Abstract
Purpose
The purpose of this systematic review is to discuss recent studies and ongoing trials of nab-paclitaxel in breast cancer and to examine the potential role of nab-paclitaxel as a backbone for immuno-oncology therapies.
Methods
PubMed and selected congress proceedings were searched for studies of nab-paclitaxel in breast cancer published between 2013 and 2015. All phase II and III clinical trials, retrospective analyses, and institutional studies were included. Active, ongoing, phase II or III trials on nab-paclitaxel that were listed on ClinicalTrials.gov were also included.
Results
Sixty-three studies, including 23 in early-stage and 30 in metastatic breast cancer (some studies not classifiable by setting), were included in this analysis. Trials of neoadjuvant nab-paclitaxel–containing regimens have reported pathological complete response rates ranging from 5.7 to 53%. Median overall survival in metastatic breast cancer studies ranged from 10.8 to 23.5 months, depending on dose and regimen. Adverse event profiles of nab-paclitaxel were generally similar to those reported from previous studies. Several ongoing trials are evaluating nab-paclitaxel in the early-stage and metastatic settings, including in combination with immuno-oncology agents.
Conclusions
nab-Paclitaxel continues to demonstrate promising efficacy in breast cancer. Recent studies demonstrate high pathological complete response rates in early-stage breast cancer, particularly in triple-negative breast cancer, an area of high unmet need, and encouraging overall survival in metastatic breast cancer across doses and schedules. Ongoing trials will provide further insights into the role of nab-paclitaxel in breast cancer including use as a potential backbone chemotherapy agent for immuno-oncology therapies such as checkpoint inhibitors.
Similar content being viewed by others
Background
Breast cancer remains the most commonly diagnosed cancer among women in the United States and worldwide [https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf, http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-044738.pdf]. Globocan estimated that 1.7 million new cases of breast cancer were diagnosed and that more than half a million women died from breast cancer in 2012 [http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-044738.pdf]. The majority of patients (61%) present with localized disease [http://seer.cancer.gov/statfacts/html/breast.html]. Regional disease is diagnosed in 32% of patients, and 6% present with distant metastatic disease [http://seer.cancer.gov/statfacts/html/breast.html]. The overall 5-year survival for all stages combined is 89% [http://seer.cancer.gov/statfacts/html/breast.html]. However, survival rates vary by stage. Localized disease is associated with a 5-year survival rate of 99%, regional disease is associated with a 5-year survival rate of 85%, and metastatic disease is associated with a 5-year survival rate of 26% [http://seer.cancer.gov/statfacts/html/breast.html].
Treatments for localized breast cancer consist of surgical resection with or without radiation therapy [1]. Neoadjuvant chemotherapy is recommended for large tumors (stage IIA-B or T3N1M0). The primary approach for managing metastatic breast cancer (MBC) is systemic therapy, consisting of cytotoxic chemotherapy, endocrine therapy for hormone receptor–positive disease, and HER2-targeted agents for HER2-positive cancers. Many of the neoadjuvant, adjuvant, and metastatic chemotherapy regimens preferred by the National Comprehensive Cancer Network include paclitaxel [1]. One disadvantage of paclitaxel is the development of hypersensitivity reactions to the solvent, Kolliphor EL (formerly called Cremophor EL) [2]. Nanoparticle albumin-bound paclitaxel (nab ®-paclitaxel, Celgene Corporation, Summit, NJ) is solvent-free, minimizing hypersensitivity reactions and potentially other solvent-related toxicities, such as neutropenia [3,4,5]. Due to minimal risk of hypersensitivity, premedication with prophylactic steroids is not required [3, 4]. Another advantage of nab-paclitaxel vs standard paclitaxel is the increased rate of transport across endothelial cell layers, greater and faster tissue penetration, and slower elimination of paclitaxel [6,7,8]. nab-Paclitaxel also demonstrates increased intratumoral delivery and retention, resulting in 33% higher intratumoral drug concentrations [6]. Compared with paclitaxel, nab-paclitaxel yields a 10-fold higher mean maximal concentration of free paclitaxel [8].
nab-Paclitaxel is currently approved for locally advanced or metastatic non-small cell lung cancer, metastatic pancreatic cancer, and MBC that has progressed on combination chemotherapy or relapsed within 6 months of adjuvant chemotherapy [2]. The approval in MBC was based on a randomized phase III trial of nab-paclitaxel 260 mg/m2 vs paclitaxel 175 mg/m2 every 3 weeks (q3w). nab-Paclitaxel demonstrated a significantly higher overall response rate (ORR; 33 vs 19%; P = 0.001) and longer time to tumor progression (23 vs 17 weeks; hazard ratio [HR], 0.75; P = 0.006) vs paclitaxel in the intention-to-treat (ITT) population [5]. Overall survival (OS) was not significantly different between the 2 treatment groups in the ITT population; however, in the second- or later-line setting, OS was significantly longer for nab-paclitaxel vs paclitaxel (median, 56 vs 47 weeks; HR, 0.73; P = 0.024). Significantly less grade 4 neutropenia (9 vs 22%; P < 0.001) and more grade 3 sensory neuropathy (10 vs 2%; P < 0.001) were reported with nab-paclitaxel [5]. Grade 3 sensory neuropathy in patients who received nab-paclitaxel improved to a lower grade after a median of 22 days of treatment interruption.
