Abstract
School-aged children (SAC) have a considerable burden of intestinal schistosomiasis in Madagascar yet its burden in pre-school aged children (PSAC) is currently overlooked. To assess the at-risk status of PSAC, we undertook a pilot epidemiological survey in June 2019 examining children (n = 89), aged 2–4-years of balanced gender, in six remote villages in Marolambo District, Madagascar. Diagnosis included use of urine-circulating cathodic antigen (CCA) dipsticks and coproscopy of stool with duplicate Kato-Katz (K-K) thick smears. Prevalence of intestinal schistosomiasis by urine-CCA was 67.4% (95% confidence interval [CI]: 56.5–77.2%) and 35.0% (95% CI: 24.7–46.5%) by K-K. The relationship between faecal eggs per gram (epg) and urine-CCA G-scores (G1 to G10) was assessed by linear regression modelling, finding for every increment in G-score, epg increased by 20.4 (6.50–34.4, P = 0.006). Observed proportions of faecal epg intensities were light (78.6%), moderate (17.9%) and heavy (3.6%). Soil-transmitted helminthiasis was noted, prevalence of ascariasis was 18.8% and trichuriasis was 33.8% (hookworm was not reported). Co-infection of intestinal schistosomiasis and soil-transmitted helminthiasis occurred in 36.3% of PSAC. These results provide solid evidence highlighting the overlooked burden of intestinal schistosomiasis in PSAC, and they also offer technical guidance for better surveillance data for the Madagascan national control programme.

Similar content being viewed by others
Background
Despite substantial control efforts, schistosomiasis remains to be a significant global public health problem, particularly in sub-Saharan Africa and Madagascar [1]. In Madagascar, 107/114 districts are endemic for schistosomiasis, with the national control programme offering praziquantel to school-aged children (SAC), aged 5-15, only through school-based mass drug administration (MDA) [2]. Given the remoteness of some disease endemic villages, many logistical challenges exist and perpetuate high rates of (re)infection. Recent epidemiological studies of SAC have documented very high burdens of infection and disease [3]. Both Schistosoma haematobium and Schistosoma mansoni exist on Madagascar; S. haematobium causes urogenital schistosomiasis and is present in northern and western districts; S. mansoni, which causes intestinal schistosomiasis, is present in eastern and southern districts; there are areas of co-endemicity in four regions in north-central and south-western parts of the country [2]. Schistosoma mansoni infection typically presents with bloody diarrhoea, abdominal pain and bloating [4]. With application of portable ultrasonography, Malagasy children as young as six have detectable periportal liver fibrosis, inferring that even younger children have developed chronic intestinal schistosomiasis [5].
Across mainland Africa, an estimated 50 million African pre-school aged children (PSAC) live with schistosomiasis [6], but PSAC are not yet formally included in national control strategies [7,8,9]. Feasible treatment options for PSAC include praziquantel crushed tablets administered on a test-and-treat basis or can be delivered in the WHO preventive strategy of ‘Integrated Management of Childhood Illnesses’ (IMCI) clinics [10, 11]. In contrast to Africa, there are no published reports of schistosomiasis among PSAC on Madagascar meaning their plight is currently overlooked. To address this and better inform the national intervention strategy, our pilot study builds on prior findings from SAC by MADEX research expeditions [3], to assess the current prevalence and infection intensity of intestinal schistosomiasis and soil-transmitted helminthiasis (STH) among PSAC in Marolambo District, Madagascar.
Methods
Study site and population
A cross-sectional survey was conducted between June-July 2019 in the hard-to-reach Marolambo District, eastern Madagascar. The population is estimated to be 250 000 people, of whom 95% are farmers. The primary source of water is the Nosivolo River (Fig. 1). We recruited 89 children aged 2–4-years from six villages: Marolambo, Ampasimbola, Ambohitelo, Marofatsy, Vohidamba, Betampona; the same sites of previous MADEX studies on S. mansoni associated morbidity [3, 5]. In each village 14–15 PSAC, aged 2–4-years old, were recruited through convenience sampling. Following community engagement announcing research intentions, parents with PSAC were asked if they would be willing to volunteer their children for the investigation. Owing to logistical constraints of available diagnostic urine-CCA dipsticks, only the first 15 children in each village were examined.
