Abstract
Background
Biological agents have allowed remarkable improvement in controlling autoimmune arthropathies, although none of the numerous biologics readily available represent a universal treatment standard. Moreover, classical and genetic predictors are currently unsatisfactory to predict individual response to a biologic, and the best treatment selection is still based on a trial-and-error approach. Here, we report a clinical case demonstrating the usefulness of examining the leukocytes’ secretome of patients. We set up and standardized a protocol that examines a patient’s immune responses to establish the secretome of the blood mononuclear leukocytes and personalize the biotherapy.
Case presentation
A 24-year-old woman was diagnosed with active early rheumatoid arthritis. The initial treatment regimen (prednisone, methotrexate, hydroxychloroquine, naproxen) was inefficient, as well as the anti-TNF adalimumab. The diagnosis was revised as possible rheumatoid arthritis-like psoriatic arthritis and adalimumab was replaced by abatacept (IgG1 Fc-CTLA-4) to no avail. Five years later, abatacept was replaced by the anti-IL-12/IL-23 ustekinumab with no objective control over the symptoms. The patient was thus enrolled in a prospective study based on the quantification of cytokines secreted by peripheral blood leukocytes stimulated with well-known immune activators of pattern recognition receptors and cytokine signalling. The results of this study revealed that plasma concentrations of cytokines were similar between the patient and healthy donors. In comparison to leukocytes from healthy donors, the patient’s secretome showed a unique overproduction of IL-6. The anti-IL-6 receptor tocilizumab was, therefore, administered with a rapid improvement of her active psoriatic arthritis that remained dependent on low prednisone dosage. Clinical parameters progressively returned to normal levels and her quality of life was greatly improved, despite the major delay to begin the present personalized treatment.
Conclusions
An efficient way to effectively treat patients with complex autoimmune arthropathies, and avoid irreversible disability, is to know their leukocytes’ secretome to identify abnormally secreted cytokines and personalize their biotherapy, as exemplified by this case report.
Similar content being viewed by others
Background
Autoimmune arthropathies are treated with biologics that allowed remarkable advancement in the control of disease alteration [1]. However, some patients do not respond to the first biologic, or even to the others successively given with or without methotrexate and studies to identify predictors of response to biologic therapy are still limited [2]. The classical predictors such as the presence of auto-antibodies [the rheumatoid factor (RF) and the anti-cyclic citrullinated peptide (anti-CCP)], genotypes encoding the shared epitope HLA-DRB1 gene, smoking and/or periodontitis are largely insufficient to foresee the patient response to a biologic [3]. Genetic predictors represent an ongoing field of research and bear the potential to contribute to the development of a precision medicine approach in the management of autoimmune arthropathies [4, 5]. Nonetheless, the identification of genetic markers of disease outcome and response to treatment is still at its infancy and has been somewhat disappointing so far [6].
The most fruitful findings to treat autoimmune arthropathies remain the characterization of immune mediators involved in the disease. This is greatly supported by the numerous biologics readily available to treat most of the autoimmune diseases, including biotherapies targeting specific immune cells, such as the cytotoxic T-lymphocyte-associated protein-4 (CTLA-4, also known as CD152) or the B-lymphocyte antigen CD20, or secreted mediators like the pro-inflammatory cytokines tumor necrosis factor (TNF), interleukin (IL)-1, IL-6, IL-12/IL-23 and IL-17 [7]. Although a range of treatment options can be addressed with biologics, none of them are universally effective and the best treatment selection is still based on a trial-and-error approach, where the most suitable one is determined when a drug reduces disease activity or remission is identified [8]. Considering the severity of these life-threatening diseases and the high cost of biologics, the best treatment option should target, from the start, the patients’ own pattern of cytokines [9].
Here, we report a clinical case demonstrating the usefulness of examining the leukocytes’ secretome of patients. We set up and standardized a protocol that investigates the immune responses of the patients to establish the secretome of their blood mononuclear leukocytes. The results were used to personalize the biotherapy of a patient suffering from an autoimmune arthropathy, providing insights on how to tailor the best treatment option and therefore avoid definitive disability and loss of quality of life.
