Abstract
Background
Drug resistance is one of the greatest challenges of malaria control programmes, with the monitoring of parasite resistance to artemisinins or to Artemisinin Combination Therapy (ACT) partner drugs critical to elimination efforts. Markers of resistance to a wide panel of antimalarials were assessed in natural parasite populations from southwestern Cameroon.
Methods
Individuals with asymptomatic parasitaemia or uncomplicated malaria were enrolled through cross-sectional surveys from May 2013 to March 2014 along the slope of mount Cameroon. Plasmodium falciparum malaria parasitaemic blood, screened by light microscopy, was depleted of leucocytes using CF11 cellulose columns and the parasite genotype ascertained by sequencing on the Illumina HiSeq platform.
Results
A total of 259 participants were enrolled in this study from three different altitudes. While some alleles associated with drug resistance in pfdhfr, pfmdr1 and pfcrt were highly prevalent, less than 3% of all samples carried mutations in the pfkelch13 gene, none of which were amongst those associated with slow artemisinin parasite clearance rates in Southeast Asia. The most prevalent haplotypes were triple mutants Pfdhfr I 51 R 59 N 108 I 164(99%), pfcrt- C72V73 I 74 E 75 T 76 (47.3%), and single mutants PfdhpsS436 G 437K540A581A613(69%) and Pfmdr1 N86 F 184D1246 (53.2%).
Conclusions
The predominance of the Pf pfcrt CVIET and Pf dhfr IRN triple mutant parasites and absence of pfkelch13 resistance alleles suggest that the amodiaquine and pyrimethamine components of AS-AQ and SP may no longer be effective in their role while chloroquine resistance still persists in southwestern Cameroon.
Similar content being viewed by others
Multilingual abstracts
Please see Additional file 1 for translations of the abstract into the five official working languages of the United Nations.
Background
Malaria is still a leading cause of illness and death especially in sub-Saharan African children under the age of five [1]. Case management currently relies largely on the use of a few effective antimalarials and is being compromised by the development and spread of resistance [2]. Parasite resistance to antimalarial drugs represents a major obstacle to malaria containment efforts [1, 3, 4]. Indeed the policy change to artemisinin-based combination therapies (ACT) for treatment of uncomplicated malaria [5], was due to the emergence and spread of resistance to chloroquine (CQ), sulphadoxine-pyrimethamine (SP) and other monotherapies [6, 7]. However, the emergence of artemisinin resistance in Western Cambodia of Southeast Asia (SEA) [1, 3] has prompted global concern given that CQ and SP resistance arose in the same region and then spread to Sub-Saharan Africa (SSA) [8]. Recent studies also suggest that resistant mutations may emerge independently in SEA and SSA [9,10,11,12,13] necessitating regional molecular monitoring of markers for the control and containment of resistant parasites. Information on parasite resistance to artemisinins, ACT partner drugs or to previously withdrawn antimalarials is vital for malaria control [14] and could justify the re-introduction of abandoned drugs [15] since drug-sensitive populations of Plasmodium falciparum resurge following long-term drug withdrawal.
Single nucleotide polymorphisms (SNPs) have been fundamental in monitoring existing or predicting emerging drug resistance patterns. Chloroquine resistance is linked to mutations in the P. falciparum chloroquine resistance transporter (Pfcrt) [16,17,18], and is associated with mutations in codons 72–76. The Pfcrt Lysine to Threonine substitution at position 76 (K76T) [16] is considered to be critical to CQ resistance as well as to the structurally and similarly acting drug, amodiaquine (AQ) [19]. SNPs in the P. falciparum multidrug resistance 1 (Pfmdr1) gene, notably the Pfmdr1 N86Y substitution [20], have been associated with resistance to CQ [21], mefloquine, halofantrine, and quinine [22]. Artemether lumefantrine (AL), the most commonly used ACT in SSA [1] seems to select pfcrt and pfmdr1 SNPs in parasite reinfections [23, 24], with a high proportion of pfmdr1 - N86 alleles recorded in AL treated patients with recurrent parasites [25]. SP resistance is due to point mutations in the parasite dihydrofolate reductase (dhfr) and dihydpteroate synthetase (dhps) genes that confer resistance to pyrimethamine and sulphadoxine respectively [26, 27].
Mutations in P. falciparum Kelch13 have been shown to underlie artemisinin resistance [13, 28], with nonsynonymous polymorphisms in the propeller domain validated as molecular markers for determining the emergence and spread of artemisinin-resistant P. falciparum [28, 29]. While the four core mutations have not been detected in Africa, several other non-synonymous K13 mutations have been identified and the effect of these and markers of previous antimalarial resistance remains largely unknown. The A481V and G533C substitutions, for instance, have been confirmed to be adjacent to these four major SNPs and may affect the tertiary structure and thus the function of the propeller [29, 30].