Recent studies have examined nab-paclitaxel in early-stage breast cancer, primarily as a neoadjuvant agent. Studies also continue to evaluate the efficacy of nab-paclitaxel in MBC in combination and across doses and schedules. This review summarizes data from recent studies of nab-paclitaxel across breast cancer settings, discusses ongoing trials, and provides perspectives on the future role of nab-paclitaxel in breast cancer.
Methods
PRISMA guidelines were followed in this systematic review. PubMed was searched for articles published between January 1, 2013 and February 28, 2016. Abstracts from the American Society for Clinical Oncology (ASCO) annual meeting and the ASCO Breast Cancer Symposium 2013–2015 were included. The entry terms for the search were “nab-paclitaxel” and “breast.” Abstracts from the 2014 European Breast Cancer Conference and the 2013–2015 San Antonio Breast Cancer Symposium proceedings were searched using the term “nab-paclitaxel.” Phase II and III clinical trials, retrospective analyses, and institutional studies were included. Duplicate studies, topic reviews, case studies, nonhuman or preclinical studies, and non-English articles were excluded. One article in PubMed was embargoed and inaccessible.
Results
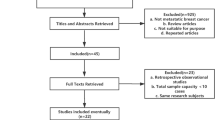
The publication selection process is depicted in Fig. 1. Twenty-three studies of nab-paclitaxel in early-stage breast cancer were retrieved, including 21 neoadjuvant and 2 adjuvant studies. Three post hoc analyses of previous neoadjuvant trials were included in the 21 retrieved neoadjuvant studies. Studies of neoadjuvant nab-paclitaxel in early-stage breast cancer are presented in Table 1. There were also 30 studies of nab-paclitaxel in MBC, including 3 health economic analyses. Studies of nab-paclitaxel in MBC that reported progression-free survival (PFS) or OS are presented in Table 2. Only studies with ≥50 patients are detailed in the text; however, all reports on early-stage HER2+ disease were included because of the small number of studies.
Studies of nab-paclitaxel in early-stage breast cancer
Unselected (all subtypes)
Among 7 phase II and III studies of neoadjuvant nab-paclitaxel (majority administered weekly) that did not select for specific disease subtype, the pathological complete response (pCR) rate ranged from 22 to 40%; 4 phase II studies included <50 patients (Table 1).
A trend toward benefit for stage II disease was observed with nab-paclitaxel 125 mg/m2 once weekly (qw) plus carboplatin vs paclitaxel 80 mg/m2 qw plus carboplatin in a phase II trial with a pCR rate of 36.8 vs 15.8% (odds ratio [OR], 3.11; 95% CI 0.963–10.053; P = 0.051); however, no such trend was observed in the overall population [9]. More grade 4 neutropenia was observed with nab-paclitaxel than with paclitaxel (56.7 vs 21.1%; P < 0.001).
A phase II trial of nab-paclitaxel 260 mg/m2 q3w and cyclophosphamide 600 mg/m2 followed by fluorouracil, epirubicin, and cyclophosphamide (FEC) q3w for operable breast cancer resulted in a pCR rate of 37% (95% CI 24–50%) [10]. Hormone receptor–positive/HER2-negative tumors demonstrated the lowest pCR rate (8%), whereas all other molecular subgroups had pCR rates ranging from 56 to 63%. Hormone receptor negativity (HR, 11.9; 95% CI 2.8–52.6; P = 0.001) and HER2 positivity (HR, 6.8; 95% CI 1.5–32.0; P = 0.015) were independent predictors of pCR.
The large phase III GeparSepto trial compared neoadjuvant paclitaxel 80 mg/m2 qw vs nab-paclitaxel 125 mg/m2 (150 mg/m2 before amendment) qw followed by epirubicin and cyclophosphamide (EC) for early-stage breast cancer, with trastuzumab plus pertuzumab added for HER2-positive cancers [11]. The original dose of nab-paclitaxel (150 mg/m2) was amended to 125 mg/m2 due to the frequency of treatment discontinuations and sensory neuropathy. Overall, patients achieved a significantly higher pCR rate with nab-paclitaxel vs paclitaxel (38.4 vs 29.0%; P < 0.001). The higher pCR rate for nab-paclitaxel vs paclitaxel was maintained in the set of patients who received treatment after the nab-paclitaxel dose amendment (41.4 vs 32.4%; P = 0.013). The largest difference between treatment arms was observed in the triple-negative breast cancer (TNBC) subgroup, with nab-paclitaxel achieving a pCR rate of 48.2 vs 26.3% with paclitaxel (P < 0.001).