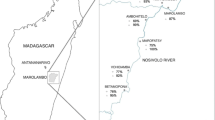
Map of Marolambo District, Madagascar showing the prevalence of Schistosoma mansoni among 2–4 year-old pre-school children in each village. Prevalence by both CCA and Kato-Katz are shown in addition to the arithmetic mean epg of positive PSAC in each village. CCA Circulating cathodic antigen, PSAC Pre-school-aged children
Parasitology
Children were provided with individual sterile sample pots labelled with their unique identifiable number (IN) which they returned the following day containing their mid-stream urine samples and stool samples. Urine was not assessed for Schistosoma haematobium as this species is not endemic in Marolambo District [3]. Urine samples were tested for circulating cathodic antigen (CCA; Rapid Medical Diagnostics Tests, Pretoria, South Africa) and recorded by the G-scores (G1 to G10) system (Fig. 3); a G-score of 1 was considered negative, all other scores were considered positive and ranked in ascending order with increasing visual intensity of the test band [12]. Urine CCA is a rapid, point-of-care test for S. mansoni, with a sensitivity of 83% and a specificity of between 81 and 90%, and when used in conjunction with coproscopy gains in specificity [13, 14]. Faecal coproscopy was undertaken by inspection of duplicate thick Kato-Katz (K-K) smears (Vestergaard-Frandsen, Lausanne, Switzerland) for S. mansoni and for soil-transmitted helminthiasis. According to World Health Organization (WHO) guidelines, S. mansoni infection was classified by intensity as light (1–99 eggs per gram; epg), moderate (100–399 epg), or heavy (≥ 400 epg); Ascaris lumbricoides infection intensity was classified as light (1–4999 epg), moderate (5000–49 999 epg) and heavy (≥ 50 000 epg); and Trichuris trichiura infection intensity was classified as light (1–999 epg), moderate (1000–9999 epg) and heavy (≥ 10 000 epg) [15]. The presence of hookworms was not recorded for it was not possible to read all K-K slides within the required one-hour window from slide preparation. As a diagnostic quality control, 10% of K-K slides were cross-checked by The Helminthiases Unit of the Institut Pasteur de Madagascar (IPM).
All S. mansoni infected children were treated with crushed praziquantel at 40 mg/kg under direct supervision. STH infected children were treated with a single dose of albendazole. Parents were requested to report back to the survey team should the child experience any immediate discomfort.
Statistical analysis
All results were recorded on electronic tablets in the field. Statistical analyses were performed on Stata 2017 (Stata Statistical Software 15. College Station, TX: StataCorp LLC). Chi-squared and Fisher’s exact tests were used to assess for association between prevalence by both urine-CCA and K-K, with age, gender and village.
Results
S. mansoni prevalence and infection intensity
Our PSAC cohort consisted of balanced gender (45M:44F). The prevalence of S. mansoni infection was 58/86 (67.4%; 95% CI: 56.5–77.2%) by CCA and 28/80 (35.0%; 95% CI: 24.7–46.5%) by Kato-Katz (Table 1). There were no statistically significant differences in prevalence by age, gender or location, from either urine-CCA or by K-K (Table 1). The arithmetic mean infection intensity of positive individuals was 74.6 epg on K-K (95% CI: 35.7–113.4). Of the 28 K-K positive children, light infections were observed in 22/28 (78.6%), moderate intensity infections in 5/28 (17.9%) and heavy intensity infections in 1/28 (3.6%). The youngest age of infection for any parasite was 2-years, noting 2-year old children could have moderate intensity infections across all sampled villages (Fig. 2). CCA G-score results were skewed towards lower scores, where 17.4% had a G-score of G2 and 1.2% had a G-score of G10 (Fig. 3).
