Abstract
Nicotinamide adenine dinucleotide (NAD+) is a central metabolic cofactor in eukaryotic cells that plays a critical role in regulating cellular metabolism and energy homeostasis. NAD+ in its reduced form (i.e. NADH) serves as the primary electron donor in mitochondrial respiratory chain, which involves adenosine triphosphate production by oxidative phosphorylation. The NAD+/NADH ratio also regulates the activity of various metabolic pathway enzymes such as those involved in glycolysis, Kreb’s cycle, and fatty acid oxidation. Intracellular NAD+ is synthesized de novo from l-tryptophan, although its main source of synthesis is through salvage pathways from dietary niacin as precursors. NAD+ is utilized by various proteins including sirtuins, poly ADP-ribose polymerases (PARPs) and cyclic ADP-ribose synthases. The NAD+ pool is thus set by a critical balance between NAD+ biosynthetic and NAD+ consuming pathways. Raising cellular NAD+ content by inducing its biosynthesis or inhibiting the activity of PARP and cADP-ribose synthases via genetic or pharmacological means lead to sirtuins activation. Sirtuins modulate distinct metabolic, energetic and stress response pathways, and through their activation, NAD+ directly links the cellular redox state with signaling and transcriptional events. NAD+ levels decline with mitochondrial dysfunction and reduced NAD+/NADH ratio is implicated in mitochondrial disorders, various age-related pathologies as well as during aging. Here, I will provide an overview of the current knowledge on NAD+ metabolism including its biosynthesis, utilization, compartmentalization and role in the regulation of metabolic homoeostasis. I will further discuss how augmenting intracellular NAD+ content increases oxidative metabolism to prevent bioenergetic and functional decline in multiple models of mitochondrial diseases and age-related disorders, and how this knowledge could be translated to the clinic for human relevance.
Similar content being viewed by others
Introduction
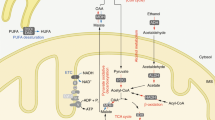
Mitochondria are highly dynamic intracellular organelles that play crucial roles in energy production, metabolism, intracellular signaling and apoptosis [87, 112]. These organelles are maternally inherited and semiautonomous containing their own DNA (mtDNA) which is a circular double-stranded molecule of ~16.5 kb in mammals encoding 13 polypeptide subunits, 22 transfer RNAs and 2 ribosomal RNAs. The rest of the mitochondrial proteome, consisting of ~1500 additional polypeptides is encoded by the nuclear DNA (nDNA), translated in the cytosol and imported into the organelles by an active process [87]. Mitochondria have the ability to change their morphology, number and function in response to various physiological stimuli (e.g. exercise, diet, temperature, or hormones) and stress [91]. Proper mitochondrial function is therefore critical for the maintenance of metabolic homeostasis and activation of appropriate stress responses. A principal bioenergetic function of mitochondria is to generate adenosine triphosphate (ATP) from nutrient breakdown (e.g. glucose, fatty-acids and amino-acids) through a process termed as oxidative phosphorylation (OXPHOS). This process involves transport of electrons from reduced equivalents [e.g. nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FADH2)] along the respiratory chain protein complexes (CI-IV) via the electron carriers (e.g. coenzyme Q10 and cytochrome c) to the terminal electron acceptor i.e. oxygen (O2) which is ultimately reduced to water (Fig. 1) [34]. The electron flow is coupled with the translocation of protons from the matrix to the intermembrane space (via complexes I, III and IV) which in turn generates an electrochemical proton gradient or membrane potential (ΔΨm) across the inner mitochondrial membrane. The energy in this gradient is subsequently harnessed by complex V or ATP synthase to generate ATP from adenosine diphosphate (ADP) and inorganic phosphate (Pi), during when the protons flow back from the intermembrane space to the matrix (Fig. 1) [34]. Under normal conditions ~1 to 2 % of electrons leak from the electron transport chain and reduce O2 to superoxide radical (O •−2 ) thereby producing reactive oxygen species (ROS), which is detoxified by the action of antioxidant enzymes such as superoxide dismutase, catalase, and glutathione peroxidase [44, 95]. However, when the balance between ROS production overrides the antioxidant capability of the cells, it leads to oxidative stress which is highly damaging to cellular macromolecules (i.e. DNA, lipids and proteins), and is linked to multiple pathologies including neurodegenerative diseases, diabetes, cancer and premature aging [44, 95]. Mitochondrial dysfunction caused by genetic mutations in mtDNA or nDNA encoded OXPHOS proteins affects the electron transport chain (ETC) function and impairs ATP production leading to the onset of mitochondrial diseases wherein the high energy demanding tissues such as brain, heart, retina and skeletal muscle are predominantly affected [34, 93]. Mitochondrial dysfunction is not only a hallmark of mitochondrial disorders, but is also implicated in aging and age-related disorders such as diabetes, obesity, neurodegeneration and cancer.
Schematic illustration of mammalian oxidative phosphorylation system. The mammalian OXPHOS comprises of five multimeric enzyme complexes (CI–V). Electrons from reducing equivalents i.e. NADH and FADH2 enter mitochondrial electron transport chain (ETC) and reduce complex I and complex II, respectively. An inner membrane electron carrier, coenzyme Q10 or ubiquinone accepts an electron from either complex I or complex II and donates it to complex III. Cytochrome c, another electron carrier in the intermembrane space accepts an electron from complex III and donates it to complex IV, which in turn reduces molecular O2 to H2O. During the electron flow, complex I, III and IV simultaneously pump protons from the matrix towards intermembrane space generating an electrochemical gradient or membrane potential (Ψm) across the inner mitochondrial membrane. The energy in this gradient is harnessed by complex V to generate ATP from ADP and inorganic phosphate (Pi), a phenomenon termed as OXPHOS. Approximately 1–2 % electrons leak from the ETC and reduce O2 to superoxide radical (O •−2 ) thereby producing reactive oxygen species (ROS)
Sirtuins or silent information regulator 2 (Sir2) proteins are a family of evolutionarily conserved nicotinamide adenine dinucleotide (NAD+)-dependent protein deacylases harboring lysine deacetylase, desuccinylase, demalonylase, demyristoylase and depalmitoylase activity [37, 40, 88, 104], or an ADP-ribosyltransferase activity [36, 48]. Mammals contain seven sirtuins (SIRT1–7) that are located in different subcellular compartments i.e. nucleus (SIRT1, SIRT6 and SIRT7), cytosol (SIRT2), and mitochondria (SIRT3, SIRT4 and SIRT5) [49, 114], and are implicated in a wide variety of biological functions including control of cellular metabolism and energy homeostasis, aging and longevity, transcriptional silencing, cell survival, proliferation, differentiation, DNA damage response, stress resistance, and apoptosis [2, 49, 102, 110, 114]. Since sirtuins are NAD+-dependent enzymes, the availability of NAD+ is one of the key mechanisms that regulate their activity. Sirtuins therefore serve as “metabolic sensors” of the cells as their activity is coupled to changes in the cellular NAD+/NADH redox state, which is largely influenced by the availability and breakdown of nutrients [20]. Thus, NAD+ is not only a vital cofactor/coenzyme but also a signaling messenger that can modulate cell metabolic and transcriptional responses. Changes in cellular NAD+ levels can occur due to modulation of pathways involved in NAD+ biosynthesis and consumption. Reduced NAD+ levels have been reported in mitochondrial and age-related disorders, and NAD+ levels also decline with age [17, 26, 45, 53, 60, 67, 71]. Boosting cellular NAD+ levels serves as a powerful means to activate sirtuins, and as a potential therapy for mitochondrial as well as age-related disorders.
