Abstract
Background
Strongyloidiasis, caused by the nematodes Strongyloides stercoralis and Strongyloides fuelleborni, is estimated to affect over 600 million individuals worldwide. The disease is endemic in Southeast Asia, where a warm-humid climate and socio-economic conditions maintain the parasite’s life cycle and transmission. However, the current diagnostic methods may not be sufficiently sensitive, suggesting that the true prevalence of strongyloidiasis could be seriously underestimated in this. This study aims to determine the prevalence of strongyloidiasis in Southeast Asia through a systematic review and meta-analysis and to discuss the implications of the estimated prevalence on diagnostic approaches and control strategies.
Methods
Following PRISMA guidelines, we conducted a systematic literature search in PubMed and Google Scholar databases to identify studies reporting Strongyloides prevalence data in the 11 Southeast Asian countries up to December 2022. A random effects model was employed to estimate the pooled prevalence of S. stercoralis at both regional and country levels.
Results
Out of 3722 articles identified, 224 met our inclusion criteria. For S. stercoralis specifically, we found 187 articles, of which 52.4% were from Thailand. All Southeast Asian countries, except Brunei, had at least one study on Strongyloides prevalence. The estimated pooled prevalence of S. stercoralis regionally was 12.7% (95% CI 10.70–14.80%), ranging from 0.4 to 24.9% at the country level. Cambodia had the highest pooled prevalence (24.9%, 95% CI 15.65–35.38%), followed by Lao PDR (16.5%, 95% CI 9.50–24.95%). Moreover, we obtained a pooled prevalence of 10% (95% CI 7.06–13.52%) in a group comprising immigrants, workers, and veterans from Southeast Asian countries. S. stercoralis infects various host types, including nonhuman primates, domestic dogs and cats, rodents, and transport carriers such as cockroaches and vegetables.
Conclusions
A high prevalence of strongyloidiasis in Southeast Asia was revealed, highlighting the importance of the region’s ongoing research, surveillance, and control efforts. Factors contributing to the strongyloidiasis transmission include the role of animal hosts, the impact of global connectivity, and the significance of the co-endemicity of other Strongyloides species. Based on these findings, a multi-pronged One-Health approach is essential for sustainable intervention and control.
Similar content being viewed by others
Background
Strongyloidiasis is a neglected tropical disease that mainly affects humans in tropical and subtropical regions, with an estimated global prevalence of over 600 million people [1]. The disease is caused by the nematodes Strongyloides stercoralis and Strongyloides fuelleborni [2]. Clinical manifestation of S. stercoralis infection in human can range from asymptomatic to chronic, depending on the infection intensity. The parasite can live in human body for many cycles if left untreated through autoinfection, where larval infectivity can be achieved without leaving the host [3, 4]. Immunocompromised individuals are particularly susceptible to hyperinfection, leading to mortality [5, 6]. On the other hand, S. fuelleborni primarily infects various nonhuman primates, and although cases of human infection have been reported in African and Southeast Asian countries, autoinfection is unlikely as eggs are excreted in feces and hatch in the external environment [4, 7,8,9,10].
Despite the medical significance of strongyloidiasis, there is a consensus that the global prevalence was underestimated due to the low sensitivity of current diagnostic techniques. Currently, the optimal parasitological method for S. stercoralis diagnosis is the detection of larvae in fecal samples [3, 11] using methods such as the Baermann-Mores, agar plate culture (APC), and Harada-Mori culture. However, other parasitological techniques unsuitable for detecting larvae but more suited for detecting eggs in fecal samples are still widely used due to their ease of application. These parasitological techniques can lead to underestimating light infections when larval output is intermittent and low [12].
Southeast Asian region comprises 11 countries, collectively inhabiting more than 600 million people [13]. The countries include Thailand, Cambodia, Lao People’s Democratic Republic (Lao PDR), Myanmar, Vietnam, Indonesia, Malaysia, The Philippines, Singapore, Timor-Leste, and Brunei. Southeast Asia is rapidly emerging as a global economic hub for technology and innovation, enhancing its popularity as an ideal destination [14, 15]. Furthermore, an estimated 23.6 million Southeast Asian migrants reside outside their country of origin, with labor migration being the dominant trend in recent decades [16]. With increased global connectivity, patterns of human migration, and favorable climatic, ecological, and socio-economic conditions, Southeast Asia is an ideal environment for transmitting strongyloidiasis. The World Health Organization (WHO) has recently included strongyloidiasis as a soil-transmitted helminth (STH) targeted for control, emphasizing its medical importance and the risk of transmission [17].
This study aims to estimate the prevalence of S. stercoralis infection in 11 Southeast Asian countries using a systematic review and meta-analysis and to discuss the implications of the estimated prevlance for diagnosis and control strategies. In view of the WHO’s inclusion of S. stercoralis as another STH targeted for control in 2030, the results can provide valuable data for strongyloidiasis control strategies in Southeast Asia.
Methods
Search strategy
The systematic review and meta-analysis followed the PRISMA guidelines (http://www.prisma-statment.org/) (Additional file 1). In December 2022, identification of all publications regarding Strongyloides in Southeast Asia was searched in PubMed and Google Scholar databases. The keywords used were Strongyloides AND the 11 countries in Southeast Asia, namely, Thailand, Laos, Cambodia, Myanmar, Vietnam, Timor-Leste, Indonesia, Malaysia, Brunei, Philippines, Singapore. Two researchers performed the data search in the databases, and the titles and abstracts were evaluated independently.
Exclusion and inclusion criteria
The articles from the search were input into a Microsoft Office Excel (Microsoft Corporation, Albuquerque, USA) spreadsheet, and duplicates were removed. Articles were eligible for inclusion if they met the following criteria: (1) data showing the prevalence of Strongyloides from any of the countries in Southeast Asia regardless of host species, (2) articles related to immigrants, workers, veterans (IWV) in Southeast Asian countries, (3) peer-reviewed articles containing original data, and (4) articles with full-text access with abstract. Articles not meeting the above criteria were excluded, such as review articles, case reports, those with no full-text and abstract, and those not in English (excluding Thai). In case of discrepancies regarding the inclusion or exclusion of a study, the team discussed the matter to reach a consensus agreement.