This ever evolving parasite population dynamics necessitates antimalarial resistance monitoring in distinct transmission contexts. Although drug pressure is the primary driver of anti-malarial drug resistance, alterations in malaria transmission has also been implicated [31]. In areas where drug policy has changed and the insecticide treated net coverage has been scaled up, molecular monitoring of current and previously used drugs could provide a better understanding of the impact of these factors on drug resistance alleles [6]. In Cameroon, CQ, AQ and SP were administered as monotherapies during 1999–2004, with CQ used as first line drug for treatment of malaria until 2002, when an interim policy was adopted involving the use of AQ as the alternative first line drug for uncomplicated malaria while SP was the second line drug [7]. Due to the declining efficacy of P. falciparum to AQ and SP, the Cameroon Ministry of Public Health revised its treatment policy in 2004 to artemisinin-based combination therapy (ACT) and adopted AS-AQ as first line drug for uncomplicated malaria while quinine (QN), injectable Arthemeter (or QN) and SP were recommended for P. falciparum treatment failure, severe malaria and intermittent preventive treatment of malaria in pregnancy (IPTp), respectively [1]. A number of other ACT options are available for treatment of mild malaria in Cameroon [32], with artemether-lumefantrine (AL) reportedly prescribed by up to 36.6% of health workers in a recent study [33].
The government of Cameroon embarked on a scaling-up of ITN coverage in 2011, in line with the Roll Back Malaria recommendation of universal coverage [34]. In the study area, where malaria parasitaemia is higher in the rainy seasons [35] and at lower altitude [36], significant increases in ITN ownership and usage have been reported [37]. This, together with infrastructural development in the area may have altered the structure of the vector population, transmission of infection, genetic diversity of circulating parasites and the efficacy of antimalarials. However, other factors such as host immunity may also be important determinants of treatment failure and the emergence and transmission potential of resistant parasites [38,39,40].
Reports on the monitoring of antimalarial resistance markers in Cameroon have been limited manly to the pfcrt: K76T [41,42,43,44] and pfmdr1: N86Y [42, 44]. The pfcrt: K76, for instance, has remained relatively fixed at 12% in 2000 [41] compared to 13% in 2012 [45]. The only such study in the mount Cameroon area [44] revealed that 87% and 76% of samples between 2004 and 2006 carried the pfcrt: K76T and pfmdr1: N86Y alleles, respectively. Furthermore, there have been no reports on the prevalence of molecular markers of artemisinin resistance in the area. In this study, the prevalence of mutations in genes associated with drug resistance were assessed in natural parasite populations across different altitudinal zones from southwestern Cameroon, enriching data on parasite antimalarial resistance, with implications for the control of the disease.
Methods
Study area
The study was conducted in localities on the eastern slope of Mt. Cameroon, with varying altitudes as described [37]. The area is categorized by an equatorial climate comprising two seasons: a short dry season (November–March) and a long rainy season (March–November) [35], intense and perennial Plasmodium spp. transmission and higher parasite prevalence in the rainy season and at lower altitude [36, 46]. P. falciparum is responsible for most of the malaria infections [1] while Anopheles gambiae (Anopheles coluzzii M form) is the main malaria vector species, with overall Entomological Inoculation Rates (EIR) as high as 287 infective bites/person/year [35]. There is a substantial level of human migration between localities, mainly for educational, recreational and commercial purposes.
Study design and selection of sampling sites and participants
This was a cross-sectional community - and hospital – based study conducted between May 2013 and March 2014. Individuals with asymptomatic parasitaemia (AP) were enrolled through surveys from selected rural and semi-urban communities at varying altitudes as described [37] based on previous reports of variation in malaria parasitaemia [38, 46]. Three communities, Mutengene, Ombe and Tiko below 200 m were considered to be at low altitude while Mile 14, 15, 16, Muea and Molyko located between 385 and 575 m were considered to be at intermediate altitude. Individuals residing from checkpoint to Buea Town and Tole above 636 m were considered to be at high altitude. Uncomplicated Malaria (UM) subjects were also registered from health facilities within these communities. All local residents, with a minimum of 1000 asexual parasites per microliter of peripheral blood, who had not travelled out of the target sites within the last 3 weeks were eligible for enrollment. A structured questionnaire was used to record demographic and clinical data such as age, area of residence and drug history of all participants. All patients were given oral antimalarial, based on their weight, by the attending clinician and according to national guidelines.
Sample collection and parasite detection
Prospective participants were prescreened by light microscopy using giemsa-stained thick and thin blood smears of the peripheral blood as described previously [37]. A smear was only considered negative if no malaria parasites were seen in 100 high power fields. The level of parasitaemia in positive smears was estimated by counting the parasites against a minimum of 200 white blood cells and assuming a leucocyte count of 8000 per microliter of blood [36, 47]. Quality control was ensured in accordance with the World Health Organisation’s protocol [47]. Venous blood (3–5 ml) was then collected from P. falciparum positive participants into EDTA tubes for molecular analysis.
DNA extraction
Leucocytes were depleted from whole blood using CF11 cellulose columns (4021–050) following a modified WorldWide Antimalarial Resistance Network (WWARN) MOL02 protocol (www.wwarn.org). Parasite genomic DNA was then extracted using a commercial kit (Qiagen, UK) according to manufacturer’s instructions, eluted with 100 μl TE (10 mM Tris–HCl; 0.5 mM EDTA; pH 9.0) elution buffer (Qiagen, UK) and kept at −34 °C until genotyping.
Genotyping of mutations in drug resistance genes
Samples with >50 ng DNA and <80% human DNA contamination (239/259, 92.3%) were sequenced on the Illumina HiSeq platform (Illumina, San Diego, USA), and subsequently genotyped using well established methods, as described previously [13, 48] without any modification. In brief, samples were genotyped at each SNP on the basis of sequencing read counts, with at least 5 reads required to emit a genotype and at least 2 reads to call an allele. Pfkelch13 alleles were determined by identifying any variation across the gene that would result in a non-synonymous change in the protein, as described [49].