Review
NAD+ biosynthesis, consumption and compartmentalization
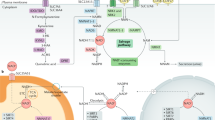
The mammalian NAD+ biosynthesis occurs via de novo and salvage pathways, and involves four major substrates including the essential amino acid l-tryptophan (Trp), nicotinic acid (NA), nicotinamide (NAM), and nicotinamide riboside (NR) [25, 54]. De novo biosynthesis of NAD+ starts from dietary Trp which is catalytically converted to N-formylkynurenine by either indoleamine 2,3-dioxygenase (IDO) or tryptophan 2,3-dioxygenase (TDO) and is the first rate limiting step. N-formylkynurenine is then converted by a series of four enzymatic reactions to α-amino-β-carboxymuconate-ε-semialdehyde (ACMS) which is unstable and hence undergoes either complete enzymatic oxidation or non-enzymatic cyclization to quinolinic acid (Fig. 2). The second rate limiting step involves the catalytic conversion of quinolinic acid to nicotinic acid mononucleotide (NAMN) by quinolinate phosphoribosyl transferase (QPRT). Next, NAMN is converted to nicotinic acid adenine dinucleotide (NAAD) by one of the three isoforms of nicotinamide mononucleotide adenylyltransferase (NMNAT) enzyme. The human NMNAT1 is localized in the nucleus, NMNAT2 is found in the Golgi and cytosol, whereas NMNAT3 is localized in both mitochondria and cytosol [13, 54]. The final step of de novo biosynthesis is the amidation of NAAD by NAD synthase (NADS) enzyme (Fig. 2) [25, 54]. The de novo pathway contributes only a minor fraction to the total NAD+ pool, however, its importance is stressed by the human disease pellagra which is caused by dietary deficiency of Trp and NAM intermediate, leading to diarrhea, dermatitis, dementia and ultimately death [51]. However, pellagra is easily treated by dietary supplementation of Trp or niacin (i.e. NA, NAM and NR). The primary source of NAD+ biosynthesis is the salvage or Preiss-Handler pathway which utilizes dietary niacin as precursors (Fig. 2). The salvage pathway involves catalytic conversion of NA to NAMN by nicotinic acid phosphoribosyltransferase (NAPT), which is subsequently converted to NAD+ by the action of NMNAT and NADS enzymes. The NAM and NR are converted to NMN by the action of nicotinamide phosphoribosyltransferase (NAMPT) and nicotinamide riboside kinase (NRK) enzymes respectively. Finally, NMN is enzymatically converted to NAD+ by NMNAT (Fig. 2) [25, 54].
Schematic representation of de novo and salvage pathways for NAD+ biosynthesis. In mammals, the de novo biosynthesis starts from l-tryptophan (Trp) which is enzymatically converted in a series of reactions to quinolinic acid (QA). Through quinolinate phosphoribosyltransferase (QPRT) enzyme activity, QA is converted to nicotinic acid mononucleotide (NAMN), which is then converted to nicotinic acid adenine dinucleotide (NAAD) by nicotinamide mononucleotide adenylyltransferase (NMNAT) enzyme. The final step in de novo biosynthesis is the amidation of NAAD by NAD synthase (NADS) which generates NAD+. The salvage pathway involves NAD+ synthesis from its precursors, i.e. Nicotinic acid (NA), nicotinamide (NAM) or nicotinamide riboside (NR). NA is catalytically converted to NAMN by the action of nicotinic acid phosphoribosyltransferase (NAPT). NAM is converted by nicotinamide phosphoribosyltransferase (NAMPT) to nicotinamide mononucleotide (NMN), which is also the product of phosphorylation of NR by nicotinamide riboside kinase (NRK) enzyme. Finally, NAMN is converted to NAD by the action of NMNAT and NADS enzymes, whereas NMN is converted to NAD by the NMNAT enzyme. Multiple enzymes break-down NAD+ to produce NAM and ADP-ribosyl moiety, however only sirtuins are depicted in this figure
The cellular abundance of NAD+ is also regulated by its breakdown since NAD+ serves as a degradation substrate for multiple enzymes including sirtuins, poly ADP-ribose polymerases (PARPs) and cyclic ADP (cADP) ribose synthases which cleave NAD+ to produce NAM and an ADP-ribosyl product [29, 49, 54, 56, 96]. For instance, the deacetylase activity of mammalian sirtuins uses NAD+ to cleave the acetyl group from ε–acetyl lysine residues of target proteins to generate NAM and 2′O-acetyl-ADP-ribose. Sirtuins are activated in response to nutrient deprivation or energy deficit which triggers cellular adaptations to improve metabolic efficiency. PARP’s are activated in response to DNA damage (e.g. DNA strand breaks) and genotoxic stress, and use NAD+ to catalyze a reaction in which the ADP ribose moiety is transferred to a substrate protein. The cADP-ribose synthases (e.g. CD38 and CD157) use NAD+ to generate cADP-ribose which serves as an intracellular second messenger. The members of PARP and cADP-ribose synthase family show increased affinity and lower Km for NAD+ compared to sirtuins, indicating that their activation critically impacts intracellular NAD+ levels and determines if it reaches a permissive threshold for sirtuin activation [54]. Multiple studies also suggested that PARP activity constitutes the main NAD+ catabolic activity, which drives cells to synthesize NAD+ from de novo or salvage pathways [14, 98].
Intracellular NAD+ has a short half-life, which is estimated to be ~1 to 2 h [38, 84], and is not evenly distributed in subcellular compartments i.e. nucleus, cytosol and mitochondria. Studies report that mitochondrial NAD+ levels are higher than in other compartments, for example in mouse skeletal muscles and cardiac myocytes, the mitochondrial NAD+ levels were found to be approximately twofold and fourfold higher respectively, than the rest of the cell [1, 77]. Multiple studies indicate that mitochondrial NAD+ concentration is ≥250 μM whereas nuclear NAD+ concentration is ~70 μM [73, 115], and the nuclear NAD+ levels are also lower than the cytosolic NAD+ levels [41, 122]. Also, the NAD+ pool in each subcellular compartment is partially sequestered from free NAD+ by binding to proteins. NAD+ cannot diffuse through mitochondrial membranes, therefore changes in cytosolic NAD+ levels cannot directly alter the mitochondrial NAD+/NADH ratio [9, 79, 109, 115]. Mammalian mitochondria have their own NAD biosynthetic machinery which plays a key role in maintaining mitochondrial NAD pool [13, 115]. However, in yeast NAD is not synthesized in mitochondria but instead transported across the mitochondrial membranes via membrane NAD transporters [106]. A mammalian mitochondrial NAD transporter however has yet to be found. Interestingly, a recent study demonstrated that exogenous NAD can cross the plasma membrane and elevate mitochondrial NAD levels in mammalian cells causing significant enhancement in mitochondrial oxygen consumption and ATP production suggesting the possibility that mitochondrial NAD transport mechanism/s might exist in mammals and that mitochondria can rapidly increase its pyridine nucleotide pool when the cytoplasmic availability of NAD and/or its precursors increases [78].