The pCR rates in 4 phase II neoadjuvant studies of nab-paclitaxel for the treatment of early-stage breast cancer of unselected subtype ranged from 22 to 40%, with 71 to 77.5% of patients having breast-conserving surgery [9,10,11,12].
HER2-negative or TNBC
Nine studies of nab-paclitaxel in early-stage HER2-negative breast cancer or TNBC were retrieved. The overall pCR rate ranged from 5.7 to 53% (Table 1). Most of these were combination studies.
The phase II Nabrax GEICAM study of neoadjuvant nab-paclitaxel 150 mg/m2 the first 3 of 4 weeks (qw 3/4) in patients with ER-positive, HER2-negative breast cancer demonstrated an ORR of 76.5% [13]. A residual cancer burden (RCB) score of 0 + 1 was achieved by 24.7% of the treated population (n = 81), and the rate of conversion to breast-conserving surgery was 40%.
The phase II SWOG S0800 trial evaluated the backbone neoadjuvant regimen of nab-paclitaxel 100 mg/m2 qw with dose-dense doxorubicin plus cyclophosphamide (AC) ± bevacizumab for the treatment of HER2-negative locally advanced or inflammatory breast cancer [14]. The overall pCR rate was 28%, with a significantly higher pCR rate reported in the bevacizumab vs non-bevacizumab arm (36 vs 21%; P = 0.021). In hormone receptor–positive disease, the difference in pCR rate between bevacizumab and no bevacizumab was not significant (25 vs 18%; P = 0.41). However, patients with hormone receptor–negative tumors demonstrated a significantly improved pCR rate with bevacizumab (59 vs 28%; P = 0.014). In addition, a significantly improved pCR rate was achieved with bevacizumab vs no bevacizumab in the locally advanced breast cancer group (37 vs 22%; P = 0.035).
Another trial that evaluated a nab-paclitaxel–containing backbone regimen was the phase II TBCRC 008 study, which compared 12 weeks of neoadjuvant carboplatin, nab-paclitaxel 100 mg/m2 qw, and vorinostat vs carboplatin, nab-paclitaxel 100 mg/m2 qw, and placebo for operable, stage II–III, HER2-negative breast cancer [15]. Similar pCR rates were reported for both arms (vorinostat, 25.8% vs placebo, 29.0%). The pCR rates in patients with TNBC were 41.7% with vorinostat and 58.3% with placebo.
The phase II ADAPT trial was designed as an umbrella trial in which patients with early-stage breast cancer had 2 sequential core biopsies during neoadjuvant therapy (baseline and 3 weeks after treatment initiation) to assess early biomarker changes and guide adjuvant therapy selection [16]. Patients were assigned to 1 of 4 subtrials based on molecular subtyping. One subtrial was the ADAPT-TN trial, which evaluated a backbone of neoadjuvant nab-paclitaxel 125 mg/m2 given the first 2 of 3 weeks (qw 2/3) with either carboplatin or gemcitabine in patients with TNBC [17, 18]. The pCR rate differed significantly between arms (carboplatin, 47.4% vs gemcitabine, 29.7%; P = 0.0045). Patients who received nab-paclitaxel plus gemcitabine vs nab-paclitaxel plus carboplatin experienced a significantly higher frequency of dose reductions (20.6 vs 11.9%; P = 0.03), treatment-related severe adverse events (13 vs 5%; P = 0.02), grade 3–4 infections (6.1 vs 1.3%; P = 0.04), and alanine aminotransferase elevations (11.7 vs 3.3%; P = 0.01).
Docetaxel 75 mg/m2 q3w was compared with nab-paclitaxel 100 mg/m2 qw 3/4 followed by FEC with epirubicin at 100 mg/m2 for stage II–III, HER2-negative breast cancer [19]. The overall pCR rate was 17%, with a higher pCR rate of 30% in patients with TNBC. Another trial that evaluated sequential nab-paclitaxel 260 mg/m2 q3w and EC demonstrated a pCR rate of 5.7% in patients with stage II–III HER2-negative breast cancer (N = 53) [20].