Haplotypes were constructed independently for each locus. As it is impossible to ascertain if any two haplotypes are coming from the same genome for complexity of infection (COI) > 1, only the frequency of haplotypes without any heterozygous call were reported. The sample should therefore carry the same DR haplotype even if multiple genomes are present in the infections.
Complexity of infection
Complexity of Infection was determined using the program COIL [50]. From the MalariaGEN Plasmodium falciparum Community Project data resource (https://www.malariagen.net/projects/p-falciparum-community-project), 101 genomic SNPs of mid-high MAF with large between-population Fst were used as a “barcode” within COIL to estimate COI. COIL was used with default parameters and population allele frequency estimate were calculated from sample data, not pre-determined.
Statistical analyses
All data were entered into Excel and analyzed using SPSS Statistics 20 for windows (SPSS Inc., Chicago, USA). The significance of difference in prevalence were explored using the Pearson’s chi square test whereas the differences in group means were assessed using Student’s t – test or analyses of variance (ANOVA). A difference giving a P value ≤0.05 was considered statistically significant.
Results
Characteristics of smear-positive participants
A total of 259 participants were enrolled in this study from three different altitudes (Table 1), most of whom had uncomplicated malaria (74.8%, 190/254), reportedly had fever in the previous 48 h (67.5%), were anaemic (47.2%) and females (52.1%). The mean age (± SD), geometric mean parasite density and complexity of infection (± SD) were 13.9 ± 13.09 years, 15,715 parasites/μl blood and 1.81 ± 1.10 respectively. The proportion of individuals with asymptomatic parasitaemia from the community surveys at low, intermediate and high altitude was 6.0%, (11/184), 7% (34/487) and 14.7% (19/129) respectively.
Prevalence of drug resistance molecular markers
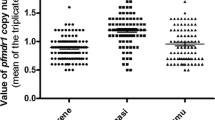
Some resistance mutations were highly prevalent; all 233 (100%) samples had pfdhfr: S108N and 232 (more than 99%) had pfdhfr: N51I and C59R mutant alleles (Table 2). One hundred and twenty one (72%) of samples harbored mutations at pfmdr1: Y184F while 120 (62.5%) mutations were detected in pfcrt: Q271K overall. CQ resistance alleles were also prevalent, with at least 115 (50%) parasites carrying mutations in pfcrt at codons 74, 75, and 76. However, 5 (less than 3% of all samples) had pfkelch13 (pk13) mutant alleles, except for pfk13: 189 T detected in 58 (36%) samples overall. Furthermore, none of the samples carried mutations in pfk13 at codons 112, 175, 217, 255, 258, 569, 573, 578 and 580, pfcrt at codon 72, pfdhfr at codons 59 and 164, pfdhps at codon 540 and pfmdr1 at codon 86 (Fig. 1).
The prevalence of pfdhps: K142N (P = 0.006) and pfmdr1: Y184F (P = 0.010) mutations, but not other markers, differed significantly among study sites, highest in the Mutengene – Tiko area (Fig. 1, Additional file 2: Table S1). However, the proportion of the alleles was similar between AP and UM individuals in all study sites, except for dhps: I431V that was higher (P = 0.039) in UM (22/86, 25.6%) compared to AP (1/22, 4.5%) at MM. Two hundred and thirty two (99.6%) samples harbored the dhfr N51I/C59R/S108 N (IRN) triple mutant, while none had the dhps A437G/K540E (GE) double mutant and therefore the IRN + GE quintuple mutant haplotype.
Pfk13 mutations
None of the candidate and validated non-synonymous K13 resistance mutations were detected in the 239 samples analyzed (Fig. 1). Furthermore, other less frequent variants that have been associated with in vivo or in vitro tests, or both were not seen in all samples analyzed.
Haplotypes of CQ and SP markers
CQ resistance haplotypes
The prevalence of pfcrt and pfmdr1 haplotypes in the study area are shown (Table 3). Two different pfcrt haplotypes were observed, with 60 (25.3%) of the samples bearing wild type alleles at all five codons, C72V73M74N75K76 (CVMNK) while the majority (112, 47.3%) carried triple mutations at codons 74, 75, and 76 (CVIET). Nevertheless, 65 (27.4%) had a mixture of the CVIET and CVMNK haplotypes. In all, 177 (74.7%) of the samples had the CVIET haplotype, which was more prevalent (P = 0.011) in semi-urban settings (50.2%) compared to rural settings (31.2%) (Table 3). The alternative South American-type mutant haplotype (SVMNT) was not detected.
A total of four pfmdr1 haplotypes were detected in the area, with 46 (33.1%) samples containing wild type alleles at codons 86, 184 and 1246, pfmdr1 N86 Y 184D1246 (NYD). Nevertheless, the predominant haplotype (74, 53.2%), contained a single mutation at codon 184 (YFD) whereas 18 (12.9%) and 1 (0.7%) of samples had haplotype variants with double (YFD) and single (YYD) mutations respectively. The proportion of the pfmdr1 haplotypes did not vary with locality (Table 3).