Case presentation
A 24-year-old woman was examined for the first time 3 months after the onset of symmetrical polyarthritis with major synovitis of 2nd, 3rd, 4th metacarpophalangeal joints of both hands, wrists, elbows, knees, ankles, forefeet, without any spinal signs. The disease activity score of 28 joints (DAS28) and DAS28 using the C-reactive protein (DAS28-CRP) were 8.09 and 7.75, respectively. Increased ferritin and thrombocytosis in the absence of detectable levels of RF, anti-CCP and antinuclear antibody (ANA) were also noticeable. Her liver function tests and lipid panel were normal and no bone erosion was visible by X-rays. She was diagnosed with active early rheumatoid arthritis (RA) (Table 1).
Initial treatments with prednisone, methotrexate, hydroxychloroquine and naproxen were without efficacy. The anti-TNF adalimumab was added to the treatment regimen for 2 years. After only mild improvement, she experienced a progressive flare-up of polyarthritis and a loss of treatment efficacy. Two years after the onset of the disease, wrist and tarsal (right and left) demineralization, as well as bone erosions of ulnar styloids (right and left), appeared. Erythrocyte sedimentation rate (ESR), CRP and ferritin were persistently increased while RF and anti-CCP remained undetectable. The diagnosis was revised as possible RA-like psoriatic arthritis (PsoA), especially as her mother has skin psoriasis. Bone lesions were increased rapidly, in particular at both wrists. Adalimumab was replaced by abatacept (IgG1 Fc-CTLA-4) with a mild effect on arthritis. Five years after disease onset, psoriatic skin lesions appeared, and diagnosis of cutaneous psoriasis was confirmed by a dermatologist. The final diagnosis was aggressive RA-like PsoA with bone erosions, without RF and anti-CCP. Abatacept was replaced by the anti-interleukin (IL)-12/IL-23 ustekinumab with an increase of prednisone dosage. A mild relieve of polysynovitis was noted, which was dependent on prednisone. Reduction of prednisone led to a major flare-up of polysynovitis associated with asthenia, and after 3 months of ustekinumab administration, no objective effect on the patient’s symptoms was noted.
The patient was enrolled in a prospective study based on the quantification of cytokines secreted by peripheral blood leukocytes. Blood (50 mL) of the patient as well as of healthy donors was drawn after informed consent was obtained. Plasma was collected following centrifugation (400×g for 10 min) of anti-coagulated blood and stored at − 80 °C for further protein quantification. Peripheral blood mononuclear cells (PBMCs) were obtained following centrifugation (600×g for 20 min) of the cellular fraction of blood over density gradient medium (Lymphocyte separation medium, density 1.077–1.080 g/mL; Wisent Bioproducts Inc., St-Bruno, Québec, Canada). Density gradient-purified PBMCs were stimulated with well-known immune activators of pattern recognition receptors and cytokine signalling for 24 h at 1 × 106/mL in RPMI 1640 (Wisent Bioproducts Inc., St-Bruno, Québec, Canada) supplemented with 10% fetal bovine serum (VWR Life Science Seradigm, Mississauga, Ontario, Canada) and 1% primocin (InvivoGen, San Diego, California, USA) in the absence (control) or presence of plate-bound mouse anti-human CD3 (1 μg/mL, clone OKT3) + anti-human CD28 (10 μg/mL, clone 9.3), lipopolysaccharides (LPS—100 ng/mL; 45 