Scatter plot of epg data points of infected PSAC by age, for each village. Note the number of dots does not reflect the total number of individuals infected in each village (as detailed in Table 1) as some children had the same epg counts (with overlapping dots). This explains why there are more dots for four-year-olds than two- or three-year-olds. PSAC Pre-school-aged children, EPG Eggs per gram
CCA G-score results: a number of PSAC with each G-score and b correlation between G-score and epg. A Frequency histogram of CCA G-scores shows the number of PSAC with each G-score. A child with a score of G1 was considered not-infected, and scores G2-10 were considered to be infected with Schistosoma mansoni. B Scatter plot of epg values against CCA G-score values, with linear regression line shown. The linear regression model estimated, that in this cohort, for every increase in G-score by a value of one, the epg increased by 20.4 (6.50–34.4, P = 0.006). CCA Circulating cathodic antigen, PSAC Pre-school-aged children
The linear regression model estimated that in our PSAC cohort, for every increase in G-score by a value of one, the epg increased by 20.4 (6.50–34.4, P = 0.006; Fig. 3). Of note, there were 4/27 (14.8%) and 1/27 (3.7%) of children with a G-score of G1 (as judged not infected) yet found to have light and moderate intensity infections by Kato-Katz, respectively (Fig. 3). Similarly, there were 30/52 (57.7%) children negative by Kato-Katz, who were found to have G-scores > 1 by CCA.
By combining the treatment naïve SAC epg results from 2015, with our treatment naïve PSAC epg results, an updated simple linear regression estimation predicts that the first age of infection is likely to be between 4–12 months (see Additional file 1: Figure S1). Although the estimates from this model assume that the epg of 2015 PSAC is the same as 2019 PSAC, for the purpose of estimating the first age of infection this is likely to be more accurate than incorporating epg data from SAC who have received PZQ due to the loss of the age-prevalence correlation [16].
Soil-transmitted helminthiasis prevalence and infection intensity
The prevalence of STH infection was 34/80 (42.5%); ascariasis was identified in 15/50 (18.8%), trichuriasis among 27/80 (33.8%), and there was 8/80 (10%) with both infections. All ascariasis was of light infection intensity, while 11/15 (73.3%) individuals with trichuriasis had light intensity infections with the remainder 4/15 (26.7%) of moderate intensity. No heavy intensity infections of STHs were identified. Co-infection with S. mansoni and STHs existed in 29/80 (36.3%) of participants (by CCA for S. mansoni and Kato-Katz for STH) and was 12/80 (15.0%, by Kato-Katz for both S. mansoni and STH).
Discussion
This pilot study is the first step in drawing attention to the burden of schistosomiasis amongst pre-school-aged (PSAC) in Madagascar. Despite a limited sample size of 15 children per village, we report on alarming levels of intestinal schistosomiasis in young children currently ignored. Upon consideration of egg-patent infections, 35.0% (95% CI: 24.7–46.5%) of 2–4-year-olds were found to have intestinal schistosomiasis. This is unsurprising given that previous studies have demonstrated the Marolambo District to be hyperendemic for intestinal schistosomiasis, with school-aged children (aged 5–14) having prevalence of 94% by CCA and 74% by Kato-Katz [3].
Given the insensitivity of Kato-Katz methodology in the detection of light or early acquired infections (where worm pairs might not have reached full fecundity), the higher recorded CCA prevalence of 67.4% (95% CI: 56.5–77.2%) is to be expected [17]. The majority of infected individuals had light egg-patent intensity infections (76.8%) and some with moderate (17.9%) and heavy intensity infections (3.6%). These prevalence and infection intensity results approximate to the statistical estimates extrapolated from treatment naïve SAC data from 2015 from the same six villages; a study found a positive correlation between age and both prevalence and infection intensity, predicting light to moderate infections in approximately 50% of 2–4-year-old pre-school children [3]. As heavy infections were found from 2-year-olds (in Ambohitelo village), this further points towards an earlier age of first exposure to schistosome cercariae, and is in keeping with observations from both Kenya and Côte d’iVoire where children as young as 5 and 6 months old (respectively) have been found to have S. mansoni infection [18, 19]. This is of importance when considering treatment age for future schistosomiasis control policies.