SP resistance haplotypes
A total of nine distinct haplotypes were detected in the study area, with the proportion of the different variants independent of locality of residence (Table 2). At least 232 (99%) and 67 (69%) of samples in all localities harbored the pfdhfr N51I/C59R/S108N/I164 (IRNI) and pfdhps S436/A437G/K540/A581/A613 (SGKAA) haplotypes respectively. Overall, 73.3% and 4.4% of isolates in the area harbored the IRNI + SGKAA and IRNI + AAKAA haplotypes carrying quadruple mutations at the key dhfr and dhps codons respectively. Furthermore, 11 and 3 samples had the sextuple mutant IRNI + AAKGS and IRNI + AGKAS respectively while 7.4% and 3.0% had the quintuple mutant IRNI + AGKAA and IRNI + SGKAA respectively. The remaining quintuple haplotypes containing the pfdhfr triplet mutant IRN and different SNP combinations at two pfdhps codons (G437 A581) were present in four samples. Only one sample had the dhfr triple mutant without any additional mutations.
Multilocus haplotypes
Multilocus haplotypes in CQ and SP markers were constructed based on pfcrt: K76T, pfmdr1: N86Y, pfdhfr N51I/C59R/S108N and dhps A437G/K540E to determine if CQ resistant parasites also tend to be SP resistant. In total, seven haplotypes were observed, with 13.7% and 59.8% of samples having variants with 6 (T + Y + IRN + GK) and 5 (T + N + IRN + GK) mutations respectively. Nevertheless, no sample had the sextuple mutant haplotype (T + Y + IRN + GE).
Discussion
Antimalarial drug resistance monitoring remains critical to malaria control and elimination, especially with the confirmation of artemisinin resistance in Cambodia [3, 28] and other foci in that region. ITNs can alter Plasmodium spp. transmission and thus indirectly influence the spread of drug resistance by changing the number of parasite clones per host and the level of community/population drug use [6]. The use of ACT is not only expected to improve the treatment efficacy, but also to delay the emergence of P. falciparum drug resistance [51]. Therefore, it is very important to monitor ACT partner drugs to ensure that national treatment policies remain effective [52]. In the mount Cameroon area, ITN ownership and usage has increased significantly following the nationwide free distribution campaign [37], possibly selecting for resistant parasites over time. The study assessed molecular markers to a wide panel of antimalarials in this area, across three transects at different altitude and varying malaria transmission intensity based on proxy measure of malaria parasitaemia.
Although artemisinin resistance has not been documented in Africa [11, 49], and pfkelch13 alleles are probably not under selection [49], monitoring is necessary, as the history of anti-malarial resistance suggests the possibility of it spreading to Africa despite global efforts in its containment. None of the nonsynonymous polymorphisms at N458Y, Y493H, R539T, I543T, R561H and C580Y in the kelch repeat region of K13 propeller domain validated as markers of artemisinin resistance [28, 29] as well as the adjacent A481V and G533C mutations thought to affect the three-dimensional structure of the K13-propeller [30] were observed in the surveyed parasite samples as reported previously [10, 53]. Furthermore, even pfk13: A578S, the most frequent allele observed in Africa [29, 53], which has recently been reported elsewhere in the country [54] was not detected, although it is not associated with clinical or in vitro resistance to artemisinin [29]. However, the K13: K189T mutation (36%, 58/161) was highly prevalent. Other k13 mutant alleles were at such low frequencies, suggesting that further measures are needed, including monitoring at 2–3 time points and investigating sweeps in flanking microsatellites around the DR markers to ascertain that ART resistant parasites are not under evolutionary selection in south western Cameroon.
The pfcrt: K76T [15] and Pfmdr1: N86Y mutations [20] are thought to be most decisive in CQ resistance, with the latter allele serving to augment CQ resistance in isolates with the former. As such, the pfcrt-K76 allele is expected to be selected after almost 15 years of change in national drug policy. The removal of chloroquine drug pressure resulted in the reemergence of chloroquine sensitive parasites in east Africa [55, 56] that reached 100% in Malawi [56] less than 10 years after chloroquine was replaced with SP and dramatically 2 years after introduction of AL [55]. This study reports a high frequency (55.2%) of pfcrt: K76T, but low proportion (13.0%) for pfmdr1: N86Y relative to previous studies prior to the large scale ITN distribution in Cameroon (pfcrt: 76 T: 71–87.1%) versus pfmdr1: N86Y (73.8–76%) [42, 44]. The slow decline in the pfcrt: K76T mutant since 2004–2009 is in line with previous reports [4] and can be explained by (i) the fixation of the allele in the parasite populations that need more time to recover CQ sensitivity in the absence of CQ pressure or (ii) the high use of amodiaquine (AQ) at the population level that selects for pfcrt: K76T alleles. As such, it is not yet possible to reintroduce CQ against P. falciparum in the study area. Nevertheless, the decreased prevalence of the pfmdr1: 86Y mutation reflects the complete withdrawal of CQ usage in the community [56] but may also be due to its selection by lumefantrine.