nM, InvivoGen, San Diego, California, USA) + adenosine triphosphate (ATP—1 mM; added for the last 30 min, Sigma-Aldrich Canada Co., Oakville, Ontario, Canada), L18-muramyl dipeptide (L18-MDP—1 μg/mL; 1.32 μM, InvivoGen, San Diego, California, USA), Poly(deoxyadenylate–thymidylate) [Poly(dA:dT)—1 μg/mL; 1.57 μM, InvivoGen, San Diego, California, USA], anisomycin (20 μM, Millipore (Canada) Ltd, Etobicoke, Ontario, Canada) or pro-inflammatory cytokines IL-1β (100 ng/mL; 5.85 nM, PeproTech US, Rocky Hill, New Jersey, USA), TNF (100 ng/mL; 5.85 nM, STEMCELL Technologies Canada Inc., Vancouver, British Columbia, Canada), IL-6 (100 ng/mL; 3.83 nM, PeproTech US, Rocky Hill, New Jersey, USA) and IFN-γ (100 U/mL; 0.30 nM, PeproTech US, Rocky Hill, New Jersey, USA) to respectively activate T cells, the NLRP3, NOD2, AIM2 and pyrin inflammasomes as well as cytokine signalling. Following stimulation, cell supernatants were collected and stored at − 80 °C for further protein quantification. Proteins involved in inflammation (IL-1α, IL-1β, IL-6, IL-9, IL-15, IL-17A, IL-18, IL-21, IL-31, TNF, LT-α, IFN-γ), immunoregulation (IL-1RA, IL-4, IL-7, IL-10, IL-12, IL-13, IL-22, IL-23, IL-27, IFN-α), chemotaxis (CCL2/MCP-1, CCL3/MIP-1α, CCL4/MIP-1β, CCL5/RANTES, CCL11/Eotaxin, CXCL1/GROα, CXCL8/IL-8, CXCL10/IP-10, CXCL12/SDF-1α) and cellular growth (IL-2, IL-5, GM-CSF) were quantified by multiplex analyses in plasma and cell supernatants using Luminex technology according to the manufacturers’ instructions (Cytokine & Chemokine 34-Plex Human ProcartaPlex™ Panel 1A, Thermo Fisher Scientific Inc., Burlington, Ontario, Canada).
The results of this study revealed that plasma concentrations of cytokines were similar between the patient and healthy donors (Fig. 1 and data not shown). In comparison to leukocytes from healthy donors, the patient’s secretome showed a unique overproduction of IL-6 in response to multiple stimuli, including the inflammasome activators LPS + ATP, MDP and poly(dA:dT), as well as the pro-inflammatory cytokines IL-1β, TNF and IFN-γ, to levels (up to 133,000 pg/mL) at least twice the ones produced by healthy donors’ cells (Fig. 2). This overproduction of IL-6 occurred without substantial increase of pro-inflammatory cytokines such as TNF, IL-12 and IL-23, which correlates with the inefficacy of the anti-TNF adalimumab and the anti-IL-12/IL-23 ustekinumab treatments. No substantial differences were observed for members of the IL-1 cytokine family (IL-1α, IL-1β, IL-18), as well as IFN-γ and IL-17. Increased secretion of the IL-1 receptor antagonist (IL-1RA) was shown upon stimulation with the AIM2 inflammasome activator poly(dA:dT) as well as IFN-γ, suggesting that the patient’s leukocytes can synthesize high amounts of IL-1RA to neutralize the production of IL-1. Of note, the stimulation of the patient’s T cells using a combination of anti-CD3 and anti-CD28 also led to increased production of IL-6, as well as IL-23, IL-1RA and IL-17, but not to the levels observed for IL-6 (Fig. 2). Finally, no substantial differences were observed between the patient and the healthy donors regarding the production of IL-2, IL-4, IL-5, IL-7, IL-9, IL-10, IL-13, IL-15, IL-21, IL-22, IL-27, IL-31, LT-α, IFN-α, CCL2/MCP-1, CCL3/MIP-1α, CCL4/MIP-1β, CCL5/RANTES, CCL11/Eotaxin, CXCL1/GROα, CXCL8/IL-8, CXCL10/IP-10 and CXCL12/SDF-1α by unstimulated or stimulated PBMCs (data not shown).