Data extraction
The articles were grouped according to each country and a group containing articles from IWV (all from Southeast Asia). The data that was retrieved from each included article were: first author name, year of publication, country and province or state of study, study population, Strongyloides species, host, a diagnostic method used, total sample size, positive samples, and prevalence (Additional files 2 and 3). If the number of positive samples or prevalence were not calculated, either value was inferred from the total sample size and prevalence or the number of positive samples. If more than one diagnostic method was used, the method with the highest prevalence was selected for meta-analysis to ensure that strongyloidiasis was not underestimated in this meta-analysis.
Statistical approach and meta-analysis
Meta-analysis was conducted for S. stercoralis, and the overall pooled prevalence was calculated with 95% confidence intervals (CI). A sub-analysis was performed according to the year of publication for the overall pooled prevalence, where the time periods selected were: year 1970 to 2000 (before millennium) and 2001 to 2022 (after millennium). The dataset from Thailand was also analysed based on these two time periods due to the high number of studies available. Another subgroup analysis was performed to calculate pooled prevalence per country. The pooled prevalence estimates were calculated using the alpha method for the random effects model based on the inverse variance method for measuring weight. Cochran Q test and the inconsistency index (I2) were used to assess the degree of heterogeneity among studies, with values of more than 75% considered high heterogeneity. Publication bias was calculated using Egger’s regression and Begg’s test, where a P-value of < 0.05 indicated the presence of publication bias.
The calculated pooled prevalence per country was plotted on a map, with the degree of prevalence indicated by the color intensity. Additionally, the diagnostic methods used for each country were summarized as percentages and plotted on the map. All statistical, meta-analysis, and map generation were conducted in R studio version 1.4.1717 (RStudio, PBC, Boston, USA) [18].
In addition to calculating pooled prevalence estimates for each country, regional prevalence rates of S. stercoralis were determined for Thailand, Cambodia, Lao PDR, and Malaysia by averaging the prevalence data from the relevant studies. These countries were selected due to their high number of S. stercoralis prevalence studies. The regions were as follows: Thailand was divided into North, Northeast, Central, South; Cambodia into Northwestern, Elephant Mountains and Cardamom, Mekong lowland and Eastern; Lao PDR into North, Central and South; and Malaysia into Peninsular and East.
The proportions of the types of diagnostic tests used to detect S. stercoralis were also calculated and summarized, with tests falling into one of four categories: parasitological only, serological only, molecular only, and a combination of any two methods (which includes any pairings of the aforementioned methods).
Results
Data characteristics
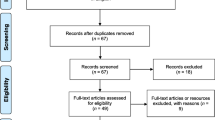
A total of 543 and 6781 records were found in PubMed and Google Scholar databases, respectively. From both databases, 3722 publications were obtained after duplicates were removed. Of these, 257 publications were screened for their eligibility. Thirty-three articles were excluded, of which seven studies presented repeat data, 10 had no record of prevalence data, 13 did not test for Strongyloides, and three were unrelated. Finally, 224 publications were included in qualitative synthesis and meta-analysis (Additional files 2 and 3). Figure 1 presents the flow diagram representing the articles selection process.
Of the 11 countries in Southeast Asia, records were found for 10 of them: Thailand, Cambodia, Lao PDR, Myanmar, Vietnam, Indonesia, Malaysia, The Philippines, Singapore, and Timor-Leste. No reports on Strongyloides prevalence were found from Brunei. Of the 224 publications, the top three were obtained from Thailand (49.5%), followed by the IWV group (10.4%) and Malaysia (9.6%). Specifically, for S. stercoralis, 187 publications were obtained, with most studies (52.4%) conducted in Thailand. Moreover, a significant proportion of studies (12.6%) were observed for the IWV group.
Strongyloides stercoralis prevalence in Southeast Asia
Among the 163 studies (excluding the IWV group) originating from the 10 countries in Southeast Asia, the overall pooled prevalence of S. stercoralis based on the random effects model was 12.7% (95% CI 10.70–14.80%). Based on the two time periods, the pooled prevalence of S. stercoralis before the millennium was 13.8% (95% CI 7.79–21.18%) and 13.1% (95% CI 11.08–15.30%) after the millennium. Subgroup analysis by country revealed that the prevalence rates ranged from 0.4 to 24.9%. Cambodia had the highest pooled prevalence (24.9%), followed by Lao PDR (16.5%). Timor-Leste, The Philippines, and Singapore each had only one study, so their prevalence data were not pooled and instead based on the single study obtained for each. Figure 2 presents the estimated prevalence of S. stercoralis in Southeast Asia, along with the proportion of diagnostic test types used per country. Parasitological tests were the preferred diagnostic method in 58% of studies.
Moreover, parasitological techniques were used as the primary diagnostic test in seven (Myanmar, Lao PDR, Thailand, Cambodia, Indonesia, Timor-Leste, and Singapore) of the 10 countries. Aside from humans, S. stercoralis was reported in other host types, including dogs, cats, rodents, and transport carriers such as cockroaches and vegetables from markets. The four countries that had studies from other hosts aside from humans are Thailand, Cambodia, Vietnam, and Indonesia.
The pooled prevalence of S. stercoralis in Southeast Asia. The estimated pooled prevalence obtained for the 11 countries is indicated in the graduated shading (0 to 100%). The stacked bar chart indicates the proportion of diagnostic techniques (Blue: parasitological, red: serological, yellow: molecular, green: a combination of any two of the above categories) used for each country. The star indicates studies reporting other host types of S. stercoralis besides humans
Thailand
A total of 98 publications were available from Thailand, with most studies conducted in the Northeast region. The Cochran Q test (P < 0.0001) and I2 index indicated a high level of heterogeneity (99.4%) among the studies. Publication bias was also detected using Egger’s test (t = 4.118, P = 0.015) and Begg’s test (P = 0.008). Based on the random effects model, the estimated country-wide pooled prevalence of S. stercoralis was 11.3% (95% CI 8.98–13.85%), slightly lower than the overall pooled prevalence of Southeast Asia (as shown in Figs. 3 and 4). Further analysis based on the four regions (Central, North, Northeast, and South) revealed that the highest prevalence of S. stercoralis was observed in the Northeast at 22.5%, followed by the North (15%), South (8.3%), and Central (6.6%) regions. Additionally, the results based on the two time periods showed a decreasing trend of S. stercoralis prevalence in the last two decades. The pooled prevalence obtained were was 17.7% (95% CI 9.19–28.13%) before the millennium and 11.3% (95% CI 8.83–13.93%) after the millennium.