HER2-positive
Three studies of nab-paclitaxel in early-stage HER2-positive breast cancer demonstrated highly consistent pCR rates, ranging from 45 to 49% (Table 1). Neoadjuvant carboplatin, nab-paclitaxel, and trastuzumab treatment in patients with stage II–III HER2-positive tumors resulted in a pCR rate of 45% in 55 evaluable patients, and a pCR plus RCB I rate of 50% in the ITT population [21]. A pCR rate of 52% was achieved in patients with ER-negative disease compared with a pCR rate of 40% in those with ER-positive disease. A phase II trial of neoadjuvant anthracycline followed by nab-paclitaxel 260 mg/m2 q3w plus trastuzumab for operable HER2-positive breast cancer reported a pCR rate of 49% in the treated population [22]. Subgroup analysis demonstrated a higher pCR rate of 71% in patients with ER-negative tumors vs 36% in those with ER-positive disease. Similar results were achieved with neoadjuvant nab-paclitaxel 260 mg/m2 every 2 weeks (q2w) followed by vinorelbine and trastuzumab for stage I–III HER2-positive breast cancer [23]. An overall pCR rate of 48.1% was reported, with a pCR rate of 68.8% in the hormone receptor–negative population and a pCR rate of 18.2% in the hormone receptor–positive population (n = 11).
Post hoc analyses
Recent post hoc biomarker analyses were reported for the Brown University Oncology Group trials BR-211A (NCT00723125), which evaluated bevacizumab, nab-paclitaxel, and carboplatin in stage II–III HER2-negative breast cancer, and BR-211B (NCT00617942), which examined trastuzumab, nab-paclitaxel, and carboplatin in stage II–III HER2-positive breast cancer [24,25,26]. Among patients with HER2-negative early-stage breast cancer in BR-211A, a strong correlation was found between triple-negative status and pCR rate after treatment with bevacizumab, nab-paclitaxel, and carboplatin (P < 0.001) [25]. In the BR-211B trial, the pCR rate was 50% among 20 patients for whom evaluable tissues and pCR data were available, and a strong correlation was reported for high baseline HER2 and pCR (P = 0.002) [24]. In addition, higher baseline levels of stromal tumor-infiltrating lymphocytes (sTILs) (median, 35 vs 25%; P = 0.018) were found in patients with HER2-positive tumors who achieved RCB 0 + 1 (defined as responders) vs RCB 2 + 3 (defined as nonresponders), respectively [26].
Adjuvant treatment with nab-paclitaxel
Two studies of adjuvant nab-paclitaxel in early-stage disease were retrieved. The combination of nab-paclitaxel 100 mg/m2 and capecitabine was compared with EC or cyclophosphamide, methotrexate, and fluorouracil (CMF) as adjuvant therapy for early-stage breast cancer in nonfrail elderly patients (age ≥65 years) in the phase II ICE II-GBG 52 trial [27]. After a median follow-up of almost 23 months, no significant difference in OS was observed between treatment arms (HR, 1.18; 95% CI 0.52–2.66). A greater percentage of patients experienced treatment discontinuations with nab-paclitaxel plus capecitabine than EC/CMF (35.8 vs 6.6%; P < 0.001). In both arms, the main reasons for discontinuation were adverse events followed by investigator or patient decision; however, grade ≥3 toxicities were less frequent with nab-paclitaxel plus capecitabine than EC/CMF (64.8 vs 90.9%; P < 0.001). Grade ≥3 hematologic events were less common (22.3 vs 88.4%; P < 0.001) and grade ≥3 nonhematologic events were more common (58.5 vs 18.7%; P < 0.001) with nab-paclitaxel plus capecitabine than with EC/CMF. The authors suggested that tolerability might have been better if a lower capecitabine dose were used in the nab-paclitaxel plus capecitabine arm.
Ongoing trials in early-stage breast cancer
The ongoing phase III GAIN-2 study (NCT01690702; planned N = 2886) compares nab-paclitaxel on a less common dose-dense schedule (330 mg/m2 q2w) plus EC vs EC followed by docetaxel using invasive disease-free survival as the primary endpoint (https://clinicaltrials.gov/ct2/show/NCT01690702, [28]). Among the 1473 patients who have been randomized, those in the nab-paclitaxel arm demonstrated higher rates of grade ≥3 febrile neutropenia (12 vs 8%) and peripheral sensory neuropathy (83 vs 68%) and required more dose reductions due to hematologic toxicities (30 vs 10%; P < 0.001).
The ETNA trial, another ongoing phase III study (NCT01822314; planned N = 632), is evaluating single-agent nab-paclitaxel 125 mg/m2 qw 3/4 vs paclitaxel 90 mg/m2 qw 3/4 as neoadjuvant therapy for high-risk HER2-negative breast cancer [https://clinicaltrials.gov/ct2/show/NCT01822314]. In each arm, patients will receive AC, EC, or FEC after initial taxane therapy. The primary endpoint is pCR.