NAD+ plays a key role in regulating cellular metabolism and energy production
NAD+ and its phosphorylated and reduced forms including NADP+, NADH, and NADPH are vital in regulating cellular metabolism and energy production. NAD+ functions as an oxidoreductase cofactor in a wide range of metabolic reactions and modulates the activity of compartment-specific pathways such as glycolysis in the cytosol, and tri-carboxylic acid (TCA) cycle, OXPHOS, fatty acid and amino acid oxidation in the mitochondria. For instance, NAD+ is converted to NADH at the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) step of glycolysis, a pathway that generates pyruvate from glucose [12, 68, 97]. In the mitochondrial compartment, NAD+ is converted to NADH at multiple steps in the TCA cycle in which acetyl-coenzyme A is oxidized to carbon dioxide. Mitochondrial NADH is then oxidized by furnishing reducing equivalents to complex I in the ETC through a series of redox reactions that generate ATP from ADP by OXPHOS. The NAD+/NADH ratio thus regulates multiple metabolic pathway enzymes including GAPDH, pyruvate dehydrogenase, isocitrate dehydrogenase, α-ketoglutarate dehydrogenase and malate dehydrogenase. In contrast to NAD+/NADH, the NADPH/NADP+ ratios are maintained high in both cytosol and mitochondrial compartments, to maintain a reducing environment [105]. NADPH plays a key role in reductive biosynthesis and cellular defense against oxidative damage [80]. For instance, NADPH serves as a cofactor for P450 enzymes that detoxify xenobiotics, acts as a terminal reductant for glutathione reductase which maintains reduced glutathione levels during oxidative defense, and also serves as a substrate for NADPH oxidase that generates peroxides for release during oxidative burst processes in the immune system [80].
Therapeutic potential of NAD+ metabolism
Since NAD+ is a rate-limiting cofactor for sirtuins, its modulation is emerging as a valuable tool to regulate sirtuin function, and consequently oxidative metabolism. SIRT1 is the most characterized among all sirtuins and is implicated in mitochondrial and metabolic homeostasis [21, 47, 50]. There are multiple targets of SIRT1 including transcriptional co-activators such as the peroxisome proliferator-activated receptor gamma coactivator-1alpha (PGC-1α) and transcription factors such as the forkhead box protein O1 (FOXO1). PGC-1α is the master regulator of mitochondrial biogenesis and function [64, 100, 101], whereas FOXO1 modulates mitochondrial fatty acid metabolism and protects against oxidative stress [108]. SIRT3 is the major mitochondrial deacetylase which targets several proteins involved in fatty acid metabolism, ketogenesis and antioxidant defense [3, 56]. Thus, modulation of NAD+ levels has profound effects on oxidative metabolism and mitochondrial function, exerted through a multitude of sirtuin targets, and serve as a promising avenue for the management and treatment of mitochondrial and age-related diseases.
Modulation of NAD+ levels by physiological processes
The intracellular NAD+ levels are typically between 0.2 and 0.5 mM in mammalian cells, and change during a number of physiological processes [54]. Since the nucleus, cytosol and mitochondria are equipped with NAD+ salvage enzymes, the compartment-specific NAD+ production activates distinct sirtuins to trigger the appropriate physiological response. The NAD+/NADH levels also vary with the availability of dietary energy and nutrients. For instance, tissue NAD+ levels decrease with energy overload such as high-fat diet [23, 118], and display circadian oscillations with a 24 h rhythm in the liver, which is regulated by feeding [4, 74, 83]. During energetic stress such as exercise, calorie restriction (CR) and fasting in mammals, the NAD+ levels increase leading to sirtuin activation, which is associated with metabolic and age-related health benefits (Fig. 3) [19, 24, 27, 30]. Decreased sirtuins (e.g. SIRT1 and SIRT3) expression is associated with various age-related pathologies [21, 58, 116, 117, 120, 123] and their overexpression has been reported to enhance overall mitochondrial and metabolic health in age-related disorders as well as mitochondrial diseases [7, 16, 26, 31, 35, 76, 82, 99].
Boosting NAD+ levels is beneficial for health and lifespan. NAD+ is a rate-limiting cofactor for the enzymatic activity of sirtuins. Boosting intracellular NAD+ levels by physiological (e.g. exercise, calorie restriction, fasting) or pharmacological [e.g. resveratrol, sirtuin activating compounds (STACs)] interventions, and inducing NAD+ biosynthesis through supplementation with precursors (e.g. NA, NAM, NR) or inhibition of NAD+ consuming enzymes (e.g. PARP-1, CD38) leads to activation of sirtuins (e.g. SIRT1, SIRT3). SIRT1 deacetylates and activates transcriptional regulators (e.g. PGC-1α, FOXO1), whereas SIRT3 deacetylates and activates multiple metabolic gene targets (e.g. succinate dehydrogenase, superoxide dismutase 2), which in turn regulate mitochondrial biogenesis and function. Supplementation with NR or PARP inhibitors extends lifespan in worms by inducing the UPRmt stress signaling response via Sir-2.1 activation, which then triggers an adaptive mitohormetic response to stimulate mitochondrial function and biogenesis. Improved mitochondrial function associated with mitohormesis or metabolic adaptation can attenuate the impact of mitochondrial diseases, aging as well as age-related metabolic and neurodegenerative disorders. The physiological and pharmacological interventions that boost NAD+ levels are highlighted in yellow and pink respectively whereas the pathways that produce and consume/decrease NAD+ levels are highlighted in green and red respectively
Modulation of NAD+ levels by pharmacological compounds
Besides physiological processes, NAD+ levels can be modulated pharmacologically. Resveratrol—a polyphenolic compound found in red wine has been shown to indirectly stimulate NAD+ production by activating the energy sensor AMP-activated protein kinase (AMPK) [22, 42]. Increased NAD+ subsequently stimulates SIRT1 activity, which in turn activates PGC-1α and FOXO family of proteins that govern mitochondrial biogenesis and function (Fig. 3) [21, 22]. SIRT1 is also amenable to intervention by small molecules such as SIRT1-activating compounds (STACs) that exert beneficial effects on age-related metabolic abnormalities [21, 71]. NAD+ levels can be directly raised by supplying NAD+ biosynthetic precursors/intermediates, or by inhibiting NAD+ consuming enzymes with specific inhibitors (Fig. 3). For instance, supplementation of NA, NR or NMN compounds increase NAD+ levels in both cultured cells and mouse tissues [21, 23, 118]. Because NR can be metabolized both in the nucleus and mitochondria, its supplementation raises the nuclear and mitochondrial NAD+ levels, thereby activating nuclear SIRT1 and mitochondrial SIRT3 respectively [21, 23]. Pharmacological activation of NAD+ thus stimulates the activity of multiple sirtuin in a compartment-specific manner to exert its beneficial effects on multiple metabolic pathways which is in contrast to STAC’s that specifically stimulate the activity of SIRT1 pathway. Treatment of mice or cultured cells with PARP and CD38 specific inhibitors has also been shown to induce NAD+ levels that activate sirtuins [6, 8].
Increased NAD+ levels protects against mitochondrial and age-related disorders
Mitochondrial disorders represent one of the most common forms of heritable metabolic disease in children [33, 70, 92]. Reduced NAD+/NADH ratio is strongly implicated in mitochondrial disorders and, age-related disorders including diabetes, obesity, neurodegeneration and cancer [26, 53, 60, 71]. NAD+ levels also decline during aging in multiple models including worms, rodents and human tissue [17, 45, 67, 72]. Increasing evidence suggests that boosting NAD+ levels could be clinically beneficial, as it activates the NAD+/sirtuin pathway which yields beneficial effects on multiple metabolic pathways.