Not only were human hosts affected, but domestic dogs, cats, and various transport carriers such as cockroaches and vegetables from markets were also found to habor S. stercoralis. Of the three studies focusing on domestic animals, S. stercoralis were also present in dogs and cats along with their owners who tested positive [19,20,21]. Regarding diagnostic tests, parasitological methods were the most commonly used (88.4%), with S. stercoralis larvae primarily detected using the agar plate culture (APC) or the formalin-ether concentration technique (FECT). Serological methods were usually employed alongside parasitological methods, with higher prevalence obtained using serology as compared to parasitological methods [22]. Aside from serum-based ELISA assays, an urine-based ELISA IgG was also employed [22, 23]. However, the limitations of serology was observed in immunocompromised patients, where Luvira et al. 2016 reported a prevalence of 5.4% in immunocompromised patients, with 43% sensitivity and 96% specificity [24].
Cambodia
A total of 18 records from Cambodia were available, with most studies conducted in Preah Vihear Province in Northwestern Cambodia. The Cochran Q test (P < 0.0001) and I2 index revealed a high level of heterogeneity (99.8%) among the studies. No publication bias was observed using Egger’s test (t = 11.760, P = 0.096) and Begg’s test (P = 0.263). Among the 10 Southeast Asia countries compared in this study, Cambodia had the highest pooled prevalence at 24.9% (95% CI 15.65–35.38%) using the random effects model (Fig. 5). Subgroup analysis showed that the highest prevalence of S. stercoralis was in the Elephant Mountains and Cardamon region (33.7%), followed by the Northwestern (31.4%), Eastern (27.75%), and Mekong lowland (23.09%) regions.
A country-wide study across all 25 provinces using urine-based ELISA IgG serology found a prevalence of 30.7% [25]. Strongyloides stercoralis was ubiquitous and distributed throughout the country, with the highest prevalence in Koh Kong Province, followed by Kampong Speu Province (both provinces are in the Elephant Mountains and Cardamon region). The study also indicated that the risk of S. stercoralis infection increases with age. Also, using multiplex bead antibody serological assays, Priest et al. 2016 surveyed women of child-bearing age across 21 provinces in Cambodia and reported a prevalence of 45.9% [26]. Apart from humans, dogs were also found to be positive for S. stercoralis infection. Jelata et al. 2017 tested both humans and dogs from the same household and discovered a high S. stercoralis prevalence of 85% in dogs using parasitological techniques such as Baermann, APC, and Kato-Katz. Meanwhile, the prevalence in humans was 27% [27].
Parasitological methods were the most common means of detecting S. stercoralis (83.9%), with studies typically employing a combination of Kato-Katz, Baermann, and APC. Two studies used serology to survey on a national scale survey [25, 26].
Lao People’s Democratic Republic
Seventeen records on the prevalence of S. stercoralis were available for Lao PDR, with studies conducted in 11 out of the 17 provinces. The Cochran Q test (P < 0.0001) and I2 index indicated a high level of heterogeneity (99.0%) among the studies. No publication bias was observed using Egger’s test (t = 9.095, P = 0.096) and Begg’s test (P = 0.916). The pooled prevalence of S. stercoralis using the random effects model was 16.5% (95% CI 9.50–24.95%), making Lao PDR the country with the second-highest pooled prevalence of S. stercoralis after Cambodia (Fig. 6). The South region had the highest prevalence at 28%, followed by the North and Central regions at 17.9% and 14.8%, respectively.
Of the 17 studies, the highest prevalence (45%) of S. stercoralis was detected in residents in Champasak Province, where the Baermann method was used to identify S. stercoralis larvae in fecal samples [28]. Co-infections of S. stercoralis with other soil-transmitted helminths such as hookworm and Trichuris trichiura were also present in the studies from Lao PDR [29,30,31]. Two studies focused on HIV-infected patients, where the prevalence of S. stercoralis was 8.5% using direct microscopy and 20.4% using a combination of direct, Kato-Katz, and concentration techniques [29, 32]. Similar to findings in Thailand and Cambodia, S. stercoralis was also detected in domestic dogs or cats, with Niamnuy et al. 2016 reporting a prevalence of 1.7% in domestic animals and 15.7% in their owners [21].
Parasitological techniques were the diagnostic method of choice in 15 studies, with none using serological methods. A comparison of parasitological and molecular techniques for S. stercoralis detection showed that combining both techniques yielded the best results. By employing a combination of Baermann, APC, and PCR, 33.7% tested positive for S. stercoralis, while using only Baermann and APC or PCR detected 20.2% and 25%, respectively [33].
Malaysia
A total of 12 articles were available for Malaysia, covering both Peninsular and East Malaysia. Nine articles were from Peninsular Malaysia, while three were from East Malaysia. The Cochran Q test (P < 0.0001) and I2 index indicated a high level of heterogeneity (99.7%) among the studies. No publication bias was observed using Egger’s test (t = 8.623, P = 0.186) and Begg’s test (P = 0.956). The estimated country-wide pooled prevalence for S. stercoralis was 11.7% (95% CI 3.99–22.58%), slightly lower than that of Southeast Asia collectively (Fig. 7). Comparing the prevalence between the two regions, East Malaysia (20.7%) had a higher prevalence than Peninsular Malaysia (13.2%).