Similar levels of cytokines in the plasma of the patient and healthy donors. Concentrations of IL-1α, IL-1β, IL-1RA, IL-18, IL-6, IL-12, IL-23, IL-17, IFN-γ and TNF were determined by multiplex assays in the plasma of healthy donors (black dots) and the patient with aggressive RA-like PsoA (red square). Results are expressed as mean ± standard deviation for the healthy donors (n = 5)
Aberrant production of IL-6 by the patient’s blood leukocytes. Density gradient-purified peripheral blood mononuclear cells were left unstimulated (unstim) or stimulated for 24 h with anti-CD3 + anti-CD28, lipopolysaccharide (LPS) + adenosine triphosphate (ATP), L18-muramyl dipeptide (L18-MDP), poly(deoxyadenylate–thymidylate) [poly(dA:dT)], anisomycin or the pro-inflammatory cytokines IL-1β, TNF, IFN-γ and IL-6 to respectively activate T cells, the NLRP3, NOD2, AIM2 and pyrin inflammasomes as well as cytokine signalling. Concentrations of IL-6, TNF, IL-12, IL-23, IL-1α, IL-1β, IL-18, IL-1RA, IL-17 and IFN-γ were determined by multiplex assays in the cells’ supernatants of healthy donors (black dots) and the patient with aggressive RA-like PsoA (red square). Results are expressed as mean ± standard deviation for the healthy donors (n = 5). ND; not determined
Thus, considering the unique major overproduction of IL-6 by the patient’s leukocytes, the anti-IL-6 receptor tocilizumab was administered with a rapid improvement of her active PsoA that remained dependent on low prednisone dosage. DAS28 and DAS28-CRP were greatly improved at 3.76 and 4.34, respectively. ESR, CRP and ferritin were progressively normalized. Her quality of life was greatly improved with, in particular, a progressive reduction of asthenia. Have the patient been enrolled in this prospective study sooner, her refractory PsoA would have probably benefited from the personalized treatment without the current associated irreversible destructive arthritis and partial functional handicap, especially at both wrists.
Discussion
The immunological disease continuum, that includes all autoinflammatory and autoimmune diseases, as proposed by McGonagle & McDermott [10], allows to better understand the complexity of immune factors that can be associated with the numerous immunological disorders. In this regard, the present case report can possibly be related to polygenic autoinflammatory diseases and mixed pattern diseases, both conceptual classification in which psoriatic arthritis can be found. The investigation of the patient’s secretome, as we report here, remains non-exhaustive, with a major focus on the biologics currently available to treat autoimmune arthropathies such as RA and PsoA (Table 2). This focus could easily be extended to other factors depending on new biologics, as well as other stimuli to activate relevant immune signalling as the field progresses further. Nonetheless, the targeted secretome allows deciphering whether a factor could be largely produced over others to be able to adapt more precisely the treatment. This case report exemplifies the personalized treatment option based on the patient’s medical history and the determination of the secretome of the patient’s blood leukocytes in response to immune activators that revealed a unique overproduction of the IL-6 cytokine. In fact, the overproduction of IL-6 in the present case report of a refractory PsoA was in line with reports of mediators from synovial inflammation of PsoA where anti-IL-6 biologics were effective treatment choices [11,12,13,14,15].
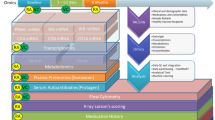
Another consideration is at the level of a unique overproduction of IL-6 in the present case of refractory PsoA, which could indicate a predominant autoinflammatory pattern over the autoimmune part of PsoA. In this regard, genomic DNA samples were isolated from the patient’s peripheral blood, and whole exome sequencing and data analysis were performed on the proband as previously described [16]. Publicly-available databases (ExAC, 1000 genomes, dbSNP, NHLBI GO Exome Sequencing Project and ClinSeq), as well as an in-house exome database, were used to filter for rare candidate variants. We did not identify any pathogenic mutations in known disease-causing genes. We analyzed the sequencing data for novel causes of immune dysregulation accounting for different modes of inheritance. Ideally, whole exome sequencing should be performed in trios to help with filtering a long list of candidate variants that are typically found when doing exome sequencing in singleton cases. Nonetheless, we did not identify any