Pharmacological activation of NAD+ production has recently been used to treat mouse models of mitochondrial diseases. For instance, treatment of cytochrome c oxidase (COX) deficiency caused by SURF1, SCO2 or COX15 genetic mutations in mice, with AMPK agonist 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR), partially rescued mitochondrial dysfunction and improved motor performance [111]. These findings could be explained by the fact that AMPK stimulates NAD+ production, consequently activating SIRT1 which promotes energy production and homeostasis [21]. Oral administration of NAD+ precursor, NR in mitochondrial myopathy mice harboring a pathogenic mutation in the mtDNA helicase—Twinkle, effectively delayed myopathy progression, by increasing mitochondrial biogenesis, preventing mitochondrial ultrastructural abnormalities, mtDNA deletion formation and activating the mitochondrial unfolded protein (UPRmt) response [60]. In addition, NR supplementation and reduction of NAD+ consumption by a specific PARP inhibitor significantly improved mitochondrial respiratory chain defect and exercise intolerance, in a mouse model of COX deficiency caused by SCO2 mutation [26].
Besides improving mitochondrial function, boosting NAD+ levels with resveratrol, NR or NMN also corrects metabolic disturbances in mice caused by high fat diet [10, 21, 62, 118]. NMN administration ameliorates glucose intolerance and insulin resistance in diet- and age-induced type 2 diabetic mice [82, 118], and rectifies glucose-stimulated insulin secretion and glucose intolerance in NAMPT-deficient animals, by restoring NAD+ levels [85]. Interventions using NAD+ precursors or PARP inhibitors were also shown to be neuroprotective. For instance treatment with NMN or NR precursors, protected against axonal degeneration and hearing loss in mice [18, 90]. Raised NAD+ levels after CR, NAM or NR treatment attenuated increase in β-amyloid content and oxidative damage, preventing cognitive decline and neurodegeneration in rodent models of Alzheimer’s disease [46, 81, 107]. PARP-1 activation also occurs in neurodegenerative DNA repair disorders including xeroderma pigmentosum group A (XPA) and Cockayne syndrome group B (CSB), and treatment with specific PARP inhibitors rescues defective phenotypes in XPA mutant worms and CSB mutant mice respectively [39, 94]. However, PARP-2 deleted mice were glucose intolerant and exhibited pancreatic dysfunction, implying that these results may interfere with other beneficial consequences of PARP inhibition, and hence warrant further investigation on the safe clinical use of these inhibitors [5]. Because PARP inhibitors enhance oxidative metabolism and improve metabolic flexibility, these compounds are being tested in phase III trials as anti-cancer agents [6, 86].
Increasing NAD+ levels by treatment with NA and NAM precursors has been shown to inhibit metastasis and breast cancer progression in response to mitochondrial complex I defect in mice [89]. However, reducing NAD+ bioavailability is reported to have an antineoplastic effect in various tumor cell types, as cancer cells rely on increased central carbon metabolism and biomass production to sustain an unrestricted growth [28, 103]. The exact role of sirtuins in cancer remains controversial with dichotomous functions being reported, for example multiple studies have shown that SIRT1, SIRT3 and SIRT5 can act as tumor promoters or tumor suppressors under different cellular conditions, tumor stage and tissue of origin [11, 32, 43, 52, 61, 63, 65, 66, 113, 119]. However, SIRT4 is only shown to have a tumor suppressor function [57, 69]. Further research is needed to understand why and how certain sirtuins have both oncogenic or tumor-suppressive roles, and how this dual action may be best exploited for cancer management.
Declining NAD+ levels during aging compromise mitochondrial function in multiple model organisms, which can be restored via NAD+ precursor supplementation or PARP inhibition. For instance, NMN or NR administration in aged mice or worms respectively, reversed mitochondrial dysfunction by restoring NAD+ levels [45, 72, 121]. Moreover, NR administration or PARP inhibition in worms extended lifespan by activating the UPRmt response via Sir-2.1 (worm SIRT1 ortholog) and mitonuclear protein imbalance, which in turn induced a mitohormetic response to improve mitochondrial function (Fig. 3) [55, 72]. Inducing UPRmt genes such as Hsp60 paralogs in Drosophila also prevented mitochondrial and age-dependent muscle dysfunction, thereby promoting longevity [75].
Conclusions and future directions
NAD+ has emerged as a vital oxidoreductase cofactor that regulates metabolism, activates sirtuins and maintains mitochondrial function by enhancing oxidative metabolism to promote healthy aging, and can extend lifespan in worms through the UPRmt stress response pathway. The control of mitochondrial and metabolic homeostasis by an evolutionarily conserved NAD+/sirtuin pathway has opened an exciting new area of research that holds great clinical potential. Based on the current evidence, both NAD+ precursors and PARP inhibitors seem as promising candidates for boosting NAD+ levels in cell culture and animal models. However, there are several key questions that remain unanswered. First, whether different pharmacological, genetic and physiological manipulations that boosts NAD+ production lead to enhanced activity of all sirtuin enzymes or whether only a few family members are activated, especially considering the fact that some sirtuins may have opposing actions? Second, how sirtuins located in different subcellular compartments differ in their enzyme kinetics towards NAD+ availability? Third, what may be the optimal dosages, routes of administration, efficacy and bioavailability of compound drugs that raise intracellular NAD+ levels for human application? Future studies that are directed towards understanding these would be highly relevant in designing therapeutic strategies aimed at selective activation of specific sirtuins, and would also aid in translating the results for human clinical application. It is possible that some of the NAD+ boosting drugs show adverse side effects in humans which could preclude their use and/or may be acceptable for only those inherited conditions that are highly devastating. It is also important to determine if NR could be valid substitute to avoid undesirable side effects of other NAD+ precursors such as NA and NAM, for instance when used as lipid lowering drugs [15, 59]. In addition, future studies are required to examine the UPRmt pathway in vivo in mammalian models to identify key signaling molecules involved in mitochondrial protective mechanisms, which will further advance our understanding of the diseases associated with mitochondrial dysfunction, and will allow discovery of new targets to modulate this pathway. Finally, it remains to be determined whether or not boosting NAD+ levels could extend lifespan in higher organisms. Although much remains to be done, based on the steadily growing evidence, the pharmacological modulation of NAD+ levels via NAD+ precursors and PARP inhibitors appears to be an attractive and valid strategy to enhance oxidative metabolism and mitochondrial biogenesis, and holds a significant therapeutic potential in the clinical management of mitochondrial and age-related disorders.