All 12 studies focused on human hosts, with the highest prevalence of S. stercoralis detected among the Orang Asli communities in the State of Selangor at 31.5%, using ELISA IgG serology [34]. Moreover, 76.5% of the S. stercoralis seropositive participants were positive for other helminths detected through microscopy. Surveying Orang Asli communities positive for tuberculosis across six states in Malaysia, Wong et al. 2019 revealed a seroprevalence of S. stercoralis was 10.9% [35]. Two more studies on Orang Asli communities reported prevalences of 22.1% and 15.8% [36, 37]. Comparing the prevalence of S. stercoralis among HIV-negative and HIV-positive prison inmates, Angal et al. 2015 found a higher prevalence among HIV-negative inmates, contrasting with other studies [38].
The studies from Malaysia employed the most diverse range of diagnostic methods, with 50% using serology and the remaining 50% using parasitological, molecular, or a combination of any two methods. Additionally, higher sensitivity of molecular methods compared to parasitological methods (without larva culture) was demonstrated, with molecular methods detecting 19.4% while Kato-Katz, FECT, and direct smear detecting only 2.5% [39]. Similarly, Othman et al. 2020 showed that S. stercoralis was not detected using FECT and direct smear, while PCR results were able to detect 22.1% [37].
Indonesia
A total of nine articles were obtained from Indonesia, with studies conducted in only six out of the 38 provinces. The Cochran Q test (P < 0.0001) and I2 index revealed a high level of heterogeneity (98.5%) among the studies. No publication bias was observed using Egger’s test (t = 9.038, P = 0.060) and Begg’s test (P = 0.089). The estimated pooled prevalence of S. stercoralis in Indonesia was 10.7% (95% CI 4.00–19.97%) using the random effects model (Fig. 8).
The highest prevalence of S. stercoralis was obtained from rodents in Surabaya Province, with a prevalence of 53%, while the highest prevalence in humans was in Papua Province [40, 41]. Moreover, 73.6% of S. stercoralis-infected participants were co-infected with other soil-transmitted helminths.
Of the nine articles, six (77.8%) used parasitological methods, usually utilizing a combination of Kato-Katz with APC or Harada-Mori culture, for diagnosis. The low sensitivity of Kato-Katz was demonstrated again, as S. stercoralis was not detected using Kato-Katz, while using APC yielded a prevalence of 16.4% [42].
Vietnam, Myanmar, Philippines, Timor-Leste, Singapore
Eleven articles were obtained from these five countries, with one study each from Timor-Leste, The Philippines, and Singapore (Additional file 2). Based on one study each, the prevalence of S. stercoralis in Timor-Leste, The Philippines, and Singapore were 0.4%, 0.8%, and 3.7%, respectively. However, as the study from Singapore was conducted in the 1970s, the actual prevalence might not reflect the current situation.
For Myanmar, two studies were available, with a pooled prevalence of 3.9% (95% CI 0.77–9.06%). They were conducted in Yangon, Ayeyarwady, and Bago-East divisions, and both studies used parasitological methods (APC and Kato-katz) for detection. For Vietnam, six studies were available, with a pooled prevalence of 13% (95% CI 5.23–23.56%). The highest prevalence of strongyloidiasis was reported in Hanoi Medical Center in Hanoi city in 2018, with a prevalence of 46.3% among visitors from 27 northern provinces using ELISA IgG [43]. Another study, conducted between 2016 and 2017 and surveying patients from the same 27 provinces, found a seroprevalence of 20%, indicating an increase in the prevalence of the disease [44].
Immigrants, workers, veterans
Among the studies analyzed, a notable proportion (12.6%) focused on individuals belonging to the following categories: immigrants, workers, and veterans. These groups encompass: (1) immigrants from Southeast Asia who have resettled in other countries, (2) migrant workers from Southeast Asia who are currently employed in other countries, and (3) veteran soldiers who previously served in the war in Southeast Asia. The Cochran Q test (P < 0.0001) and I2 index revealed a high level of heterogeneity (97.9%) among the studies. No publication bias was observed using Egger’s test (t = 6.818, P = 0.0003) and Begg’s test (P = 0.02). A pooled prevalence of 10% (95% CI 7.06–13.52%) was obtained, with people originating from six countries in Southeast Asia (Cambodia, Lao PDR, Vietnam, Myanmar, Thailand, and Indonesia) (Fig. 9). The countries where they reside or work include Australia, The United States of America (USA), Canada, Thailand, and Malaysia.
The highest S. stercoralis prevalence was from a study by Nutman et al. 1987, with a seroprevalence of 85% among new immigrants to USA from the Indochina region (Cambodia, Lao PDR and Vietnam) were reported in 1987 [45]. In 2006, a seroprevalence of 40% was detected in Cambodian communities that resettled in Australia [46]. Among the studies conducted on veteran soldiers, a seroprevalence of 11.6% was detected among Australians involved in war operations in Vietnam [47]. For migrant workers, a prevalence of 19.3% was obtained using FECT and Harada-mori culture from Laotian workers working in Thailand (Ubon Ratchathani Province) [21]. A similar high prevalence of 13.9% also resulted from using FECT from Thai workers working in Taiwan, China [48].
Strongyloides species in Southeast Asia
Aside from S. stercoralis, four other species of Strongyloides were present in Southeast Asia. These four species are S. fuelleborni, S. ratti, S. papillosus, and S. ransomi. Additional file 3 presents the other Strongyloides species found in Southeast Asia, with their respective host, country, and prevalence.
Seven studies reported S. fuelleborni originating from Lao PDR, Thailand, and Malaysia. Their hosts include humans and nonhuman primates such as long-tailed macaques, pig-tailed macaques, white-handed gibbons, and Bornean orangutans. Janwan et al. 2020 found S. fuelleborni in pig-tailed macaques and their owners [49]. The prevalence of S. fuelleborni in pig-tailed macaques was 51.1%, while S. fuelleborni was detected in one owner. Moreover, Thanchomnang et al. 2017 detected both S. stercoralis and S. fuelleborni in humans living near Kumphawapi Monkey Park in Udon Thani Province [8]. A high prevalence of S. fuelleborni found in nonhuman primates (ranging from 31.1 to 62.1%), along with the evidence of infection in human hosts, demonstrates the potential of the zoonotic transmissibility of S. fuelleborni to humans.
