbound Emerg Dis. 2016;63(6):e197–210.
Hutcheson R. Tick-borne brucellosis. NEJM. 1963;269(8):430.
Simsek F, Yildirmak MT, Gedik H, Kantürk A, Iris EN. Pulmonary involvement of brucellosis: a report of six cases. Afr Health Sci. 2011;11:112–6.
Popov G, Gotseva A. А case of brucellosis after tick exposure. Cases from practice. 2019; LXXI issue 1.
Khan MZ, Zahoor M. An overview of brucellosis in cattle and humans, and its serological and molecular diagnosis in control strategies. Trop Med Infect Dis. 2018;3(2):65.
Chen X. Insists on normalizing the quarantine and purification of zoonotic brucellosis. ChinAnim Health. 2022;24(03):3–5.
Yong Y, Pu D, Shi X, Zhao H. Prevention and control of brucellosis in laboratory infection. Dis Surveill. 2021;36(11):1203–6.
Harrison A, Bennett NCJ. The importance of the aggregation of ticks on small mammal hosts for the establishment and persistence of tick-borne pathogens: an investigation using the R0 model. Parasitology. 2012;139(12):1605–13.
Murray PR, Rosenthal KS, Pfaller MA. Medical microbiology. Edinburgh: Elsevier Health Sciences; 2015.
Richter D, Allgöwer R, Matuschka FR. Co-feeding transmission and its contribution to the perpetuation of the Lyme disease spirochete Borrelia afzelii. Emerg Infect Dis. 2002;8(12):1421.
Wikel SK. Ticks and tick-borne infections: complex ecology, agents, and host interactions. Vet Sci. 2018;5(2):60.
Shapiro SZ, Voigt W, Fujisaki K. Tick antigens recognized by serum from a guinea pig resistant to infestation with the tick Rhipicephalus appendiculatus. J Parasitol. 1986;72(3):454–63.
Yu Z, Wang H, Wang T, Sun W, Yang X, Liu J. Tick-borne pathogens and the vector potential of ticks in China. Parasit Vectors. 2015;8(1):24.
Urge B, Tadele M, Siyoum T. Infestation of ectoparasites in dairy calves reared by smallholder farmers in central areas of Ethiopia. Biomed J Sci Tech Res. 2020. https://doi.org/10.26717/BJSTR.2020.26.004360.
Dadar M, Fakhri Y, Shahali Y, Mousavi KA. Contamination of milk and dairy products by Brucella species: a global systematic review and meta-analysis. Food Res Int. 2020;128: 108775.
Capligina V, Seleznova M, Akopjana S, Freimane L, Lazovska M, Krumins R, et al. Large-scale countrywide screening for tick-borne pathogens in field-collected ticks in Latvia during 2017–2019. Parasit Vectors. 2020;13(1):351.
Buczek AM, Buczek W, Buczek A, Bartosik K. The potential role of migratory birds in the rapid spread of ticks and tick-borne pathogens in the changing climatic and environmental conditions in Europe. Int J Environ Res Public Health. 2020;17(6):2117.
Bouchard C, Dibernardo A, Koffi J, Wood H, Leighton PA, Lindsay LR. N increased risk of tick-borne diseases with climate and environmental changes. Can Commun Dis Rep. 2019;45(4):83–9.
Harrus S, Baneth G. Drivers for the emergence and re-emergence of vector-borne protozoal and bacterial diseases. Int J Parasitol. 2005;35(11–12):1309–18.
Acknowledgements
Not applicable.
Funding
This work was supported by the Inner Mongolia Autonomous Region Science and Technology leading talent team: Zoonotic disease prevention and Control Technology innovation team (2022SLJRC0023); Key Technology Project of Inner Mongolia Science and Technology Department (2021GG0171); State Key Laboratory of Reproductive Regulation and Breeding of Grassland Livestock (2020ZD0008); Study on pathogen spectrum, temporal and spatial distribution and transmission features of the important emerging and re-emerging zoonosis in Inner Mongolia Autonomous Region (U22A20526).
Author information
Authors and Affiliations
Contributions
XYF and WH conceived the study and contributed the original idea. RM, CFL, AG, NJ, JL and XYF wrote the initial draft of the paper. XYF and WH contributed to the revision of the manuscript, and the final version was reviewed by WH. All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All participants consented to have their data published.
Competing interests
We declare no competing interests.
Supplementary Information
Additional file 1.
Databases and search strategies.
Additional file 2.
Quality assessment of included literatures.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ma, R., Li, C., Gao, A. et al. Evidence-practice gap analysis in the role of tick in brucellosis transmission: a scoping review. Infect Dis Poverty 13, 3 (2024). https://doi.org/10.1186/s40249-023-01170-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40249-023-01170-4