Abbreviations
- NAD+ :
-
nicotinamide adenine dinucleotide oxidized
- NADH:
-
nicotinamide adenine dinucleotide reduced
- NADP+ :
-
nicotinamide adenine dinucleotide phosphate oxidized
- NADPH:
-
nicotinamide adenine dinucleotide phosphate reduced
- FADH2 :
-
flavin adenine dinucleotide reduced
- mtDNA:
-
mitochondrial DNA
- nDNA:
-
nuclear DNA
- OXPHOS:
-
oxidative phosphorylation
- ETC:
-
electron transport chain
- ATP:
-
adenosine triphosphate
- ADP:
-
adenosine diphosphate
- ROS:
-
reactive oxygen species
- Sir2:
-
silent information regulator 2
- Trp:
-
l-tryptophan
- NA:
-
nicotinic acid
- NAM:
-
nicotinamide
- NR:
-
nicotinamide riboside
- NMN:
-
nicotinamide mononucleotide
- IDO:
-
indoleamine 2,3-dioxygenase
- TDO:
-
tryptophan 2,3-dioxygenase
- ACMS:
-
α-amino-β-carboxymuconate-ε-semialdehyde
- QPRT:
-
quinolinate phosphoribosyl transferase
- NAMN:
-
nicotinic acid mononucleotide
- NAAD:
-
nicotinic acid adenine dinucleotide
- NMNAT:
-
nicotinamide mononucleotide adenylyltransferase
- NAPT:
-
nicotinic acid phosphoribosyltransferase
- NAMPT:
-
nicotinamide phosphoribosyltransferase
- NRK:
-
nicotinamide riboside kinase
- PARPs:
-
poly ADP-ribose polymerases
- cADP:
-
cyclic adenosine diphosphate
- GAPDH:
-
glyceraldehyde 3-phosphate dehydrogenase
- CR:
-
calorie restriction
- AMPK:
-
adenosine monophosphate-activated protein kinase
- PGC-1α:
-
peroxisome proliferator-activated receptor gamma coactivator-1alpha
- FOXO1:
-
forkhead box protein O1
- STACs:
-
SIRT1 activating compounds
- COX:
-
cytochrome c oxidase
- AICAR:
-
5-aminoimidazole-4-carboxamide ribonucleotide
- UPRmt :
-
mitochondrial unfolded protein
- XPA:
-
xeroderma pigmentosum group A
- CSB:
-
Cockayne syndrome group B
References
Alano CC, Tran A, Tao R, Ying W, Karliner JS, Swanson RA (2007) Differences among cell types in NAD(+) compartmentalization: a comparison of neurons, astrocytes, and cardiac myocytes. J Neurosci Res 85:3378–3385. doi:10.1002/jnr.21479
Anderson KA, Green MF, Huynh FK, Wagner GR, Hirschey MD (2014) SnapShot: mammalian sirtuins. Cell 159(956–956):e951. doi:10.1016/j.cell.2014.10.045S0092-8674(14)01372-5
Anderson KA, Hirschey MD (2012) Mitochondrial protein acetylation regulates metabolism. Essays Biochem 52:23–35. doi:10.1042/bse0520023bse0520023
Asher G, Reinke H, Altmeyer M, Gutierrez-Arcelus M, Hottiger MO, Schibler U (2010) Poly(ADP-ribose) polymerase 1 participates in the phase entrainment of circadian clocks to feeding. Cell 142:943–953. doi:10.1016/j.cell.2010.08.016S0092-8674(10)00940-2
Bai P, Canto C, Brunyanszki A, Huber A, Szanto M, Cen Y, Yamamoto H, Houten SM, Kiss B, Oudart H et al (2011) PARP-2 regulates SIRT1 expression and whole-body energy expenditure. Cell Metab 13:450–460. doi:10.1016/j.cmet.2011.03.013S1550-4131(11)00100-8
Bai P, Canto C, Oudart H, Brunyanszki A, Cen Y, Thomas C, Yamamoto H, Huber A, Kiss B, Houtkooper RH et al (2011) PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab 13:461–468. doi:10.1016/j.cmet.2011.03.004S1550-4131(11)00091-X
Banks AS, Kon N, Knight C, Matsumoto M, Gutierrez-Juarez R, Rossetti L, Gu W, Accili D (2008) SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab 8:333–341. doi:10.1016/j.cmet.2008.08.014
Barbosa MT, Soares SM, Novak CM, Sinclair D, Levine JA, Aksoy P, Chini EN (2007) The enzyme CD38 (a NAD glycohydrolase, EC 3.2.2.5) is necessary for the development of diet-induced obesity. FASEB J 21:3629–3639. doi:10.1096/fj.07-8290com
Barile M, Passarella S, Danese G, Quagliariello E (1996) Rat liver mitochondria can synthesize nicotinamide adenine dinucleotide from nicotinamide mononucleotide and ATP via a putative matrix nicotinamide mononucleotide adenylyltransferase. Biochem Mol Biol Int 38:297–306
Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K et al (2006) Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444:337–342. doi:10.1038/nature05354
Bell EL, Emerling BM, Ricoult SJ, Guarente L (2011) SirT3 suppresses hypoxia inducible factor 1alpha and tumor growth by inhibiting mitochondrial ROS production. Oncogene 30:2986–2996. doi:10.1038/onc.2011.37onc201137
Berg JM, Tymoczko JL, Stryer L (2002) Biochemistry, 5th edn. W.H. Freeman, New York
Berger F, Lau C, Dahlmann M, Ziegler M (2005) Subcellular compartmentation and differential catalytic properties of the three human nicotinamide mononucleotide adenylyltransferase isoforms. J Biol Chem 280:36334–36341. doi:10.1074/jbc.M508660200
Berger NA (1985) Poly(ADP-ribose) in the cellular response to DNA damage. Radiat Res 101:4–15
Bogan KL, Brenner C (2008) Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu Rev Nutr 28:115–130. doi:10.1146/annurev.nutr.28.061807.155443
Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, Czopik A, Steele AD, Crowe H, Marmor S, Luo J et al (2007) SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell 6:759–767. doi:10.1111/j.1474-9726.2007.00335.x
Braidy N, Guillemin GJ, Mansour H, Chan-Ling T, Poljak A, Grant R (2011) Age related changes in NAD+ metabolism oxidative stress and Sirt1 activity in wistar rats. PLoS ONE 6:e19194. doi:10.1371/journal.pone.0019194
Brown KD, Maqsood S, Huang JY, Pan Y, Harkcom W, Li W, Sauve A, Verdin E, Jaffrey SR (2014) Activation of SIRT3 by the NAD(+) precursor nicotinamide riboside protects from noise-induced hearing loss. Cell Metab 20:1059–1068. doi:10.1016/j.cmet.2014.11.003S1550-4131(14)00500-2
Canto C, Auwerx J (2009) Caloric restriction, SIRT1 and longevity. Trends Endocrinol Metab 20:325–331. doi:10.1016/j.tem.2009.03.008
Canto C, Auwerx J (2011) NAD+ as a signaling molecule modulating metabolism. Cold Spring Harb Symp Quant Biol 76:291–298. doi:10.1101/sqb.2012.76.010439sqb.2012.76.010439
Canto C, Auwerx J (2012) Targeting sirtuin 1 to improve metabolism: all you need is NAD(+)? Pharmacol Rev 64:166–187. doi:10.1124/pr.110.003905pr.110.003905
Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J (2009) AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458:1056–1060. doi:10.1038/nature07813nature07813
Canto C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, Yamamoto H, Andreux PA, Cettour-Rose P et al (2012) The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab 15:838–847. doi:10.1016/j.cmet.2012.04.022
Canto C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M, Zierath JR, Auwerx J (2010) Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab 11:213–219. doi:10.1016/j.cmet.2010.02.006
Canto C, Menzies KJ, Auwerx J (2015) NAD(+) metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metab 22:31–53. doi:10.1016/j.cmet.2015.05.023
Cerutti R, Pirinen E, Lamperti C, Marchet S, Sauve AA, Li W, Leoni V, Schon EA, Dantzer F, Auwerx J et al (2014) NAD(+)-dependent activation of Sirt1 corrects the phenotype in a mouse model of mitochondrial disease. Cell Metab 19:1042–1049. doi:10.1016/j.cmet.2014.04.001