Discussion
This systematic review and meta-analysis provided a comprehensive overview of the prevalence of Strongyloides stercoralis and other Strongyloides species in Southeast Asia. Our findings demonstrate considerable heterogeneity in prevalence rates among countries in the region, with variations in diagnostic methods used across studies. These results underscore the importance of continued research and surveillance efforts to understand better and control Strongyloides infections in Southeast Asia.
The overall pooled prevalence of S. stercoralis from this systematic review and meta-analysis was estimated at 12.7% in Southeast Asia, based on the data obtained from 187 publications across 10 countries. Additionally, the results based on the division into two time periods (before and after the millennium) showed that the prevalence of S. stercoralis did not change significantly in the region over time. We also revealed that S. stercoralis in Southeast Asia ranged from 0.4 to 24.9%, with the highest prevalence in Cambodia. A recent estimate conducted by Buonfrate et al. 2020 on the global prevalence of S. stercoralis through modeling was 8.1%, with a 10–15% prevalence estimated for Southeast Asia [1]. However, the prevalence of S. stercoralis could still be underestimated. One possible reason is that most studies used parasitological methods, such as direct microscopy, Kato-Katz, and FECT, instead of parasitological methods involving larva culture as they are more convenient and less time-consuming [50, 51]. Another reason for the underestimation is the skewed proportion of studies originating from some countries in Southeast Asia. Our results demonstrated that more than 50% of the studies were from Thailand, while the other 50% was shared between the nine other countries in Southeast Asia. For example, many regions were not covered in Indonesia, where studies were conducted in six out of 38 provinces, and in The Philippines, where only one was conducted in Laguna Province.
Of the countries in Southeast Asia, the two countries with the highest pooled prevalence of S. stercoralis was Cambodia (24.9%) and Lao PDR (16.5%). Also, most of the studies in these two countries were conducted after the millennium. This could be a reason why the overall prevalence of S. stercoralis in Southeast Asia did not decrease in spite of the general improvements in santitiaton and hygiene in other countries regionally, whereas a decrease in S. stercoralis prevalence was observed in Thailand [52]. The high prevalence of S. stercoralis in Cambodia can be attributed to various factors such as climate, sanitation and hygiene, socio-economic conditions, occupation demographics, and the presence of domestic and free-roaming dogs [53]. Eslahi et al. 2022 conducted a meta-analysis of the global prevalence of S. stercoralis in dogs and found that the highest prevalence was in Cambodia, with more than 8% pooled prevalence [54]. Moreover, the genetic analysis also revealed a close relationship between type A (including humans, dogs, and other hosts) and type B (dog only) S. stercoralis genotypes [20, 55]. These two genotypes have been documented in Cambodia and Myanmar [27, 56]. Although the transmissibility and infectivity of the Type B S. stercoralis genotype to humans from dogs are currently not known, the risk of transmission is still plausible and a multi-faceted one-health approach for S. stercoralis control may be useful.
In Thailand, although the overall prevalence was 11.3%, there was a decrease in the prevalence of S. stercoralis before and after the millennium. Since the 2000s, emphasis was placed by the government to reduce helminthic infections in the country. The Thai Department of Disease Control has adopted an active control in high-risk areas (areas with parasite prevalence > 10%) through guidelines for endemic areas [57, 58]. These guidelines include screening, identification, treatment, and follow-up protocols. Additionally, through a Health Education and Preventive Equipment Package (HEPEP), health education, mass screening, and treatment for strongyloidiasis was successfully implemented in the Northeast region [59]. The decrease in strongyloidiasis after the millennium was also evidenced in a retrospective study performed using patient data from 2004 to 2014 [60]. The number of strongyloidiasis patients decreased from 22.4% to 2004 to 12.9% in 2014. The improvements in sanitation and hygiene, along with successful government policies and interventions could have led to the overall decrease in S. stercoralis prevalence in Thailand.
Although improvements have been made in Southeast Asia, S. stercoralis presence in the environment is still prevalent. Aside from being present in the soil and fecal material, S. stercoralis transmission can be facilitated by transport carriers such as cockroaches and vegetables [61,62,63]. Larvae were found in samples of various Periplanta species from three fresh markets in Chachoengsao Province and 18 open-air shopping markets in Samut Prakan Province, with 2.6% and 0.8% prevalence rates, respectively [61, 62]. Also, Punsawad et al. 2013 reported the presence of S. stercoralis larva on fresh vegetables obtained from open-air markets in Nakhon Si Thammarat Province [63]. Other possible factors driving the risk of transmission include global connectivity leading to increased people movement between and within countries and climate changes. First, the impact of global connectivity on the transmission risk of strongyloidiasis was observed with a significant proportion of studies in the IWV group having a pooled prevalence of 10%. With barriers reduced due to global connectivity and the ease and affordability of travel, strongyloidiasis is not only limited to tropical regions. Angheben et al. 2011 described two cases of strongyloidiasis in Italian tourists after traveling in Malaysia, Singapore, and Thailand [64]. A Swiss traveler also reported strongyloidiasis after visiting Vietnam and Cambodia [65]. With geographical barriers reduced, the classification of strongyloidiasis as a tropical and subtropical disease can be misleading. Second, although the favorable conditions for transmission of S. stercoralis are warm and moist soil, studies have demonstrated that the larva can survive lower temperatures [66]. The increasing global average temperatures may also increase the chances of the larva’s survival in temperate countries. Beknazarova et al. 2016 explored the global prevalence of strongyloidiasis and provided evidence to show that infection is primarily determined by the socio-economic status of communities rather than by geography or climate [67]. With an increase in global connectivity coupled with climate changes, the importance of screening programs and accurate diagnosis for strongyloidiasis is warranted.
Currently, there is no standard requirement for the diagnosis of strongyloidiasis. Although larva culture along with serology is recommended by the WHO, no stringent protocols are in place for screening [17]. Also, molecular tests should be clinically validated, especially in large-scale field-based settings [17]. Molecular identification of Strongyloides is also essential to accurately assess the risk of transmission and their zoonotic potential since both animals and humans can be infected with the same Strongyloides species.