strong candidate variants to explain the phenotype. We also performed Sanger sequencing to analyze specific non-coding polymorphisms in the IL6 promoter and 5′UTR that could potentially account for some of the patient’s phenotype since the clinical spectrum of PsoA, more precisely the active peripheral arthritis, has been linked to IL6 (-174G/C) polymorphism [17]. Coding regions as well as flanking 5′ untranslated regions of the IL6 gene (RefSeq: NM_000600.5) were amplified by AmpliTaq Gold Fast PCR Master Mix (Thermo Fisher Scientific Inc., Waltham, Massachusetts, USA) and sequenced on SeqStudio Genetic Analyzer (Applied Biosystems Inc., Foster City, California, USA). We found that the patient had reference alleles at -597G, -572G, -473A8/T12 and -174G [18]. We finally analyzed the data for common low-penetrance coding variants associated with psoriatic or rheumatoid arthritis. We found that the patient is a heterozygous carrier for the single nucleotide polymorphism (SNP) in TRAF3IP2 rs33980500 (NM_001164281.2:c.28G>A; p.Asp10Asn; NM_147200.2:c.55G>A, NP_671733.2;p.Asp19Asn), which was identified by two genome-wide association studies (GWAS). The susceptibility allele rs33980500 has been shown to cause altered TRAF6 binding and thereby affect multiple immune pathways [19, 20]. Because TRAF3IP2 binding motifs have been associated with multiple TRAF proteins, the inhibition of the TRAF6 pathway could upregulate the TRAF2/5 pathway, leading to an enhanced immune response. Interestingly, studies showed that, in the TRAF6-independent pathway, TRAF2 and TRAF5 transduce the IL-17 signals to stabilize mRNA transcripts of chemokines and cytokines, such as CXCL1/GROα and IL-6 [21, 22]. Although these results did not identify a high penetrance genetic cause, they did identify a risk allele in TRAF3IP2, which in combination with additional environmental and genetic factors may help to explain this patient’s phenotype and suggest a further link between the PsoA, IL-6 production and TRAF3IP2/TRAFs signalling (Fig. 3).
IL-6 production by blood leukocytes is altered in the RA-like PsoA patient in response to immune activators. In healthy individuals (top panel), the stimulation of peripheral blood mononuclear cells with immune activators leads to the activation of intracellular multiprotein oligomers (e.g. inflammasomes) and specific receptors that activate signalling pathways, including TRAF proteins, translocation of transcription factors and subsequent cytokine response. In the patient with aggressive RA-like PsoA (bottom panel), the activation of blood cells with anti-CD3 + anti-CD28, pro-inflammatory cytokines IL-1β, TNF and IFN-γ, muramyl dipeptide (MDP), lipopolysaccharide + adenosine triphosphate (LPS + ATP) and poly(deoxyadenylate–thymidylate) [poly(dA:dT)] led to a unique overproduction of IL-6 (red arrows). As the patient is a heterozygous carrier for a single nucleotide polymorphism p.Asp10Asn (p.D10N) in TRAF3IP2, this susceptibility allele causes altered TRAF6 binding (double red lines with an oblique stroke). The inhibition of the TRAF6 pathway could upregulate the TRAF2/5 pathway, leading to an enhanced immune response [21, 22] (dashed red arrow)
While we examined the secretome of blood cells, one limitation of this study is that many other cell types in autoimmune arthritis are known to secrete cytokines, such as synoviocytes, fibroblasts, chondrocytes, osteoblasts/osteoclasts and epithelial cells (the latter ones in psoriatic arthritis for example), and that these cells, through their cytokine secretion, could contribute to the local pathology [23,24,25,26]. The cytokine pattern could evidently be different from the ones produced by the blood cells. However, the use of these cells often requires invasive techniques to obtain them and their purification, for subsequent activation, is more labour-intensive than blood cells [27]. It is also useful to stress that the presence of a SNP in TRAF3IP2 rs33980500, as shown in this case report, should be present in all cell types using this signalling pathway [28, 29].
Conclusion
As a corollary and conclusion, an efficient way to effectively treat patients with complex autoimmune arthropathies, and to avoid irreversible disability, is to determine their blood leukocytes’ secretome to identify abnormally secreted cytokines to personalize their biotherapy. Another concluding detail should also be noted as the finding of an abnormal patients’ secretome can justify pursuing a causal investigation such as a genetic evaluation, as exemplified by this case report.