In line with previous reports in Cameroon [41, 42, 44], pfcrt polymorphisms scanning revealed that the mutant pfcrt CVIET (Southeast Asian CQ-resistant) haplotype was still the more predominant in the parasite population while the pfcrt SVMNT haplotype was not detected in any of the samples analyzed as reported elsewhere [43]. The remaining isolates had the wild type (CQ-sensitive) pfcrt CVMNK form, distributed in all three transects in variable frequencies (Table 3). In all, 25.3% (60/237) were of CVMNK type—suggesting that one quarter of P. falciparum isolates are still susceptible to chloroquine in the area, slightly higher than previous reports [57]. Such genetic reformation might have been propelled by the selection pressure exerted by the amodiaquine component of the AS-AQ artemisinin combined therapy recommended for the treatment of uncomplicated P. falciparum malaria in Cameroon [44]. This can be justified by the fact that AQ has a very similar genetic target (Pfcrt) to chloroquine [43]. With close to three quarters of the population carrying this CQR haplotype, however, CQ and AQ cannot be effective treatment options in the area. Taken together, these findings suggest that the intensification of control has not affected the diversity of the parasite population. Nevertheless, that only 47.3% of parasites were of the reversible CQ resistant haplotype (CVIET) phenotype suggests the possibility of CQ re-use over time.
Sulphadoxine-pyrimethamine remains the drug of choice by the World Health Organization for intermittent preventive treatment in pregnancy (IPTp) [58], although, resistance is reportedly increasing in stable transmission areas [59, 60]. The dhfr IRN triple mutant and dhps double GE mutant combination associated with in vivo SP treatment failure [61] was not recorded in any of the samples analyzed. However, up to 99.6% of samples harbored the dhfr triple mutant in this study (Table 3) while none of the isolates carried the dhps: K540E mutant. This suggests that resistance to pyrimethamine but not sulphadoxine is widespread in the study area, although it may also be due to trimethoprim and sulfamethoxaxole (Cotimoxazole), a commonly used antibiotic that is known to select for dhfr/dhps resistant alleles [62]. This suggests that IPTp with SP may no longer be effective in the area, although further measures are needed to confirm the local prevalence of dhfr/dhps genotypes/haplotypes. Additionally, the overall impact of these alleles on the IPTp-SP routine can only be ascertained through in vivo efficacy studies in pregnancy.
The pfdhps: 142N and pfmdr1: 184F mutations were highest at a low altitudes compared to medium and high altitudes. However, there were no significant differences in the prevalence of the critical pfcrt: 76T and pfmdr1: 86Y mutations as well as CQ pfcrt and SP pfdhfr/dhps haplotypes among the three transects (Table 3). Although variability in malaria parasitaemia with altitude has been reported [36, 46] in the region, the prevalence of the markers does not mirror this. The similarity in the prevalence of the markers among the three areas could be explained by the small relative differences in transmission intensity between areas as well as gene flow due to migration of human and vector populations [6].
This study had a few limitations. First the small number of samples analysed in this study might have also reduced the statistical power. Secondly, the geographic proximity of the three study areas and evaluation of the effect of transmission intensity on drug resistance at a single time point may have limited the ability to detect differences in the molecular profiles of drug resistance among the areas [6]. Thirdly, the fact that only individuals with asymptomatic parasitaemia or uncomplicated malaria were enrolled may have limited the diversity of the parasite population analysed.
Conclusions
None of the candidate and validated K13 resistance mutations were detected in southwestern Cameroon, although other non-synonymous mutations were observed. Parasites in the area, however, remain largely resistant to CQ, with only a slow decline in the pfcrt: K76T mutant since 2004–2009 suggesting the fixation of the allele in the populations that need more time to recover CQ sensitivity in the absence of CQ pressure. Resistance to pyrimethamine but not sulphadoxine is also widespread in the study area.
Abbreviations
- ACT:
-
Artemisinin-based combination therapies
- AL:
-
Artemether lumefantrine
- ANOVA:
-
Analyses of variance
- AP:
-
asymptomatic parasitaemia
- AQ:
-
Amodiaquine
- CQ:
-
Chloroquine
- dhfr:
-
Dihydrofolate reductase
- dhps:
-
Dihydpteroate synthetase
- EIR:
-
Entomological inoculation rates
- GMPD:
-
Geometric mean parasite density
- IPTp:
-
Intermittent preventive treatment of malaria in pregnancy
- ITNs:
-
Insecticide-treated nets
- K76 T:
-
Lys to Thr at position 76
- LM:
-
lumefantrine
- MQ:
-
mefloquine
- Pfcrt:
-
Plasmodium falciparum chloroquine resistance transporter
- Pfmdr1:
-
Plasmodium falciparum multidrug resistance 1
- QN:
-
Quinine
- SEA:
-
Southeast Asia
- SNPs:
-
Single nucleotide polymorphisms
- SP:
-
Sulphadoxine-pyrimethamine
- SSA:
-
Sub-Saharan Africa
- UM:
-
Uncomplicated Malaria
- WWARN:
-
WorldWide Antimalarial Resistance Network
References
WHO. World Malaria Report 2016. Available from: http://www.who.int/malaria/publications/world-malariareport-2016/report/en/.
White NJ. Antimalarial drug resistance. J Clin Invest. 2004;113:1084–92.
Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM, et al. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359:2619–20.
Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–67.
WHO. Guidelines for the treatment of malaria. Third Edition. World Health Organization, 2015. Available from: http://www.who.int/malaria/publications/atoz/9789241549127/en/.
Shah M, Omosun Y, Lal A, Odero C, Gatei W, Otieno K, et al. Assessment of molecular markers for anti-malarial drug resistance after the introduction and scale-up of malaria control interventions in western Kenya. Malar J. 2015;14:75.
Basco LK, Ngane VF, Ndounga M, et al. Molecular epidemiology of malaria in Cameroon. XXI. Baseline therapeutic efficacy of chloroquine, amodiaquine, and sulfadoxine pyrimethamine monotherapies in children before national drug policy change. Am J Trop Med Hyg. 2006;75(3):388–95.