For sustainable control of Strongyloides infections, a multifaceted approach is necessary. Strategies include improving sanitation and hygiene practices and implementing health education programs to raise awareness about the risks and associated preventive measures. Integrating Strongyloides screening into routine health services for vulnerable populations should also be considered. In addition, strengthening surveillance systems and fostering regional collaborations can facilitate sharing of best practices and resources to address the challenges associated with Strongyloides control.
Conclusions
This systematic review and meta-analysis highlight the importance of the region’s ongoing research, surveillance, and control efforts. The use of inadequate diagnostic methods remains a significant challenge, resulting in low detection sensitivity and the underestimation of disease burden. Additionally, the impact of global connectivity and climate changes on transmission necessitates a multi-pronged approach for effective intervention and control. To address strongyloidiasis in Southeast Asia, we recommend (1) widespread adoption of appropriate diagnostic tests, (2) screening programs especially those requiring immunosuppressive drug treatments and travelers, (3) mass drug administration for humans [68], and (4) integration across various sectors for a One Health approach to strongyloidiasis control. Progress in these areas at the regional level could significantly contribute to sustainable control of strongyloidiasis worldwide.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its additional information files.
Abbreviations
- APC:
-
Agar plate culture
- ELISA:
-
Enzyme-linked-immunosorbant assay
- FECT:
-
Formalin-ether concentration technique
- HIV:
-
Human immunodeficiency virus
- Lao PDR:
-
Lao People’s Democratic Republic
- PCR:
-
Polymerase chain reaction
- STH:
-
Soil-transmitted helminth
- WHO:
-
World Health Organization
- USA:
-
The United States of America
References
Buonfrate D, Bisanzio D, Giorli G, Odermatt P, Fürst T, Greenaway C, et al. The global prevalence of Strongyloides stercoralis infection. Pathogens. 2020;9:6.
Krolewieck A, Nutman TB. Strongyloidiasis: a neglected tropical disease. Infect Dis Clin North Am. 2019;33:1.
Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis. 2001;33:7.
Nutman TB. Human infection with Strongyloides stercoralis and other related Strongyloides species. Parasitology. 2017;144:3.
Karanam LS, Basavraj GK, Papireddy CKR. Strongyloides stercoralis hyper infection syndrome. Indian J Surg. 2020;83(Suppl 3):582-586.
Lemos Cabral S, Deveza N, Baptista JP, Martins P. Disseminated Strongyloides stercoralis infection associated with endogenous hypercortisolism – a case report. Eur J Case Rep Intern Med. 2020;7:7.
Ashford RW, Barnish G, Viney ME. Strongyloides fuelleborni kellyi: infection and disease in Papua New Guinea. Parasitol Today. 1992;8:9.
Thanchomnang T, Intapan PM, Sanpool O, Rodpai R, Tourtip S, Yahom S, et al. First molecular identification and genetic diversity of Strongyloides stercoralis and Strongyloides fuelleborni in human communities having contact with long-tailed macaques in Thailand. Parasitol Res. 2017;116:7.
Potters I, Micalessi I, van Esbroeck M, Gils S, Theunissen C. A rare case of imported Strongyloides fuelleborni infection in a Belgian student. Clin Infect Pract. 2020;7:100031.
Olsen A, van Lieshout L, Marti H, Polderman T, Polman K, Steinmann P, et al. Strongyloidiasis – the most neglected of the neglected tropical diseases? Trans R Soc Trop Med Hyg. 2009;103:10.
Requena-Méndez A, Chiodini P, Bisoffi Z, Buonfrate D, Gotuzzo E, Muñoz J. The laboratory diagnosis and follow up of strongyloidiasis: a systematic review. PLoS Negl Trop Dis. 2013;7:1.
Buonfrate D, Formenti F, Perandin F, Bisoffi Z. Novel approaches to the diagnosis of Strongyloides stercoralis infection. Clin Microbiol Infect. 2015;21:6.
Coker RJ, Hunger BM, Rudge JW, Liverani M, Hanvoravongchai P. Emerging infectious diseases in Southeast Asia: regional challenges to control. Lancet. 2011;377:9765.
Degelsegger A, Remøe S. Spotlight on: stimulating innovation in Southeast Asia. 1st ed. Vienna: Centre for Social Innovation, Austria; 2014.
Stephensona M, Dobson G. Deciphering the development of smart and sustainable tourism cities in Southeast Asia: a call for research. Austrian J South-East Asian Stud. 2020;13:1.
Fong E, Shibuya K. Migration patterns in East and Southeast Asia: causes and consequences. Annu Rev Sociol. 2020;46:1.
World Health Organization. Diagnostic methods for the control of strongyloidiasis, virtual meeting, 29 September 2020. World Health Organization. 2021. https://www.who.int/publications/i/item/9789240016538. Accessed 01 Jan 2023.
Team R, RStudio. Integrated development for R. 2020. RStudio, PBC, Boston.
Sedionoto B, Wasessombat S, Punsawad C, Anamnar W. Diagnosis and prevalence of hookworm and Strongyloides stercoralis infections among schoolchildren in rural southern Thailand. Walailak Procedia. 2019;2019:1.
Sanpool O, Intapan PM, Rodpai R, Laoraksawong P, Sadaow L, Tourtip S, et al. Dogs are reservoir hosts for possible transmission of human strongyloidiasis in Thailand: molecular identification and genetic diversity of causative parasite species. J Helminthol. 2019;94:e110.
Niamnuy NK, Kaewthamasorn M, Congpuong K, Phatanavanh B, Lohsoonthorn V. Prevalence and associated risk factors of intestinal parasites in humans and domestic animals across borders of Thailand and Lao PDR: focus on hookworm and threadworm. Southeast Asian J Trop Med Public Health. 2016;47:5.
Ruantip S, Eamudomkarn C, Kopolrat KY, Sithithaworn J, Laha T, Sithithaworn P. Analysis of daily variation for 3 and 30 days of parasite-specific IgG in urine for diagnosis of strongyloidiasis by enzyme-linked immunosorbent assay. Acta Trop. 2021;100:1.
Ruantip S, Eamudomkarn C, Techasen A, Wangboon C, Sithithaworn J, Bethony JM, et al. Accuracy of urine and serum assays for the diagnosis of strongyloidiasis by three enzyme-linked immunosorbent assay protocols. Am J Trop Med Hyg. 2019;100:1.