Abbreviations
- ANA:
-
antinuclear antibody
- Anti-CCP:
-
anti-cyclic citrullinated peptide
- DAS28:
-
disease activity score of 28 joints
- DAS28-CRP:
-
DAS28 using the C-reactive protein
- GWAS:
-
genome-wide association study
- IL:
-
interleukin
- PsoA:
-
psoriatic arthritis
- RA:
-
rheumatoid arthritis
- RF:
-
rheumatoid factor
- SNP:
-
single nucleotide polymorphism
- TNF:
-
tumor necrosis factor
References
Littlejohn EA, Monrad SU (2018) Early diagnosis and treatment of rheumatoid arthritis. Prim Care 45(2):237–255
Hyrich KL, Watson KD, Silman AJ, Symmons DP, British Society for Rheumatology Biologics R (2006) Predictors of response to anti-TNF-alpha therapy among patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Rheumatology (Oxford) 45(12):1558–1565
Wijbrandts CA, Tak PP (2017) Prediction of response to targeted treatment in rheumatoid arthritis. Mayo Clin Proc. 92(7):1129–1143
Cherlin S, Plant D, Taylor JC, Colombo M, Spiliopoulou A, Tzanis E et al (2018) Prediction of treatment response in rheumatoid arthritis patients using genome-wide SNP data. Genet Epidemiol 42(8):754–771
Bluett J, Barton A (2017) Precision medicine in rheumatoid arthritis. Rheum Dis Clin North Am 43(3):377–387
Viatte S, Barton A (2017) Genetics of rheumatoid arthritis susceptibility, severity, and treatment response. Semin Immunopathol. 39(4):395–408
Her M, Kavanaugh A (2016) Alterations in immune function with biologic therapies for autoimmune disease. J Allergy Clin Immunol. 137(1):19–27
Smolen JS, Landewe R, Breedveld FC, Buch M, Burmester G, Dougados M et al (2014) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 73(3):492–509
Turner MD, Nedjai B, Hurst T, Pennington DJ (2014) Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta 1843(11):2563–2582
McGonagle D, McDermott MF (2006) A proposed classification of the immunological diseases. PLoS Med. 3(8):e297
Alenius GM, Eriksson C, Rantapaa Dahlqvist S (2009) Interleukin-6 and soluble interleukin-2 receptor alpha-markers of inflammation in patients with psoriatic arthritis? Clin Exp Rheumatol 27(1):120–123
Costa L, Caso F, Cantarini L, Del Puente A, Scarpa R, Atteno M (2014) Efficacy of tocilizumab in a patient with refractory psoriatic arthritis. Clin Rheumatol 33(9):1355–1357
FitzGerald O (2016) Spondyloarthropathies: IL-6 blockade in psoriatic arthritis—a new therapeutic option? Nat Rev Rheumatol 12(6):318–319
Mease PJ, Gottlieb AB, Berman A, Drescher E, Xing J, Wong R et al (2016) The efficacy and safety of clazakizumab, an anti-interleukin-6 monoclonal antibody, in a phase IIb study of adults with active psoriatic arthritis. Arthritis Rheumatol. 68(9):2163–2173
van Kuijk AW, Reinders-Blankert P, Smeets TJ, Dijkmans BA, Tak AA (2006) Detailed analysis of the cell infiltrate and the expression of mediators of synovial inflammation and joint destruction in the synovium of patients with psoriatic arthritis: implications for treatment. Ann Rheum Dis. 65(12):1551–1557
Zhou Q, Wang H, Schwartz DM, Stoffels M, Park YH, Zhang Y et al (2016) Loss-of-function mutations in TNFAIP3 leading to A20 haploinsufficiency cause an early-onset autoinflammatory disease. Nat Genet 48(1):67–73
Cubino N, Montilla C, Usategui-Martin R, Cieza-Borrela C, Carranco T, Calero-Paniagua I et al (2016) Association of IL1Beta (-511 A/C) and IL6 (− 174 G>C) polymorphisms with higher disease activity and clinical pattern of psoriatic arthritis. Clin Rheumatol 35(7):1789–1794
Terry CF, Loukaci V, Green FR (2000) Cooperative influence of genetic polymorphisms on interleukin 6 transcriptional regulation. J Biol Chem 275(24):18138–18144
Ellinghaus E, Ellinghaus D, Stuart PE, Nair RP, Debrus S, Raelson JV et al (2010) Genome-wide association study identifies a psoriasis susceptibility locus at TRAF3IP2. Nat Genet 42(11):991–995
Huffmeier U, Uebe S, Ekici AB, Bowes J, Giardina E, Korendowych E et al (2010) Common variants at TRAF3IP2 are associated with susceptibility to psoriatic arthritis and psoriasis. Nat Genet 42(11):996–999