There are a number of limitations to consider when interpreting our findings. The convenience sampling may have resulted in selection bias. Specifically, those who volunteered may have been more engaged with schistosomiasis education and prevention activities and therefore skewed results towards lower disease burdens. Alternatively, parents with concerns about their child’s health may have been more willing to volunteer their children for participation in the study, skewing the results towards higher prevalence and infection intensity. This pilot study enrolled a small sample of 80 participants as it was the first step in assessing prevalence amongst PSAC. This small sample size is reflected by large confidence intervals around both prevalence estimates as well as for the association between G score and epg. A larger and more robust study design would likely yield more accurate and representative associations between epg and G scores in this population. Despite these limitations, this study provides important preliminary data about the prevalence of schistosomiasis in PSAC. There is a need for further studies into the optimal dosing, frequency and timing of paediatric praziquantel treatment as well as the need for efforts to develop other interventions that can bring broader benefit to these afflicted communities, not forgetting the fine-scale spatial heterogeneities of typical schistosomiasis here and elsewhere.
To our knowledge, this is the first study to present the measurement accuracy of CCA G-score on PSAC. A proportion of G1-scores (18.5%; as judged not infected) were reported in children with evidence of egg-patent infection. Studies among SAC have previously reported the CCA test specificity to be between 81 and 90%, where CCA tests have yielded negative results in egg-patent individuals with evidence of light infections, and less commonly with moderate or heavy infections [13, 14]. The reason for this remains unclear but may be due to physiological differences in urinary excretion of CCA between children.
Across sub-Saharan Africa there is increasing recognition of schistosome infection among PSAC [20, 21]. Untreated, chronic infection leads to significant and detrimental effects on health, including stunting, wasting, anaemia, reduced exercise tolerance, impaired cognitive development or even death from the consequences of periportal liver fibrosis [4, 10]. Calprotectin and faecal occult blood tests have shown to be effective biomarkers for morbidity in S. mansoni in children as young as 3-years who have been found to have associated anaemia and should be attempted to be used in this setting here to better describe the burden of disease in PSAC [22]. Nonetheless, evident progression towards liver disease is clear since our 2016 study identified 11% of SAC had sonographic evidence of liver fibrosis, from 6-years of age [5]. Similarly, research in Côte d’Ivoire has demonstrated sonographic evidence of bladder lesions in PSAC infected with S. haematobium that intensifies without treatment [23]. As PSAC in Madagascar are not yet included in the national control programme [24], many already suffer chronic disease which will only progress further if left untreated before primary school age. To prevent disease progression, access and delivery of praziquantel to PSAC is needed; waiting to provide treatment until children are over 5-years of age is not justifiable in the context of already evident chronic disease [11].
The anthelmintic praziquantel is well recognised to be both a safe and efficacious treatment among PSAC [6]. PSAC dosing is extrapolated from adult dosing regimens using 40 mg/kg praziquantel crushed in a syrup drink to reduce the bitter taste of praziquantel [25, 26], although pharmacokinetic and pharmacodynamic data suggests higher doses up to 60 mg/kg may be needed [27], and are currently being investigated [28]. Efforts towards developing a paediatric orally dispersible praziquantel tablet for children aged 3-months to 6-years are underway with ongoing Phase III clinical trials at the time of writing [29, 30]. All infected children in our age-group were treated after the study with appropriate dose of praziquantel and the recommended single dose of albendazole for STH treatment in line with current dosing recommendations and we observed that there were no reported adverse effects.
Although WHO guidance from 2010 recommended a case-by-case approach to treatment of PSAC with S. mansoni infection, realising the pervasive nature of infections, preventive chemotherapy approaches through IMCI strategy, or MDA with praziquantel to include PSAC are reasonable [10]. Inclusion of PSAC in the Madagascar national control programme, for example first with expanded surveillance then inclusion in treatment campaigns using crushed praziquantel, whilst waiting for deployment of the paediatric praziquantel formula; this is justified as a much-needed step towards reducing its public health burden in both SAC and PSAC by 2025 [31]. There were 36.3% of children co-infected with schistosomiasis and STH in our study, which suggests that mass treatment with both albendazole and PZQ may be more practical and beneficial than test-and-treat.