Chen D, Bruno J, Easlon E, Lin SJ, Cheng HL, Alt FW, Guarente L (2008) Tissue-specific regulation of SIRT1 by calorie restriction. Genes Dev 22:1753–1757. doi:10.1101/gad.1650608gad.1650608
Chiarugi A, Dolle C, Felici R, Ziegler M (2012) The NAD metabolome—a key determinant of cancer cell biology. Nat Rev Cancer 12:741–752. doi:10.1038/nrc3340nrc3340
Chini EN (2009) CD38 as a regulator of cellular NAD: a novel potential pharmacological target for metabolic conditions. Curr Pharm Des 15:57–63
Costford SR, Bajpeyi S, Pasarica M, Albarado DC, Thomas SC, Xie H, Church TS, Jubrias SA, Conley KE, Smith SR (2010) Skeletal muscle NAMPT is induced by exercise in humans. Am J Physiol Endocrinol Metab 298:E117–126. doi:10.1152/ajpendo.00318.2009ajpendo.00318.2009
Cote CD, Rasmussen BA, Duca FA, Zadeh-Tahmasebi M, Baur JA, Daljeet M, Breen DM, Filippi BM, Lam TK (2015) Resveratrol activates duodenal Sirt1 to reverse insulin resistance in rats through a neuronal network. Nat Med 21:498–505. doi:10.1038/nm.3821nm.3821
Deng CX (2009) SIRT1, is it a tumor promoter or tumor suppressor? Int J Biol Sci 5:147–152
DiMauro S, De Vivo DC (1999) Basic neurochemistry: molecular, cellular and medical aspects, 6th edn. Lippincott-Raven, Philadelphia
DiMauro S, Schon EA (2003) Mitochondrial respiratory-chain diseases. N Engl J Med 348:2656–2668. doi:10.1056/NEJMra022567348/26/2656
Donmez G, Wang D, Cohen DE, Guarente L (2010) SIRT1 suppresses beta-amyloid production by activating the alpha-secretase gene ADAM10. Cell 142:320–332. doi:10.1016/j.cell.2010.06.020
Du J, Jiang H, Lin H (2009) Investigating the ADP-ribosyltransferase activity of sirtuins with NAD analogues and 32P-NAD. Biochemistry 48:2878–2890. doi:10.1021/bi802093g10.1021/bi802093g
Du J, Zhou Y, Su X, Yu JJ, Khan S, Jiang H, Kim J, Woo J, Kim JH, Choi BH et al (2011) Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science 334:806–809. doi:10.1126/science.1207861334/6057/806
Elliott G, Rechsteiner M (1975) Pyridine nucleotide metabolism in mitotic cells. J Cell Physiol 86(Suppl 2):641–651
Fang EF, Scheibye-Knudsen M, Brace LE, Kassahun H, SenGupta T, Nilsen H, Mitchell JR, Croteau DL, Bohr VA (2014) Defective mitophagy in XPA via PARP-1 hyperactivation and NAD(+)/SIRT1 reduction. Cell 157:882–896. doi:10.1016/j.cell.2014.03.026S0092-8674(14)00362-6
Feldman JL, Baeza J, Denu JM (2013) Activation of the protein deacetylase SIRT6 by long-chain fatty acids and widespread deacylation by mammalian sirtuins. J Biol Chem 288:31350–31356. doi:10.1074/jbc.C113.511261C113.511261
Fjeld CC, Birdsong WT, Goodman RH (2003) Differential binding of NAD+ and NADH allows the transcriptional corepressor carboxyl-terminal binding protein to serve as a metabolic sensor. Proc Natl Acad Sci U S A 100:9202–9207. doi:10.1073/pnas.16335911001633591100
Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V (2008) Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell 14:661–673. doi:10.1016/j.devcel.2008.02.004S1534-5807(08)00074-9
George J, Nihal M, Singh CK, Zhong W, Liu X, Ahmad N (2016) Pro-proliferative function of mitochondrial sirtuin deacetylase SIRT3 in human melanoma. J Invest Dermatol 136:809–818. doi:10.1016/j.jid.2015.12.026
Glasauer A, Chandel NS (2013) Ros. Curr Biol 23:R100–102. doi:10.1016/j.cub.2012.12.011S0960-9822(12)01451-0
Gomes AP, Price NL, Ling AJ, Moslehi JJ, Montgomery MK, Rajman L, White JP, Teodoro JS, Wrann CD, Hubbard BP et al (2013) Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 155:1624–1638. doi:10.1016/j.cell.2013.11.037
Gong B, Pan Y, Vempati P, Zhao W, Knable L, Ho L, Wang J, Sastre M, Ono K, Sauve AA et al (2013) Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-gamma coactivator 1alpha regulated beta-secretase 1 degradation and mitochondrial gene expression in Alzheimer’s mouse models. Neurobiol Aging 34:1581–1588. doi:10.1016/j.neurobiolaging.2012.12.005S0197-4580(12)00620-3
Haigis MC, Guarente LP (2006) Mammalian sirtuins—emerging roles in physiology, aging, and calorie restriction. Genes Dev 20:2913–2921. doi:10.1101/gad.1467506
Haigis MC, Mostoslavsky R, Haigis KM, Fahie K, Christodoulou DC, Murphy AJ, Valenzuela DM, Yancopoulos GD, Karow M, Blander G et al (2006) SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell 126:941–954. doi:10.1016/j.cell.2006.06.057
Haigis MC, Sinclair DA (2010) Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol 5:253–295. doi:10.1146/annurev.pathol.4.110807.092250
Hall JA, Dominy JE, Lee Y, Puigserver P (2013) The sirtuin family’s role in aging and age-associated pathologies. J Clin Invest 123:973–979. doi:10.1172/JCI6409464094
Hegyi J, Schwartz RA, Hegyi V (2004) Pellagra: dermatitis, dementia, and diarrhea. Int J Dermatol 43:1–5
Herranz D, Serrano M (2010) SIRT1: recent lessons from mouse models. Nat Rev Cancer 10:819–823. doi:10.1038/nrc2962nrc2962
Houtkooper RH, Auwerx J (2012) Exploring the therapeutic space around NAD+. J Cell Biol 199:205–209. doi:10.1083/jcb.201207019jcb.201207019
Houtkooper RH, Canto C, Wanders RJ, Auwerx J (2010) The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocr Rev 31:194–223. doi:10.1210/er.2009-0026er.2009-0026
Houtkooper RH, Mouchiroud L, Ryu D, Moullan N, Katsyuba E, Knott G, Williams RW, Auwerx J (2013) Mitonuclear protein imbalance as a conserved longevity mechanism. Nature 497:451–457. doi:10.1038/nature12188nature12188
Houtkooper RH, Pirinen E, Auwerx J (2012) Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol 13:225–238. doi:10.1038/nrm3293nrm3293
Jeong SM, Xiao C, Finley LW, Lahusen T, Souza AL, Pierce K, Li YH, Wang X, Laurent G, German NJ et al (2013) SIRT4 has tumor-suppressive activity and regulates the cellular metabolic response to DNA damage by inhibiting mitochondrial glutamine metabolism. Cancer Cell 23:450–463. doi:10.1016/j.ccr.2013.02.024S1535-6108(13)00078-0
Julien C, Tremblay C, Emond V, Lebbadi M, Salem N Jr, Bennett DA, Calon F (2009) Sirtuin 1 reduction parallels the accumulation of tau in Alzheimer disease. J Neuropathol Exp Neurol 68:48–58. doi:10.1097/NEN.0b013e3181922348
Kang-Lee YA, McKee RW, Wright SM, Swendseid ME, Jenden DJ, Jope RS (1983) Metabolic effects of nicotinamide administration in rats. J Nutr 113:215–221
Khan NA, Auranen M, Paetau I, Pirinen E, Euro L, Forsstrom S, Pasila L, Velagapudi V, Carroll CJ, Auwerx J et al (2014) Effective treatment of mitochondrial myopathy by nicotinamide riboside, a vitamin B3. EMBO Mol Med 6:721–731. doi:10.1002/emmm.201403943emmm.201403943
Kim JE, Chen J, Lou Z (2008) DBC1 is a negative regulator of SIRT1. Nature 451:583–586. doi:10.1038/nature06500nature06500
Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P et al (2006) Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 127:1109–1122. doi:10.1016/j.cell.2006.11.013