Another ongoing phase III trial (Nordic trip, N = 1800), will provide additional information about the potential benefit of nab-paclitaxel plus EC as a treatment for early TNBC [29]. Patients will be randomized to receive adjuvant or neoadjuvant treatment in 1 of 3 arms: nab-paclitaxel followed by EC, nab-paclitaxel plus capecitabine followed by EC plus capecitabine, or nab-paclitaxel plus carboplatin followed by EC. The primary endpoint is invasive disease-free survival.
Studies of nab-paclitaxel in MBC
Unselected (all subtypes)
Four studies of nab-paclitaxel in MBC of unselected subtype reported median OS ranging from 10.8 months with nab-paclitaxel 260 mg/m2 q3w to 26.9 months with nab-paclitaxel 125 mg/m2 qw 3/4 combined with cisplatin (Table 2).
The phase III CALGB 40502 trial evaluated first-line bevacizumab combined with paclitaxel 90 mg/m2, nab-paclitaxel 150 mg/m2, or ixabepilone 16 mg/m2 qw 3/4 for locally recurrent or metastatic breast cancer [30]. A protocol amendment made the use of bevacizumab optional; however, 97% of patients had already received bevacizumab at that time. The majority (93%) of patients had HER2-negative disease. Median PFS (primary endpoint) was 11 months for the paclitaxel arm vs 9.3 months with nab-paclitaxel (HR, 1.20; 95% CI 1.00–1.45; P = 0.054) and 7.4 months with ixabepilone (HR, 1.59; 95% CI 1.31–1.93; P < 0.001). Median OS significantly differed between ixabepilone and paclitaxel (23.6 vs 27.4 months, respectively; HR, 1.31; 95% CI 1.03–1.66; P = 0.027), but not between nab-paclitaxel and paclitaxel (23.5 vs 26.5 months, respectively; HR, 1.17; 95% CI 0.92–1.47; P = 0.20). Grade ≥3 nonhematologic toxicities were more common in the nab-paclitaxel vs paclitaxel arm (65 vs 49%; P < 0.001), with grade ≥2 sensory neuropathy affecting more patients treated with nab-paclitaxel vs paclitaxel (54 vs 46%; P = 0.031). Compared with paclitaxel, nab-paclitaxel demonstrated worse hematologic and nonhematologic toxicity (both, P < 0.001). Ixabepilone caused less hematologic toxicity (P = 0.004) but not significantly different nonhematologic toxicity (P = 0.14) than paclitaxel. Dose reductions occurred more frequently and earlier in the nab-paclitaxel arm: by cycle 3, 45% of patients receiving nab-paclitaxel had undergone a dose reduction compared with 15% of those receiving paclitaxel and 15% of those receiving ixabepilone. Only 28% of patients received full-dose nab-paclitaxel at cycle 5 vs 76% of patients receiving paclitaxel and 65% receiving ixabepilone.
A phase II trial of nab-paclitaxel 125 mg/m2 qw 3/4 and cisplatin for MBC reported an ORR (primary endpoint) of 67.1%, with an 80.6% ORR in the first-line setting and an 80% ORR for those who were not previously treated with taxanes [31]. The median PFS was 9.8 months (95% CI 8.1–11.6 months) by investigator assessment, and median OS was 26.9 months. Compared with patients who were pretreated with taxanes, those who had not previously received taxanes demonstrated longer PFS (median, 8.5 vs 11.2 months; P = 0.009) by investigator assessment and longer OS (median, not reached vs 16.7 months; P < 0.001). There were no significant differences in PFS according to molecular subtype. Treatment was well tolerated in most patients, with neutropenia being the most common cause for dose adjustment. Grade ≥3 neutropenia was the most common hematologic adverse event, affecting 84.9% of patients.
A randomized phase II/III trial of women with refractory MBC compared nab-paclitaxel 260 mg/m2 q3w with paclitaxel concentrate for nanodispersion (PICN) 260 or 295 mg/m2 q3w [32]. Comparing the equal-dose regimens of nab-paclitaxel and PICN, ORR and PFS were numerically greater for nab-paclitaxel vs PICN (ORR, 43 vs 35%; PFS, median, 7.8 vs 5.3 months [P not significant for either]). ORR and PFS were not significantly different for higher-dose PICN vs lower-dose PICN or vs nab-paclitaxel. The lower-dose PICN arm had lower rates of grade ≥3 adverse events compared with the higher-dose PICN arm and the nab-paclitaxel arm.