Naidoo I, Roper C. Following the path of most resistance: dhps K540E dispersal in African Plasmodium falciparum. Trends Parasitol. 2010;26:447–56.
Talundzic E, Okoth SA, Congpuong K, Plucinski MM, Morton L, Goldman IF, et al. Selection and spread of artemisinin-resistant alleles in Thailand prior to the global artemisinin resistance containment campaign. PLoS Pathog. 2015;11:e1004789.
Takala-Harrison S, Jacob CG, Arze C, Cummings MP, Silva JC, Dondorp AM, et al. Independent emergence of artemisinin resistance mutations among Plasmodium falciparum in Southeast Asia. J Infect Dis. 2015;211:670–9.
Taylor SM, Parobek CM, DeConti DK, Kayentao K, Coulibaly SO, Greenwood BM, et al. Absence of putative artemisinin resistance mutations among plasmodium falciparum in sub-Saharan Africa: a molecular epidemiologic study. J Infect Dis. 2014;467:1–9.
Kamau E, Campino S, Amenga-Etego L, Drury E, Ishengoma D, Johnson K, et al. K13-propeller polymorphisms in Plasmodium falciparum parasites from sub-Saharan Africa. J Infect Dis. 2014;211:1352e1355.
Miotto O, Amato R, Ashley EA, MacInnis B, Almagro-Garcia J, Amaratunga C, et al. Genetic architecture of artemisinin-resistant Plasmodium falciparum. Nat Genet. 2015;47(3):226–34.
Plowe CV, Roper C, Barnwell JW, Happi CT, Joshi HH, Mbacham W, et al. World Antimalarial resistance network (WARN) III: molecular markers for drug resistant malaria. Malar J. 2007;6:121.
Payne D. Spread of chloroquine resistance in Plasmodium falciparum. Parasitol Today. 1987;3:241–6.
Fidock DA, Nomura T, Talley AK, Cooper RA, Dzekunov SM, Ferdig MT, et al. Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol Cell. 2000;6:861–71.
Djimde A, Doumbo OK, Cortese JF, Kayentao K, Doumbo S, Diourte Y, et al. A molecular marker for chloroquine-resistant falciparum malaria. N Engl J Med. 2001;344:257–63.
Ursing J, Kofoed PE, Rodrigues A, Rombo L, Gil JP. Plasmodium falciparum genotypes associated with chloroquine and amodiaquine resistance in Guinea-Bissau. Am J Trop Med Hyg. 2007;76:844–8.
Sa JM, Twu O, Hayton K, Reyes S, Fay MP, Ringwald P, et al. Geographic patterns of Plasmodium falciparum drug resistance distinguished by differential responses to amodiaquine and chloroquine. Proc Natl AcadSci U S A. 2009;106:18883–9.
Dorsey G, Kamya MR, Singh A, Rosenthal PJ. Polymorphisms in the Plasmodium falciparum pfcrt and pfmdr-1 genes and clinical response tochloroquine in Kampala. Uganda J Infect Dis. 2001;183:1417–20.
Sidhu AB, Verdier-Pinard D, Fidock DA. Chloroquine resistance in Plasmodium falciparum malaria parasites conferred by pfcrt mutations. Science. 2002;298:210–3.
Cheruiyot J, Ingasia LA, Omondi AA, Juma DW, Opot BH, Ndegwa JM, et al. Polymorphisms in Pfmdr1, Pfcrt, and Pfnhe1 genes are associated with reduced in vitro activities of quinine in Plasmodium falciparum isolates from western Kenya. Antimicrob Agents Chemother. 2014;58(Suppl 7):3737–43.
Sisowath C, Ferreira PE, Bustamante LY, Dahlstrom S, Mårtensson A, Björkman A, et al. The role of pfmdr1 in Plasmodium falciparum tolerance to artemether-lumefantrine in Africa. Tropical Med Int Health. 2007;12:736e742.
Sisowath C, Petersen I, Veiga MI, Mårtensson A, Premji Z, Björkman A, et al. In vivo selection of plasmodium falciparum parasites carrying the chloroquine-susceptible pfcrt K76 allele after treatment with artemether-lumefantrine in Africa. J Infect Dis. 2009;199:750e757.
Venkatesan M, Gadalla NB, Stepniewska K, Dahal P, Nsanzabana C, Moriera C, et al. Polymorphisms in Plasmodium falciparum chloroquine resistance transporter and multidrug resistance 1 genes: parasite risk factors that affect treatment outcomes for P. falciparum malaria after artemether-lumefantrine and artesunate-amodiaquine. Am J Trop Med Hyg. 2014;91:833e843.
Triglia T, Menting JG, Wilson C, Cowman F. Mutations in dihydropteroate synthase are responsible for sulfone and sulphanomide resistance in Plasmodium falciparum. Proc Natl Acad Sci U S A. 1997;94:13944–9.
Duraisingh MJ, Curtis J, Warhurst DC. Plasmodium falciparum detection of polymorphisms in the dihydrofolate reductase and dihydropteroatesynthetase genes by PCR and restriction digestion. Exp Parasitol. 1998;89:1–8.
Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014;505:50e55.
WHO. Artemisinin and artemisinin-based combination therapy resistance: Status Report. World Health Organisation. 2017. Available from: http://www.who.int/malaria/publications/atoz/artemisinin-resistanceapril2017/en/.