Lai CC, Lin PM, Lin SF, Hsu CH, Lin HC, Hu ML, Hsu CM, Yang MY (2013) Altered expression of SIRT gene family in head and neck squamous cell carcinoma. Tumour Biol 34:1847–1854. doi:10.1007/s13277-013-0726-y
Lin J, Handschin C, Spiegelman BM (2005) Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab 1:361–370. doi:10.1016/j.cmet.2005.05.004
Liu C, Huang Z, Jiang H, Shi F (2014) The sirtuin 3 expression profile is associated with pathological and clinical outcomes in colon cancer patients. Biomed Res Int 2014:871263. doi:10.1155/2014/871263
Lu W, Zuo Y, Feng Y, Zhang M (2014) SIRT5 facilitates cancer cell growth and drug resistance in non-small cell lung cancer. Tumour Biol 35:10699–10705. doi:10.1007/s13277-014-2372-4
Massudi H, Grant R, Braidy N, Guest J, Farnsworth B, Guillemin GJ (2012) Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PLoS ONE 7:e42357. doi:10.1371/journal.pone.0042357PONE-D-12-10133
Meyerhof O (1951) Mechanisms of glycolysis and fermentation. Can J Med Sci 29:63–77
Miyo M, Yamamoto H, Konno M, Colvin H, Nishida N, Koseki J, Kawamoto K, Ogawa H, Hamabe A, Uemura M et al (2015) Tumour-suppressive function of SIRT4 in human colorectal cancer. Br J Cancer 113:492–499. doi:10.1038/bjc.2015.226bjc2015226
Morava E, van den Heuvel L, Hol F, de Vries MC, Hogeveen M, Rodenburg RJ, Smeitink JA (2006) Mitochondrial disease criteria: diagnostic applications in children. Neurology 67:1823–1826. doi:10.1212/01.wnl.0000244435.27645.54
Mouchiroud L, Houtkooper RH, Auwerx J (2013) NAD(+) metabolism: a therapeutic target for age-related metabolic disease. Crit Rev Biochem Mol Biol 48:397–408. doi:10.3109/10409238.2013.789479
Mouchiroud L, Houtkooper RH, Moullan N, Katsyuba E, Ryu D, Canto C, Mottis A, Jo YS, Viswanathan M, Schoonjans K et al (2013) The NAD(+)/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell 154:430–441. doi:10.1016/j.cell.2013.06.016S0092-8674(13)00755-1
Nakagawa T, Lomb DJ, Haigis MC, Guarente L (2009) SIRT5 Deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell 137:560–570. doi:10.1016/j.cell.2009.02.026S0092-8674(09)00202-5
Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P (2009) Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science 324:654–657. doi:10.1126/science.11708031170803
Owusu-Ansah E, Song W, Perrimon N (2013) Muscle mitohormesis promotes longevity via systemic repression of insulin signaling. Cell 155:699–712. doi:10.1016/j.cell.2013.09.021S0092-8674(13)01158-6
Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschop MH (2008) Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci USA 105:9793–9798. doi:10.1073/pnas.0802917105
Pirinen E, Canto C, Jo YS, Morato L, Zhang H, Menzies KJ, Williams EG, Mouchiroud L, Moullan N, Hagberg C et al (2014) Pharmacological Inhibition of poly(ADP-ribose) polymerases improves fitness and mitochondrial function in skeletal muscle. Cell Metab 19:1034–1041. doi:10.1016/j.cmet.2014.04.002S1550-4131(14)00165-X
Pittelli M, Felici R, Pitozzi V, Giovannelli L, Bigagli E, Cialdai F, Romano G, Moroni F, Chiarugi A (2011) Pharmacological effects of exogenous NAD on mitochondrial bioenergetics, DNA repair, and apoptosis. Mol Pharmacol 80:1136–1146. doi:10.1124/mol.111.073916mol.111.073916
Pittelli M, Formentini L, Faraco G, Lapucci A, Rapizzi E, Cialdai F, Romano G, Moneti G, Moroni F, Chiarugi A (2010) Inhibition of nicotinamide phosphoribosyltransferase: cellular bioenergetics reveals a mitochondrial insensitive NAD pool. J Biol Chem 285:34106–34114. doi:10.1074/jbc.M110.136739M110.136739
Pollak N, Dolle C, Ziegler M (2007) The power to reduce: pyridine nucleotides—small molecules with a multitude of functions. Biochem J 402:205–218. doi:10.1042/BJ20061638
Qin W, Yang T, Ho L, Zhao Z, Wang J, Chen L, Zhao W, Thiyagarajan M, MacGrogan D, Rodgers JT et al (2006) Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol Chem 281:21745–21754. doi:10.1074/jbc.M602909200
Ramsey KM, Mills KF, Satoh A, Imai S (2008) Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in beta cell-specific Sirt1-overexpressing (BESTO) mice. Aging Cell 7:78–88. doi:10.1111/j.1474-9726.2007.00355.x
Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C et al (2009) Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science 324:651–654. doi:10.1126/science.11716411171641
Rechsteiner M, Hillyard D, Olivera BM (1976) Turnover at nicotinamide adenine dinucleotide in cultures of human cells. J Cell Physiol 88:207–217. doi:10.1002/jcp.1040880210
Revollo JR, Korner A, Mills KF, Satoh A, Wang T, Garten A, Dasgupta B, Sasaki Y, Wolberger C, Townsend RR et al (2007) Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab 6:363–375. doi:10.1016/j.cmet.2007.09.003
Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, Poirier GG (2010) PARP inhibition: PARP1 and beyond. Nat Rev Cancer 10:293–301. doi:10.1038/nrc2812nrc2812
Ryan MT, Hoogenraad NJ (2007) Mitochondrial-nuclear communications. Annu Rev Biochem 76:701–722. doi:10.1146/annurev.biochem.76.052305.091720
Sack MN, Finkel T (2012) Mitochondrial metabolism, sirtuins, and aging. Cold Spring Harb Perspect Biol 4:a013102. doi:10.1101/cshperspect.a013102a013102
Santidrian AF, Matsuno-Yagi A, Ritland M, Seo BB, LeBoeuf SE, Gay LJ, Yagi T, Felding-Habermann B (2013) Mitochondrial complex I activity and NAD+/NADH balance regulate breast cancer progression. J Clin Invest 123:1068–1081. doi:10.1172/JCI6426464264
Sasaki Y, Araki T, Milbrandt J (2006) Stimulation of nicotinamide adenine dinucleotide biosynthetic pathways delays axonal degeneration after axotomy. J Neurosci 26:8484–8491. doi:10.1523/JNEUROSCI.2320-06.2006
Scarpulla RC (2002) Nuclear activators and coactivators in mammalian mitochondrial biogenesis. Biochim Biophys Acta 1576:1–14.
Schaefer AM, Taylor RW, Turnbull DM, Chinnery PF (2004) The epidemiology of mitochondrial disorders—past, present and future. Biochim Biophys Acta 1659:115–120. doi:10.1016/j.bbabio.2004.09.005
Schapira AH (2006) Mitochondrial disease. Lancet 368:70–82. doi:10.1016/S0140-6736(06)68970-8
Scheibye-Knudsen M, Fang EF, Croteau DL, Bohr VA (2014) Contribution of defective mitophagy to the neurodegeneration in DNA repair-deficient disorders. Autophagy 10:1468–1469. doi:10.4161/auto.2932129321
Schieber M, Chandel NS (2014) ROS function in redox signaling and oxidative stress. Curr Biol 24:R453–462. doi:10.1016/j.cub.2014.03.034S0960-9822(14)00326-1
Schreiber V, Dantzer F, Ame JC, de Murcia G (2006) Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol 7:517–528. doi:10.1038/nrm1963
Sirover MA (1999) New insights into an old protein: the functional diversity of mammalian glyceraldehyde-3-phosphate dehydrogenase. Biochim Biophys Acta 1432:159–184.