A number of regional retrospective analyses have also been conducted recently on the use of nab-paclitaxel for the treatment of unselected MBC. A retrospective analysis of patients with breast cancer in British Columbia who received nab-paclitaxel from 2007 to 2011 was performed [33]. Most patients had metastatic disease, and 2 had regional relapse only. Approximately one-fourth of patients had prior taxane exposure. Time to relapse was significantly shorter in patients with prior exposure to adjuvant taxanes vs those without (median, 2.7 vs 4.5 years, P < 0.001). No significant differences in time to treatment failure (defined as time from first to last cycle of nab-paclitaxel) or dose reduction rates were found between these 2 groups. Thus, nab-paclitaxel may result in clinical benefit in patients with MBC regardless of whether they have had prior taxane exposure. A retrospective German survey of national chemotherapy practices demonstrated that nab-paclitaxel was used less frequently than paclitaxel and docetaxel for first-line treatment of MBC [34]. However, retrospective analysis of a US claims database of patients with MBC who were treated with taxanes (n = 2599 docetaxel; n = 1643 paclitaxel; n = 261 nab-paclitaxel) demonstrated that patients remained on nab-paclitaxel 50% longer than on other taxanes (127 vs 85 days; P < 0.05), possibly due to lower incidence of discontinuation for neutropenia with nab-paclitaxel vs docetaxel or paclitaxel (6.9% vs docetaxel, 29.4% or paclitaxel, 17.5%; P < 0.001) [35]. A separate analysis of a US claims database revealed that nab-paclitaxel was most often administered as second- or later-line therapy, as monotherapy, or on a weekly schedule [36].
HER2-negative or TNBC
A phase II trial of nab-paclitaxel 100 mg/m2 qw 3/4 with or without anti–death receptor 5 monoclonal antibody tigatuzumab (10 mg/kg loading, 5 mg/kg q2w) was performed in patients with metastatic TNBC [37]. ORR was 28% in the combination arm vs 38% in the nab-paclitaxel–alone arm. PFS was not significantly different between arms (median, 3.7 months for nab-paclitaxel monotherapy vs 2.8 months for the combination; P = 0.3152). Five patients in the combination arm demonstrated long-term PFS (334–1025+ days) compared with 1 patient in the nab-paclitaxel arm (1004+ days).
There was one study of nab-paclitaxel in patients with HER2-negative MBC (unselected for hormone receptor status) that reported median PFS [38]. None of the studies on HER2-negative breast cancer or TNBC reported OS.
A prospective trial of second-line nab-paclitaxel 260 mg/m2 q3w in patients with HER2-negative MBC with prior taxane exposure reported an ORR of 48% (95% CI 31.5–61.3%) and a median PFS of 8.9 months (95% CI 8.0–11.6 months; range, 5–21+ months) [38]. Response rate by subgroup demonstrated a higher response in TNBC (68.8%) vs other subgroups (ER+/PR−, 55.6%; ER−/PR+, 50.0%; ER+/PR+, 32.0%). Median OS was not reached.
Health economic analyses
Three cost-effectiveness analyses compared nab-paclitaxel with other taxanes in patients with MBC.
The Spanish COSTABRAX cost-effectiveness analysis demonstrated that nab-paclitaxel q3w was cost-effective compared with paclitaxel q3w as a second-line treatment for MBC [39]. Efficacy data from the phase III CA012 trial were used in a Markov model expanded to a time horizon of 5 years. The cost of life-year gained with nab-paclitaxel q3w vs paclitaxel q3w was €11,088, and the cost per quality-adjusted life-year (QALY) gained was €17,808. Compared with paclitaxel qw, nab-paclitaxel q3w showed a savings of €711 per patient. Another study combined cost data in China with a meta-analysis of 10 randomized phase III trials of nab-paclitaxel 260 mg/m2 q3w or docetaxel 100 mg/m2 q3w in comparison with paclitaxel 175 mg/m2 q3w [40]. The cost per course of treatment was $19,752 for nab-paclitaxel, $8940 for paclitaxel, and $13,741 for docetaxel. The cost per QALY gained for nab-paclitaxel vs docetaxel as alternatives to paclitaxel was $57,900 vs $130,600. Thus, nab-paclitaxel appeared to be a more cost-effective alternative to docetaxel as initial therapy for MBC in a Chinese healthcare setting. The Italian COSTANza study, which used a Markov model, also suggested that nab-paclitaxel was cost-effective, with a gain of 0.165 QALY compared with paclitaxel [41].