Li J, Chen J, Xie D, Eyi UM, Matesa RA, Obono MMO, et al. Limited artemisinin resistance-associated polymorphisms in plasmodium falciparum K13-propeller and PfATPase6 geneisolated from Bioko Island, Equatorial Guinea. Int J Parasitol Drugs Drug Resist. 2016;6:54e59.
Hastings IM, Watkins WM. Intensity of malaria transmission and the evolution of drug resistance. Acta Trop. 2005;94:218–29.
National Malaria Control Programme. Directorates for Management and Use of Artemisinine-Based Combination Therapies (ACT) in Cameroon. Yaounde: Ministry of Public Health Report; 2007. p. 22.
Kuete T, Mvoa EE, Yinyang J, Priso AE, Chamabe PCG, Mpondo EM, et al. Pattern of uncomplicated malaria treatment and antimalarial prescription practices among health workers in the littoral region of Cameroon: an assessment of ten years post-malaria treatment policy change. Pharmacol Pharm. 2016;7:217–25.
Kilian A, Boulay M, Koenker H, Lynch M. How many mosquito nets are needed to achieve universal coverage? Recommendations for the quantification and allocation of long-lasting insecticidal nets for mass campaigns. Malar J. 2010;9:330.
Wanji S, Tanke T, Atanga SN, Ajonina C, Nicholas T, Fontenille D. Anopheles species of the mount Cameroon region; biting habits, feeding behavior and entomological inoculation rates. Trop Med IntHlth. 2003;8:643–9.
Achidi EA, Apinjoh TO, Mbunwe E, Besingi R, Yafi CN, Awah WN, et al. Febrile status, malaria parasitaemia and gastrointestinal helminthiasis in school children resident at different altitudes. Ann Trop Med Parasitol. 2008;102:103–18.
Apinjoh TO, Anchang-Kimbi JK, Mugri RN, Nyingchu RV, Tangoh DA, Chi HF, et al. The effect of insecticide treated nets (ITNs) on Plasmodium falciparum infection in rural and semi-urban communities in the south westregion of Cameroon. PLoS One. 2015;10(2):e0116300.
Greenhouse B, Slater M, Njama-Meya D, Nzarubara B, Maiteki-Sebuguzi C, Clark TD, et al. Decreasing efficacy of antimalarial combination therapy in Uganda explained by decreasing host immunity rather than increasing drug resistance. Infect Dis. 2009;199(5):758–65.
Ataide R, Ashley EA, Powell R, Chan J, Malloy MJ, O’Flaherty K, et al. Host immunity to Plasmodium falciparum and the assessment of emerging artemisinin resistance in a multinational cohort. PNAS. 2017;114(13):3515–20.
O’Flaherty K, Maguire J, Simpson JA, Fowkes FJI. Immunity as a predictor of anti-malarial treatment failure: a systematic review. Malar J. 2017;16:158.
Basco LK. Molecular epidemiology of malaria in Cameroon. Xiii. Analysis of Pfcrt mutations and in vitro chloroquine resistance. Am J Trop Med Hyg. Hyg. 2002;67(4):388–91.
Menard S, Morlais I, Tahar R, et al. Molecular monitoring of plasmodium falciparum drug susceptibility at the time of the introduction of artemisinin based combination therapy in Yaounde´, Cameroon: implications for the future. Malar J. 2012;11:113.
Mbenda HGN, Das A. Occurrence of multiple chloroquine-resistant Pfcrt haplotypes and emergence of the S(agt)VMNT type in Cameroonian plasmodium falciparum. J Antimicrob Chemother. 2014;69:400–3.
Mbacham WF, Evehe MS, Netongo PM, et al. Efficacy of amodiaquine, sulphadoxine-pyrimethamine and their combination for the treatment of uncomplicated Plasmodium falciparum malaria in children in Cameroon at the time of policy change to artemisinin-based combination therapy. Malar J. 2010;9:34.
Zhou RM, Zhang HW, Yang CY, Liu Y, Zhao YL, Li SH, et al. Molecular mutation profile of pfcrt in Plasmodium falciparum isolates imported from Africa in Henan province. Malar J. 2016;15:265.
Kimbi HK, Sumbele IU, Nweboh M, Anchang-Kimbi JK, Lum E, Nana Y, et al. Malaria and haematologic parameters of pupils at different altitudes along the slope of Mount Cameroon: a cross-sectional study. Malar J. 2013;12:193.
WHO. Basic malaria microscopy. Secondth ed. Switzerland: World Health Organisation; 2010. p. 74–5.
Manske M, Miotto O, Campino S, Auburn S, Almagro-Garcia J, Maslen G, et al. Analysis of Plasmodium falciparum diversity in natural infections by deep sequencing. Nature. 2012;487(7407):375–9.
MalariaGEN Plasmodium falciparum Community Project. Genomic epidemiology of artemisinin resistant malaria. elife. 2016;5:e08714.
Galinsky K, Valim C, Salmier A, de Thoisy B, Musset L, Legrand E, et al. COIL: a methodology for evaluating malarial complexity of infection using likelihood from single nucleotide polymorphism data. Malar J. 2015;14:4.
World Health Organization (WHO). Guidelines for the treatment of malaria. 2015. Available from: http://www.who.int/malaria/publications/atoz/9789241549127/en/.