Skidmore CJ, Davies MI, Goodwin PM, Halldorsson H, Lewis PJ, Shall S, Zia’ee AA (1979) The involvement of poly(ADP-ribose) polymerase in the degradation of NAD caused by gamma-radiation and N-methyl-N-nitrosourea. Eur J Biochem 101:135–142
Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA (2010) Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 143:802–812. doi:10.1016/j.cell.2010.10.002S0092-8674(10)01138-4
Srivastava S, Barrett JN, Moraes CT (2007) PGC-1alpha/beta upregulation is associated with improved oxidative phosphorylation in cells harboring nonsense mtDNA mutations. Hum Mol Genet 16:993–1005. doi:10.1093/hmg/ddm045
Srivastava S, Diaz F, Iommarini L, Aure K, Lombes A, Moraes CT (2009) PGC-1alpha/beta induced expression partially compensates for respiratory chain defects in cells from patients with mitochondrial disorders. Hum Mol Genet 18:1805–1812. doi:10.1093/hmg/ddp093ddp093
Srivastava S, Haigis MC (2011) Role of sirtuins and calorie restriction in neuroprotection: implications in Alzheimer’s and Parkinson’s diseases. Curr Pharm Des 17:3418–3433.
Tateishi K, Wakimoto H, Iafrate AJ, Tanaka S, Loebel F, Lelic N, Wiederschain D, Bedel O, Deng G, Zhang B et al (2015) Extreme vulnerability of IDH1 mutant cancers to NAD+ depletion. Cancer Cell 28:773–784. doi:10.1016/j.ccell.2015.11.006S1535-6108(15)00427-4
Teng YB, Jing H, Aramsangtienchai P, He B, Khan S, Hu J, Lin H, Hao Q (2015) Efficient demyristoylase activity of SIRT2 revealed by kinetic and structural studies. Sci Rep 5:8529. doi:10.1038/srep08529srep08529
Tischler ME, Friedrichs D, Coll K, Williamson JR (1977) Pyridine nucleotide distributions and enzyme mass action ratios in hepatocytes from fed and starved rats. Arch Biochem Biophys 184:222–236.
Todisco S, Agrimi G, Castegna A, Palmieri F (2006) Identification of the mitochondrial NAD+ transporter in Saccharomyces cerevisiae. J Biol Chem 281:1524–1531. doi:10.1074/jbc.M510425200
Turunc Bayrakdar E, Uyanikgil Y, Kanit L, Koylu E, Yalcin A (2014) Nicotinamide treatment reduces the levels of oxidative stress, apoptosis, and PARP-1 activity in Abeta(1-42)-induced rat model of Alzheimer’s disease. Free Radic Res 48:146–158. doi:10.3109/10715762.2013.857018
van der Horst A, Burgering BM (2007) Stressing the role of FoxO proteins in lifespan and disease. Nat Rev Mol Cell Biol 8:440–450. doi:10.1038/nrm2190
van Roermund CW, Elgersma Y, Singh N, Wanders RJ, Tabak HF (1995) The membrane of peroxisomes in Saccharomyces cerevisiae is impermeable to NAD(H) and acetyl-CoA under in vivo conditions. EMBO J 14:3480–3486
Verdin E, Hirschey MD, Finley LW, Haigis MC (2010) Sirtuin regulation of mitochondria: energy production, apoptosis, and signaling. Trends Biochem Sci 35:669–675. doi:10.1016/j.tibs.2010.07.003S0968-0004(10)00135-0
Viscomi C, Bottani E, Civiletto G, Cerutti R, Moggio M, Fagiolari G, Schon EA, Lamperti C, Zeviani M (2011) In vivo correction of COX deficiency by activation of the AMPK/PGC-1alpha axis. Cell Metab 14:80–90. doi:10.1016/j.cmet.2011.04.011S1550-4131(11)00211-7
Wallace DC (2005) A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet 39:359–407. doi:10.1146/annurev.genet.39.110304.095751
Wang RH, Sengupta K, Li C, Kim HS, Cao L, Xiao C, Kim S, Xu X, Zheng Y, Chilton B et al (2008) Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer Cell 14:312–323. doi:10.1016/j.ccr.2008.09.001S1535-6108(08)00294-8
Yamamoto H, Schoonjans K, Auwerx J (2007) Sirtuin functions in health and disease. Mol Endocrinol 21:1745–1755. doi:10.1210/me.2007-0079
Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, Lamming DW, Souza-Pinto NC, Bohr VA, Rosenzweig A et al (2007) Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell 130:1095–1107. doi:10.1016/j.cell.2007.07.035
Yang L, Zhang J, Xing W, Zhang X, Xu J, Zhang H, Chen L, Ning X, Ji G, Li J et al (2016) SIRT3 deficiency induces endothelial insulin resistance and blunts endothelial-dependent vasorelaxation in mice and human with obesity. Sci Rep 6:23366. doi:10.1038/srep23366srep23366
Yang W, Zou Y, Zhang M, Zhao N, Tian Q, Gu M, Liu W, Shi R, Lu Y, Yu W (2015) Mitochondrial Sirt3 expression is decreased in APP/PS1 double transgenic mouse model of Alzheimer’s disease. Neurochem Res 40:1576–1582. doi:10.1007/s11064-015-1630-1
Yoshino J, Mills KF, Yoon MJ, Imai S (2011) Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab 14:528–536. doi:10.1016/j.cmet.2011.08.014S1550-4131(11)00346-9
Yu W, Denu RA, Krautkramer KA, Grindle KM, Yang DT, Asimakopoulos F, Hematti P, Denu JM (2016) Loss of SIRT3 provides growth advantage for B cell malignancies. J Biol Chem 291:3268–3279. doi:10.1074/jbc.M115.702076M115.702076
Zeng L, Yang Y, Hu Y, Sun Y, Du Z, Xie Z, Zhou T, Kong W (2014) Age-related decrease in the mitochondrial sirtuin deacetylase Sirt3 expression associated with ROS accumulation in the auditory cortex of the mimetic aging rat model. PLoS ONE 9:e88019. doi:10.1371/journal.pone.0088019PONE-D-13-39205
Zhang H, Ryu D, Wu Y, Gariani K, Wang X, Luan P, D’Amico D, Ropelle ER, Lutolf MP, Aebersold R et al (2016) NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. doi:10.1126/science.aaf2693
Zhang T, Berrocal JG, Frizzell KM, Gamble MJ, DuMond ME, Krishnakumar R, Yang T, Sauve AA, Kraus WL (2009) Enzymes in the NAD+ salvage pathway regulate SIRT1 activity at target gene promoters. J Biol Chem 284:20408–20417. doi:10.1074/jbc.M109.016469M109.016469
Zhu Y, Yan Y, Principe DR, Zou X, Vassilopoulos A, Gius D (2014) SIRT3 and SIRT4 are mitochondrial tumor suppressor proteins that connect mitochondrial metabolism and carcinogenesis. Cancer Metab 2:15. doi:10.1186/2049-3002-2-152049-3002-2-15
Acknowledgements
This work was supported by Virginia Tech open access subvention fund.
Competing interests
The author declares that she has no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Srivastava, S. Emerging therapeutic roles for NAD+ metabolism in mitochondrial and age-related disorders. Clin Trans Med 5, 25 (2016). https://doi.org/10.1186/s40169-016-0104-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40169-016-0104-7