Luvira V, Trakulhun K, Mungthin M, Naaglor T, Chantawat N, Pakdee W, et al. Comparative diagnosis of strongyloidiasis in immunocompromised patients. Am J Trop Med Hyg. 2016;95:2.
Forrer A, Khieu V, Vounatsou P, Sithithaworn P, Ruantip S, Huy R, et al. Strongyloides stercoralis: spatial distribution of a highly prevalent and ubiquitous soil-transmitted helminth in Cambodia. PLoS Negl Trop Dis. 2019;13:6.
Priest JW, Jenks MH, Moss DM, Mao B, Buth S, Wannemuehler K, et al. Integration of multiplex bead assays for parasitic diseases into a national, population-based serosurvey of women 15–39 years of age in Cambodia. PLoS Negl Trop Dis. 2016;10:5.
Jaleta TG, Zhou S, Bemm FM, Schär F, Khieu V, Muth S, et al. Different but overlapping populations of Strongyloides stercoralis in dogs and humans – dogs as a possible source for zoonotic strongyloidiasis. PLoS Negl Trop Dis. 2017;11:8.
Barda B, Sayasone S, Phongluxa K, Xayavong S, Keoduangsy K, Odermatt P, et al. Efficacy of moxidectin versus ivermectin against Strongyloides stercoralis infections: a randomized, controlled noninferiority trial. Clin Infect Dis. 2017;65:2.
Kaneshiro Y, Sourinphoumy K, Imaizumi N, Rasaphon M, Kuba-Miyara M, Sakihama S, et al. Intestinal helminth infections in HIV-infected patients in Savannakhet after establishment of an HIV registration network in Lao People’s Democratic Republic. Trop Med Health. 2019;47:14.
Senephansiri P, Laummaunwai P, Laymanivong S, Boonmar T. Status and risk factors of Strongyloides stercoralis infection in rural communities of Xayaburi Province, Lao PDR. J Parasitol. 2017;55:5. Korean.
Hürlimann E, Keller L, Patel C, Welsche S, Hattendorf J, Ali SM, et al. Efficacy and safety of co-administered ivermectin and albendazole in school-aged children and adults infected with Trichuris trichiura in Côte d’Ivoire, Laos, and Pemba Island, Tanzania: a double-blind, parallel-group, phase 3, randomized controlled trial. Lancet Infect Dis. 2022;22:1.
Paboriboune P, Phoumindr N, Borel E, Sourinphoumy K, Phaxayaseng S, Luangkhot E, et al. Intestinal parasitic infections in HIV-infected patients, Lao People’s Democratic Republic. PLoS ONE. 2014;9:3.
Chankongsin S, Wampfler R, Ruf MT, Odermatt P, Marti H, Nickel B, et al. Strongyloides stercoralis prevalence and diagnostics in Vientiane, Lao People’s Democratic. Repub Infect Dis Poverty. 2020;9:1.
Ahmad AF, Hadip F, Ngui R, Lim YA, Mahmud R. Serological and molecular detection of Strongyloides stercoralis infection among an Orang Asli community in Malaysia. Parasitol Res. 2013;112:8.
Wong WK, Mohd-Nor N, Noordin R, Foo PC, Mohamed Z, Haq JA, et al. Parasitic infections in malaysian aborigines with pulmonary tuberculosis: a comparative cross-sectional study. Parasitol Res. 2019;118:9.
Al-Mekhlafi HM, Nasr NA, Lim YAL, Elyana FN, Sady H, Atroosh WM, et al. Prevalence and risk factors of Strongyloides stercoralis infection among Orang Asli schoolchildren: new insights into the epidemiology, transmission and diagnosis of strongyloidiasis in Malaysia. Parasitology. 2019;146:12.
Othman N, Miswan N, Wong WK, Lim BH, Noordin R. Multiplex real-time PCR revealed very high prevalence of soil-transmitted helminth infections among aborigines in Peninsular Malaysia. Asian Pac J Trop Med. 2020;13:12.
Angal L, Mahmud R, Samin S, Yap NJ, Ngui R, Amir A, et al. Determining intestinal parasitic infections (IPIs) in inmates from Kajang Prison, Selangor, Malaysia for improved prison management. BMC Infect Dis. 2015;15:467.
Basuni M, Mhui J, Othman N, Verweij JJ, Ahmad M, Miswan N, et al. A pentaplex real-time polymerase chain reaction assay for detection of four species of soil-transmitted helminths. Am J Trop Med Hyg. 2011;84:2.
Prasetyo RH. Survey of house rat intestinal parasites from Surabaya district, East Java, Indonesia that can cause opportunistic infections in humans. Southeast Asian J Trop Med Public Health. 2016;47:2.
Kridaningsih TN, Sukmana DJ, Mufidah H, Diptyanusa A, Kusumasari RA, Burdam FH, et al. Epidemiology and risk factors of Strongyloides stercoralis infection in Papua, Indonesia: a molecular diagnostic study. Acta Trop. 2020;209:105575.
Sedionoto BW, Wasessombat S, Punsawad C, Anamnart W. Environmental factors and prevalence of hookworm infection and strongyloidiasis in rural East Kalimantan, Indonesia. E3S Web of Conferences. 2019;125;04001.
De NV, Minh PN, Bich NN, Chai JY. Seroprevalence of tissue and luminal helminths among patients in Hanoi Medical University Hospital, Vietnam, 2018. Korean J Parasitol. 2020;58:4.
De VN, Minh PH, Duyet VL, Mas-Coma S. Strongyloidiasis in northern Vietnam: epidemiology, clinical characteristics and molecular diagnosis of the causal agent. Parasit Vectors. 2019;12:1.
Nutman TB, Ottesen EA, Leng S, Samuels J, Kimball E, Lutkoski M, et al. Eosinophilia in Southeast Asian refugees: evaluation at a referral center. J Infect Dis. 1987;155:2.