A recent questionnaire-based study of healthcare providers (N = 22) in Sweden indicated that mean infusion times for nab-paclitaxel vs paclitaxel were 42.1 ± 20.7 vs 104.3 ± 43.3 min, respectively [42]. Total patient times in clinic per infusion were 82.2 ± 40.9 and 183.9 ± 34.8 min, respectively. The study suggested that the corresponding time required for a 12-week treatment of nab-paclitaxel q3w, nab-paclitaxel qw, and paclitaxel qw would be 2.8, 8.4, and 20.9 h, respectively. The corresponding time for patient hospital visits would be 5.5, 16.4, and 36.8 h, respectively. Thus, nab-paclitaxel may require less time for drug administration, potentially reducing cost.
Ongoing trials in MBC
Table 3 lists selected ongoing trials of nab-paclitaxel regimens for the treatment of MBC. There are several ongoing trials evaluating combinations of nab-paclitaxel with HER2-targeted agents for the treatment of metastatic HER2-positive breast cancer. The single-arm phase III PERUSE trial (NCT01572038; planned N = 1500) is evaluating the safety of first-line pertuzumab combined with trastuzumab and a taxane of the investigator’s choice, as its primary endpoint, in patients with metastatic or locally recurrent HER2-positive breast cancer [https://clinicaltrials.gov/ct2/show/NCT01572038]. Secondary endpoints include PFS, OS, ORR, and quality of life. Interim safety results indicated that grade ≥3 adverse events occurred in 53.4% of patients in the docetaxel group (n = 320), 41.1% in the paclitaxel group (n = 331), and 26.7% in the nab-paclitaxel group (n = 45) [43]. The most common grade ≥3 toxicities included neutropenia (approximately 12, 6, and 2% in docetaxel, paclitaxel, and nab-paclitaxel groups, respectively) and diarrhea (approximately 9, 6, and 6%). The phase IIIb SAPPHIRE trial (NCT02019277; N = 50) is evaluating the safety and efficacy of trastuzumab combined with intravenous pertuzumab and a taxane of investigator’s choice in patients with metastatic HER2-positive breast cancer [44, 45]. Interim results showed that 50 patients had been enrolled, with the majority (72%) receiving nab-paclitaxel as the taxane of choice. Grade ≥3 adverse events were reported in 52% of patients, although toxicities were not categorized according to type of taxane received. In addition, the phase I/II STELA trial (NCT02073916; planned N = 45) will combine trastuzumab emtansine (T-DM1), nab-paclitaxel, and lapatinib to treat metastatic HER2-positive MBC (https://clinicaltrials.gov/ct2/show/NCT02073916, [46]).
A number of ongoing trials are also evaluating nab-paclitaxel in HER2-negative MBC. The phase II/III tnAcity trial (NCT01881230) is comparing nab-paclitaxel plus gemcitabine with nab-paclitaxel plus carboplatin as first-line treatment for metastatic TNBC. The phase II portion (N = 240) has 3 arms: nab-paclitaxel 125 mg/m2 plus gemcitabine 1000 mg/m2 on days 1 and 8 q3w, nab-paclitaxel 125 mg/m2 plus carboplatin area under the curve (AUC) of 2 days 1 and 8 q3w, and gemcitabine 1000 mg/m2 plus carboplatin AUC of 2 days 1 and 8 q3w (https://clinicaltrials.gov/ct2/show/NCT01881230, [47]). In the phase III portion (N = 550), the nab-paclitaxel plus gemcitabine or nab-paclitaxel plus carboplatin arm will be selected based on phase II trial results and compared with gemcitabine 1000 mg/m2 plus carboplatin AUC of 2 q3w. The phase II SNAP trial (NCT01746225; planned N = 258) will evaluate different schedules of first-line nab-paclitaxel for the treatment of HER2-negative MBC (https://clinicaltrials.gov/ct2/show/NCT01746225, [48]). All patients will receive induction nab-paclitaxel 125 mg/m2 qw 3/4 followed by nab-paclitaxel 150 mg/m2 on days 1 and 15 of a 28-day cycle, 100 mg/m2 qw 3/4, or 75 mg/m2 qw. PFS will be assessed as the primary endpoint. An ongoing phase I/II study (NCT01938833; planned N = 47) is evaluating the combination of nab-paclitaxel plus the histone deacetylase inhibitor romidepsin in recurrent or metastatic HER2-negative inflammatory breast cancer [https://clinicaltrials.gov/ct2/show/NCT01938833]. Results from the phase I portion (n = 9) demonstrated that the regimen was well tolerated and resulted in an ORR of 33%, including 1 complete response [49].