Afsharpad M, Zakeri S, Pirahmadi S, Djadid ND, et al. Molecular monitoring of Plasmodium falciparum resistance to antimalarial drugs after adoption of sulfadoxine–pyrimethamine plus artesunate as the first line treatment in Iran. ActaTrop. 2012;121:13–8.
Conrad MD, LeClair N, Arinaitwe E, Wanzira H, Kakuru A, Bigira V, et al. Comparative impacts over 5 years of artemisinin-based combination therapies on Plasmodium falciparum polymorphisms that modulate drug sensitivity in Ugandan children. J Infect Dis. 2014;210:344e353.
Ménard D, Khim N, Beghain J, Adegnika AA, Shafiul-Alam M, Amodu O, et al. A Worldwide map of plasmodium falciparum K13-propeller polymorphisms. N Engl J Med. 2016;374:25.
Achieng AO, Muiruri P, Ingasia LA, Opot BH, Juma DW, Yeda R, et al. Temporal trends in prevalence of Plasmodium falciparum molecular markers selected for by artemether lumefantrine treatment in pre-ACT and post-ACT parasites in western Kenya. Int J Parasitol Drugs Drug Resist. 2015;5:92e99.
Kublin JG, Cortese JF, Njunju EM, Mukadam RA, Wirima JJ, Kazembe PN, et al. Reemergence of chloroquine-sensitive Plasmodium falciparum malaria after cessation of chloroquine use in Malawi. J Infect Dis. 2003;187:1870–5.
Menegon M, Sannella AR, Severini C, Paglia MG, Matteelli A, Caramello P, et al. Molecular epidemiology of imported malaria in Italy: the use of genetic markers and in vitro sensitivity test in a study of chloroquine resistance in Plasmodium falciparum. Ann Ist Super Sanita. 2006;42(2):203–10.
WHO. A strategic framework for malaria prevention and control during pregnancy in the African region. World Health Organization, 2004. Available from: http://www.who.int/malaria/publications/atoz/afr_mal_04_01/en/.
Mockehaupt FP, Bedu-Addo G, Eggeltte TA, Hommerich L, Holmberg V, Von Oertzen C, et al. Rapid increase in the prevalence of sulphadoxine pyrimethamine resistance among Plasmodium falciparum isolated from pregnant women in Ghana. J Infect Dis. 2008;198:1545–9.
Nnaemeka C, Shah M, Gatei W, Van Eyk AM, Ayisi J, Kanuki S, et al. Temporal trends of sulphadoxine-pyrimethamine (SP) drug resistance molecular markers in Plasmodium falciparum parasites in western Kenya. Malar J. 2012;11:34.
Kublin JG, Dzinjalamala FK, Kamwendo DD, Malkin EM, Cortese JF, Martino LM, et al. Molecular markers for failure of sulfadoxine-pyrimethamine and chlorproguanil-dapsone treatment of Plasmodium falciparum malaria. J Infect Dis. 2002;185:380–8.
Jelinek T, Kilian AH, Curtis J, Duraisingh MT, Kabagambe G, von Sonnenburg F, Warhurst DC. Plasmodium falciparum: selection of serine 108 of dihydrofolate reductase during treatment of uncomplicated malaria with co-trimoxazole in Ugandan children. Am J Trop Med Hyg. 1999;61(1):125–30.
Chauvin P, Menard S, Iriart X, Nsango SE, Tchioffo MT, Abate L, et al. Prevalence of Plasmodium falciparum parasites resistant to sulfadoxine/pyrimethamine in pregnant women in Yaoundé, Cameroon: emergence of highly resistant pfdhfr/pfdhps alleles. J Antimicrob Chemother. 2015;70(9):2566–71.
Acknowledgements
We thank the participants from the communities who made this study possible; and the Chiefs, Quarter Heads and health personnel who assisted with this work.
Funding
TOA received funding from the UK Medical Research Council—Grant no. G0600718 through the Centre for Genomics and GlobalHealth (http://www.cggh.org) while sequencing was done at the Sanger Institue thanks to the Wellcome Trust Sanger Instutte grant n0. 098051 to DK.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Author information
Authors and Affiliations
Contributions
TOA conceived, designed and coordinated the study, performed the experiments, contributed reagents, performed the statistical analysis and drafted the manuscript. RNM designed and performed the experiments. OM designed and performed the experiments, HFC, RBT, JKA, EFM, DAT and RVN participated in the field surveys. CJ and RA participated in the molecular genetics studies. AD, DK, EAA and AA contributed reagents/materials and participated in the coordination. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Institutional Review Board of the Faculty of Health Sciences, University of Buea, Cameroon (No. 2013–03-0153) while administrative authorization was obtained from the South West Regional Delegation of Public Health. Written informed consent or assent was obtained from all participants or their parents/guardians for those below 21 years of age.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional files
Additional file 1:
Multilingual abstracts in the five official working languages of the United Nations. (PDF 805 kb)
Additional file 2:
Comparision of alleles across altitudinal zones along the slope of mount Cameroon. (DOCX 12 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Apinjoh, T.O., Mugri, R.N., Miotto, O. et al. Molecular markers for artemisinin and partner drug resistance in natural Plasmodium falciparum populations following increased insecticide treated net coverage along the slope of mount Cameroon: cross-sectional study. Infect Dis Poverty 6, 136 (2017). https://doi.org/10.1186/s40249-017-0350-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40249-017-0350-y