Caruana SR, Kelly HA, Ngeow JY, Ryan NJ, Bennett CM, Chea L, et al. Undiagnosed and potentially lethal parasite infections among immigrants and refugees in Australia. J Travel Med. 2006;13:4.
Rahmanian H, MacFarlane AC, Rowland KE, Einsiedel LI, Neuhaus SJ. Seroprevalence of Strongyloides stercoralis in a south australian Vietnam veteran cohort. Aust N Z J Public Health. 2015;39:4.
Cheng HS, Shieh YH. Investigation on subclinical aspects related to intestinal parasitic infections among thai laborers in Taipei. J Travel Med. 2000;7:6.
Janwan P, Rodpai R, Intapan PM, Sanpool O, Tourtip S, Maleewong W, et al. Possible transmission of Strongyloides fuelleborni between working Southern pig-tailed macaques (Macaca nemestrina) and their owners in Southern Thailand: molecular identification and diversity. Infect Genet Evol. 2020;85:104516.
Hailu T, Nibret E, Amor A, Munshea A, Anegagrie M. Agar plate culture: an alternative sensitive routine laboratory detection method for Strongyloides stercoralis and hookworm parasites. Iran J Parasitol. 2021;16:1.
Hailu T, Amor A, Nibret E, Munshea A, Anegagrie M, Flores-Chavez MD, et al. Evaluation of five diagnostic methods for Strongyloides stercoralis infection in Amhara National Regional State, northwest Ethiopia. BMC Infect Dis. 2022;22:1.
Chakravarty I, Bhattacharya A, Das SK. Water, sanitation and hygiene: the unfinished agenda in the World Health Organization South-East Asia region. WHO South East Asia J Public Health. 2017;6:22.
Schär F, Giardina F, Khieu V, Muth S, Vounatsou P, Marti H, et al. Occurrence of and risk factors for Strongyloides stercoralis infection in South-East Asia. Acta Trop. 2016;159:227.
Eslahi AV, Hashemipour S, Ofatifar M, Houshmand E, Haajialilo E, Mahmoudi R, et al. Global prevalence and epidemiology of Strongyloides stercoralis in dogs: a systematic review and meta-analysis. Parasites & Vectors. 2022;15:1.
Bradbury RS, Pafčo B, Nosková E, Hasegawa H. Strongyloides genotyping: a review of methods and application in public health and population genetics. Int J Parasitol. 2021;51:13.
Nagayasu E, Aung MP, Hortiwakul T, Hino A, Tanaka T, Higashiarakawa M, et al. A possible origin population of pathogenic intestinal nematodes, Strongyloides stercoralis unveiled by molecular phylogeny. Sci Rep. 2017;7:1.
Bureau of General Communicable Disease, Ministry of Public Health. Guidelines for Helminthiasis Control under the Royal Initiative Projects of Her Royal Highness Princess Maha Chakri Sirindhorn: For Public Health Personnel. 2018. http://dn.core-website.com/public/dispatch_upload/backend/core_dispatch_190145_3.pdf. Accessed 20 Jun 2023.
Boonjaraspinyo S, Boonmars T, Ekobol N, Artchayasawat A, Sriraj P, Aukkanimart R, et al. Prevalence and associated risk factors of intestinal parasitic infections: a population-based study in Phra Lap Sub-District, Mueang Khon Kaen District, Khon Kaen Province, northeastern Thailand. Trop Med Infect Dis. 2022;8:22.
Laoraksawong P, Sanpool O, Rodpai R, Thanchomnang T, Kanarkard W, Maleewong W, et al. Impact of the health education and preventive equipment package (HEPEP) on prevention of Strongyloides stercoralis infection among rural communities in Northeast Thailand: a cluster randomized controlled trial. BMC Public Health. 2018;18:1.
Prasongdee TK, Laoraksawong P, Kanarkard W, Kraiklang R, Sathapornworachai K, Naonongwai S, et al. An eleven-year retrospective hospital-based study of epidemiological data regarding human strongyloidiasis in northeast Thailand. BMC Infect Dis. 2017;17:627.
Dokmaikaw A, Suntaravitun P. Prevalence of parasitic contamination of cockroaches collected from fresh markets in Chachoengsao Province, Thailand. Kobe J Med Sci. 2020;65:4.
Chamavit P, Sahaisook P, Niamnuy N. The majority of cockroaches from the Samut Prakarn Province of Thailand are carriers of parasitic organisms. Excli J. 2011;10:218.
Punsawad C, Phasuk N, Thongtup K, Nagavirochana S, Viriyavejakul P. Prevalence of parasitic contamination of raw vegetables in Nakhon Si Thammarat province, southern Thailand. BMC Public Health. 2019;19:1.
Angheben A, Mistretta M, Gobbo M, Bonafini S, Iacovazzi T, Sepe A, et al. Acute strongyloidiasis in italian tourists returning from Southeast Asia. J Travel Med. 2011;18:2.
Kling K, Kuenzli E, Blum J, Neumayr A. Acute strongyloidiasis in a traveler returning from South East Asia. Travel Med Infect Dis. 2016;14:5.
Grove DI. Human strongyloidiasis. Adv Parasitol. 1996;38:259.
Beknazarova M, Whiley H, Ross K, Strongyloidiasis: a disease of socioeconomic disadvantage. Int J Environ Res Public Health. 2016;13:5.
Amato VS, Tuon FF. Mass drug administration for the control of Strongyloides stercoralis infection: progress and challenges. Clin Infect Dis. 2020;71:12.
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
UT and AHEC conceived and designed the study. UT, AHEC and WP performed data extraction, synthesis and analysis. UT, AHEC, WP, TK, DW and PND interpretated the results. All authors reviewed and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Supplementary Information
Additional file 1.
PRISMA checklist.
Additional file 2.
Strongyloides stercoralis data extracted from articles.
Additional file 3.
Other Strongyloides species data extracted from articles.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chan, A.H.E., Kusolsuk, T., Watthanakulpanich, D. et al. Prevalence of Strongyloides in Southeast Asia: a systematic review and meta-analysis with implications for public health and sustainable control strategies. Infect Dis Poverty 12, 83 (2023). https://doi.org/10.1186/s40249-023-01138-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40249-023-01138-4