Alternative interventions are also desired such as improving access to safe water, sanitation and hygiene (WASH) facilities, health education for specific behavioural change as well as focal use of molluscicides [15]. In Marolambo, communities rely on the Nosivolo river as their main water source, for drinking, washing, cooking and transport; safer, alternative water sources are limited [3]. PSAC are at increased risk of infection and re-infection through passive water contact if caregivers regularly come into contact with infested water sources [21]. Strategies to combat this will have important impacts on transmission in children, particularly those less than 3-years of age [32]. In this setting, consideration of preventive chemotherapy for ascariasis and trichuriasis is also needed which could synergise future drug delivery campaigns to improve health of PSAC more broadly. In short, hyper-endemic regions such as this should be amongst the first to benefit from deployment of the forthcoming paediatric praziquantel formulation.
Conclusions
This pilot study assesses the prevalence and infection intensity of S. mansoni and soil transmitted helminths amongst PSAC in a hard-to-reach region of Madagascar. It provides an important starting point to develop better evidence-based policies for control of schistosomiasis in Malagasy PSAC. We describe a significant burden of intestinal schistosomiasis which further informs policymakers of the pressing need to expand future access to praziquantel alongside developing strategies that synergise with other child health programmes.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- epg:
-
Eggs per gram
- CCA:
-
Circulating cathodic antigen
- CI :
-
Confidence interval
- IN:
-
Identifiable number
- IMCI:
-
Integrated Management of Childhood Illness
- K-K:
-
Kato-Katz
- MADEX:
-
Madagascar medical expeditions
- MDA:
-
Mass drug administration
- PC:
-
Preventive chemotherapy
- PSAC:
-
Pre-school-aged children
- SAC:
-
School-aged children
- STH:
-
Soil-transmitted helminthiasis
- WASH:
-
Water, sanitation and hygiene
- WHO:
-
World Health Organization
References
Adenowo AF, Oyinloye BE, Ogunyinka BI, Kappo AP. Impact of human schistosomiasis in sub-Saharan Africa. Braz J Infect Dis. 2015;19(2):196–205.
Ministère de la Santé Publique de Madagascar. Cartographie des Maladies Tropicales negligees a Chimiotherapie preventive Schistosomiasis-geo Helminthiases-Filariose Lymphatique. Antananarivo: Ministère de la Santé Publique de Madagascar; 2016.
Spencer SA, Penney J, Russell HJ, Howe AP, Linder C, Rakotomampianina ALD, et al. High burden of Schistosoma mansoni infection in school-aged children in Marolambo District, Madagascar. Parasit Vectors. 2017;10(1):307.
Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet. 2014;383(9936):2253–64.
Russell HJ, Penney JMS, Linder C, Joekes EC, Bustinduy AL, Stothard JR, et al. A cross-sectional study of periportal fibrosis and Schistosoma mansoni infection among school-aged children in a hard-to-reach area of Madagascar. Trans R Soc Trop Med Hyg. 2020;114(4):315–22.
Stothard JR, Sousa-Figueiredo JC, Betson M, Bustinduy A, Reinhard-Rupp J. Schistosomiasis in African infants and preschool children: let them now be treated! Trends Parasitol. 2013;29(4):197–205.
Reinhard-Rupp J, Klohe K. Developing a comprehensive response for treatment of children under 6 years of age with schistosomiasis: research and development of a pediatric formulation of praziquantel. Infect Dis Poverty. 2017;6(1):122.
Ekpo UF, Oluwole AS, Abe EM, Etta HE, Olamiju F, Mafiana CF. Schistosomiasis in infants and pre-school-aged children in sub-Saharan Africa: implication for control. Parasitology. 2012;139(7):835–41.
Mduluza T, Mutapi F. Putting the treatment of paediatric schistosomiasis into context. Infect Dis Poverty. 2017;6(1):85.