Bulek K, Liu C, Swaidani S, Wang L, Page RC, Gulen MF et al (2011) The inducible kinase IKKi is required for IL-17-dependent signaling associated with neutrophilia and pulmonary inflammation. Nat Immunol 12(9):844–852
Sun D, Novotny M, Bulek K, Liu C, Li X, Hamilton T (2011) Treatment with IL-17 prolongs the half-life of chemokine CXCL1 mRNA via the adaptor TRAF5 and the splicing-regulatory factor SF2 (ASF). Nat Immunol 12(9):853–860
Ganesan R, Rasool M (2017) Fibroblast-like synoviocytes-dependent effector molecules as a critical mediator for rheumatoid arthritis: current status and future directions. Int Rev Immunol 36(1):20–30
Danis J, Janovak L, Guban B, Goblos A, Szabo K, Kemeny L et al (2018) Differential inflammatory-response kinetics of human keratinocytes upon cytosolic RNA- and DNA-fragment induction. Int J Mol Sci 19(3):774
Mensah KA, Schwarz EM, Ritchlin CT (2008) Altered bone remodeling in psoriatic arthritis. Curr Rheumatol Rep 10(4):311–317
Veale DJ, Fearon U (2018) The pathogenesis of psoriatic arthritis. Lancet 391(10136):2273–2284
Amos PJ, Cagavi Bozkulak E, Qyang Y (2012) Methods of cell purification: a critical juncture for laboratory research and translational science. Cells Tissues Organs. 195(1–2):26–40
Matsushima Y, Kikkawa Y, Takada T, Matsuoka K, Seki Y, Yoshida H et al (2010) An atopic dermatitis-like skin disease with hyper-IgE-emia develops in mice carrying a spontaneous recessive point mutation in the Traf3ip2 (Act1/CIKS) gene. J Immunol. 185(4):2340–2349
Das NA, Carpenter AJ, Yoshida T, Kumar SA, Gautam S, Mostany R et al (2018) TRAF3IP2 mediates TWEAK/TWEAKR-induced pro-fibrotic responses in cultured cardiac fibroblasts and the heart. J Mol Cell Cardiol 121:107–123
Authors’ contributions
PEP, the rheumatologist responsible for clinically following the patient, analyzed and interpreted the results and wrote the manuscript. NP obtained participants’ consents, purified and stimulated blood cells and performed multiplex assays. MPL performed multiplex assays. NSM helped prepare samples for whole exome and Sanger sequencing. DBB and IA analyzed and interpreted the sequencing data. PAT designed the study, analyzed and interpreted the results. MP designed the study, analyzed and interpreted the results and wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We acknowledge Julie-Christine Lévesque from the Bioimaging platform of the Infectious Disease Research Centre, funded by an equipment and infrastructure grant from the Canadian Foundation for Innovation (CFI), for her technical assistance with the multiplex assays. We would like to thank Michel J. Tremblay (Centre de recherche du CHU de Québec-Université Laval) for generously providing the anti-CD3 and anti-CD28 antibodies as well as Ingrid Saba for reviewing the manuscript.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding authors on request.
Consent for publication
A consent for publication form was signed by the patient. All authors approved the manuscript for submission.
Ethics approval and consent to participate
The institutional review board of the CHU de Québec-Université Laval Research Center approved the study (2016–2519), and volunteers signed consent forms.
Funding
This research was sponsored by operating grants from La Fondation du Grand défi Pierre Lavoie, Rare Disease Foundation and the BC Children’s Hospital Foundation. The publication fees were sponsored by a grant (# 3623) from La Fondation du CHU de Québec. MP is a Junior 2 scholar from the Fonds de recherche du Québec-Santé (FRQS). The funding agencies had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data, the decision to submit for publication or preparation, review, or approval of the manuscript for publication.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Poubelle, P.E., Pagé, N., Longchamps, MP. et al. The use of leukocytes’ secretome to individually target biological therapy in autoimmune arthritis: a case report. Clin Trans Med 8, 19 (2019). https://doi.org/10.1186/s40169-019-0236-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40169-019-0236-7