Bustinduy AL, Friedman JF, Kjetland EF, Ezeamama AE, Kabatereine NB, Stothard JR, et al. Expanding Praziquantel (PZQ) Access beyond Mass Drug Administration Programs: Paving a Way Forward for a Pediatric PZQ Formulation for Schistosomiasis. PLoS Negl Trop Dis. 2016;10(9):e0004946.
Bustinduy AL, Stothard JR, Friedman JF. Paediatric and maternal schistosomiasis: shifting the paradigms. Br Med Bull. 2017;123(1):115–25.
Casacuberta-Partal M, Hoekstra PT, Kornelis D, van Lieshout L, van Dam GJ. An innovative and user-friendly scoring system for standardised quantitative interpretation of the urine-based point-of-care strip test (POC-CCA) for the diagnosis of intestinal schistosomiasis: a proof-of-concept study. Acta Trop. 2019;199:105150.
Stothard JR, Kabatereine NB, Tukahebwa EM, Kazibwe F, Rollinson D, Mathieson W, et al. Use of circulating cathodic antigen (CCA) dipsticks for detection of intestinal and urinary schistosomiasis. Acta Trop. 2006;97(2):219–28.
van Dam GJ, Wichers JH, Ferreira TM, Ghati D, van Amerongen A, Deelder AM. Diagnosis of schistosomiasis by reagent strip test for detection of circulating cathodic antigen. J Clin Microbiol. 2004;42(12):5458–61.
World Health Organization. Preventive chemotherapy in human helminthiasis: coordinated use of anthelminthic drugs in control interventions: a manual for health professionals and programme managers. Geneva: World Health Organization; 2006.
Spencer SA, Linder C, Penney J, et al. Five-year follow-up on the prevalence and intensity of infections of Schistosoma mansoni in a hard-to-reach District of Madagascar. Am J Trop Med Hyg. 2021. https://doi.org/10.4269/ajtmh.20-1433.
Stothard JR, Sousa-Figueiredo JC, Betson M, Adriko M, Arinaitwe M, Rowell C, et al. Schistosoma mansoni Infections in young children: when are schistosome antigens in urine, eggs in stool and antibodies to eggs first detectable? PLoS Negl Trop Dis. 2011;5(1):e938.
Coulibaly JT, N’Gbesso YK, Knopp S, Keiser J, N’Goran EK, Utzinger J. Efficacy and safety of praziquantel in preschool-aged children in an area co-endemic for Schistosoma mansoni and S. haematobium. PLoS Negl Trop Dis. 2012;6(12):e1917.
Sassa M, Chadeka EA, Cheruiyot NB, Tanaka M, Moriyasu T, Kaneko S, et al. Prevalence and risk factors of Schistosoma mansoni infection among children under two years of age in Mbita, Western Kenya. PLoS Negl Trop Dis. 2020;14(8):e0008473.
Osakunor DNM, Woolhouse MEJ, Mutapi F. Paediatric schistosomiasis: What we know and what we need to know. PLoS Negl Trop Dis. 2018;12(2):e0006144.
Kibira SPS, Ssempebwa JC, Ssenyonga R, Radloff S, Makumbi FE. Schistosomiasis infection in pre-school aged children in Uganda: a qualitative descriptive study to identify routes of exposure. BMC Infect Dis. 2019;19(1):165.
Bustinduy AL, Sousa-Figueiredo JC, Adriko M, Betson M, Fenwick A, Kabatereine N, et al. Fecal occult blood and fecal calprotectin as point-of-care markers of intestinal morbidity in Ugandan children with Schistosoma mansoni infection. PLoS Negl Trop Dis. 2013;7(11):e2542.
Barda B, Coulibaly JT, Hatz C, Keiser J. Ultrasonographic evaluation of urinary tract morbidity in school-aged and preschool-aged children infected with Schistosoma haematobium and its evolution after praziquantel treatment: a randomized controlled trial. PLoS Negl Trop Dis. 2017;11(2):e0005400.
Ministère de la Santé Publique de Madagascar. Plan directeur de lutte contre les maladies tropicales negligées — (MTN) 2016–2020. Antananarivo: Ministère de la Santé Publique de Madagascar; 2016.
World Health Organization. Report of a meeting to review the results of studies on the treatment of schistosomiasis in preschool-age children. Geneva: World Health Organization; 2010.
Coulibaly JT, Panic G, Silue KD, Kovac J, Hattendorf J, Keiser J. Efficacy and safety of praziquantel in preschool-aged and school-aged children infected with Schistosoma mansoni: a randomised controlled, parallel-group, dose-ranging, phase 2 trial. Lancet Glob Health. 2017;5(7):e688–98.
Bustinduy AL, Waterhouse D, de Sousa-Figueiredo JC, Roberts SA, Atuhaire A, Van Dam GJ, et al. Population Pharmacokinetics and pharmacodynamics of Praziquantel in Ugandan children with intestinal schistosomiasis: higher dosages are required for maximal efficacy. MBio. 2016. https://doi.org/10.1128/mBio.00227-16.
ClinicalTrials.gov. U.S. National Library of Medicine. 2021. https://clinicaltrials.gov/ct2/show/study/NCT03640377. Accessed 4th Jan 2021.
Lim RM, Woolhouse MEJ, Mduluza T, Chase-Topping M, Osakunor DNM, Chitsulo L, et al. Investigating a strategy for quantifying schistosome infection levels in preschool-aged children using prevalence data from school-aged children. PLoS Negl Trop Dis. 2020;14(10):e0008650.
Bergquist R, Zhou XN, Rollinson D, Reinhard-Rupp J, Klohe K. Elimination of schistosomiasis: the tools required. Infect Dis Poverty. 2017;6(1):158.
World Health Organization. Ending the neglect to attain the sustainable development goals: a road map for neglected tropical diseases 2021–2030. Geneva: World Health Organization; 2020.
Stothard JR, Gabrielli AF. Schistosomiasis in African infants and preschool children: to treat or not to treat? Trends Parasitol. 2007;23(3):83–6.
Acknowledgements
With thanks to the teachers, children and families who participated in this study and to the following people and organisations: Ministry of Health, Madagascar; University of Antananarivo Faculty of Medicine, Prof Danielle Vololontiana; National Institute of Public Health (INSPC), Madagascar; Institut Pasteur, Dr Patricia Rakotomanga and Dr Armand Rafalimanantsoa, Antananarivo; Lohorano, Marolambo, Bellarmin Ramahefasoa, Celestin; Royal Geographical Society, London; University of Manchester Medical School, Prof Anthony Freemont, Prof Sheena Cruickshank, Prof Andrew Macdonald; Dr Ed Wilkins and Prof Bertie Squire.
Funding
Funding for the expedition was generously provided by The Rivers Foundation; The Scientific Exploration Society; The University of Manchester: Global Impact Award, the Division of Development and Alumni Relations (and individuals who donated to our Hubbub Crowdfunder); The Royal College of Pathology; The Royal Society of Medicine; The Lord Mayor Grant; the Medical-Chirurgical Bursary; International Child Health Group and Altrincham Rotary Club.
Author information
Authors and Affiliations
Contributions
All authors contributed to development of the research protocol, planning and carrying out the research on the expedition or writing and editing of this paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval for the study was granted by the University of Manchester (Ref 2019-6410-10877) and the research committee for the National Institute for Public Health Madagascar. Consent to participate was gained from the parents of all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Supplementary Information
Additional file 1: Figure S1.
Scatter plot and linear regression line (with 95% CI represented by the shaded area) of faecal eggs per gram (epg) by age. The data points included in this figure are from coproscopy by Kato-Katz from stool among treatment naïve (pre-MDA) school aged children (SAC) from June 2015 (3), and treatment naïve PSAC in June 2019. The simple linear regression line (epg = -14.9 + 48.1*Age), estimates that for each increase in age by one year, the epg increases by 48.05 (95% CI: 23.0–73.1).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sheehy, C., Lawson, H., Andriamasy, E.H. et al. Prevalence of intestinal schistosomiasis in pre-school aged children: a pilot survey in Marolambo District, Madagascar. Infect Dis Poverty 10, 87 (2021). https://doi.org/10.1186/s40249-021-00871-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40249-021-00871-y