Discussion
Recent clinical data indicate that nab-paclitaxel is effective and safe across all stages of breast cancer. The results from trials in the neoadjuvant setting for early-stage TNBC or HER2-positive breast cancer were particularly encouraging. In TNBC, nab-paclitaxel monotherapy or in combination with other agents resulted in pCR rates ranging from 10.5 to 62%. In the phase III neoadjuvant GeparSepto trial, the largest difference in pCR was identified for patients with TNBC (nab-paclitaxel, 48.2% vs paclitaxel, 26.3%; P < 0.001), supporting the clinical benefit of nab-paclitaxel in early-stage TNBC [11]. The unmet need for the treatment of TNBC lends greater importance to these findings. Patients with early-stage HER2-positive breast cancer also benefited from nab-paclitaxel treatment. Neoadjuvant nab-paclitaxel combined with trastuzumab and carboplatin, anthracycline, or vinorelbine demonstrated pCR rates in the breast and lymph nodes ranging from 45 to 49%, which is comparable to those observed for other current neoadjuvant therapies [50]. In addition, neoadjuvant nab-paclitaxel resulted in breast-conserving surgery in 71 to 77.5% of patients with early-stage breast cancer.
In the phase III CALGB 40502 study, patients with MBC treated with first-line nab-paclitaxel plus bevacizumab achieved a median PFS of approximately 9 months and a median OS of 23.5 months [30]. For reference, patients with MBC who received nab-paclitaxel 260 mg/m2 q3w as first- or later-line therapy in a phase III trial demonstrated a median time to tumor progression of 5.3 months and a median OS of 15.0 months [5]. Neither PFS nor OS for the nab-paclitaxel plus bevacizumab arm of the CALGB 40502 trial was significantly different from that of the paclitaxel plus bevacizumab arm [30]. The 150 mg/m2 dose of nab-paclitaxel was not optimal, with a higher percentage of patients in the nab-paclitaxel group developing hematologic and nonhematologic toxicities. Several ongoing trials are evaluating the potential clinical benefit of nab-paclitaxel in patients with MBC, particularly the HER2-positive and TNBC subpopulations. Interim analyses from some of these trials have demonstrated promising results; once final, the findings from these trials will provide further insights into the role of nab-paclitaxel for the treatment of breast cancer across treatment settings and patient subsets.
Recent efforts to maintain efficacy while limiting toxicity have focused on the optimization of nab-paclitaxel schedule and dose. The safety profiles of nab-paclitaxel–based regimens in the recent studies included in this review were consistent with those in past studies, including the registrational phase III trial. The most common grade 3/4 hematologic and nonhematologic adverse events associated with nab-paclitaxel were neutropenia and peripheral neuropathy, respectively. The majority of recent studies have examined weekly dosing, likely because an accumulation of data in the metastatic setting suggests an advantage over every-3-week dosing in balancing efficacy and tolerability. In addition, safety and treatment-exposure results of 2 large trials (GeparSepto in the neoadjuvant setting and CALGB 40502 in the metastatic setting) have suggested that nab-paclitaxel may be more feasible at a starting dose of 125 mg/m2 compared with 150 mg/m2 [11, 30].
Future of nab-paclitaxel in breast cancer: nab-paclitaxel and immune therapy
In addition to the ongoing trials discussed above, there is interest in combining nab-paclitaxel with immuno-oncology agents (Table 3). Chemotherapy-induced cytotoxicity has been shown to activate the immune response and to release tumor antigens from cancer cells [51, 52]. Preclinical data from mouse models of multiple solid tumor types suggested potential synergy between chemotherapy and immune checkpoint inhibitors [53, 54]. Recent clinical data indicated that combining nab -paclitaxel with checkpoint inhibitors may be safe and effective in MBC. A phase Ib study of atezolizumab, a programmed death ligand 1 (PD-L1) inhibitor, combined with nab-paclitaxel qw (NCT01633970) demonstrated activity in 24 efficacy-evaluable patients with metastatic TNBC (ORR 70.8%; stable disease in 20.8%) [55]. Five patients (16%) discontinued nab-paclitaxel due to toxicity (3 for peripheral neuropathy [1 each for grades 1, 2, and 3] and 1 each for fatigue and asthenia [both grade 2]). nab-Paclitaxel plus atezolizumab is currently being compared with nab-paclitaxel plus placebo as a first-line treatment for metastatic TNBC in the randomized phase III IMpassion130 trial (NCT02425891; Table 3) (https://clinicaltrials.gov/ct2/show/NCT02425891, [56]). The PD-1 inhibitor nivolumab is also being evaluated in combination with nab-paclitaxel in MBC in an ongoing phase I trial (NCT02309177; planned N = 138) [https://clinicaltrials.gov/ct2/show/NCT02309177]. The combination of atezolizumab and nab-paclitaxel is also being evaluated as a neoadjuvant regimen for the treatment of early-stage TNBC in an ongoing phase II trial (NCT02530489; planned N = 37) [https://clinicaltrials.gov/ct2/show/NCT02530489] and a phase III trial (NeoTRIPaPDL1; NCT02620280 [nab-paclitaxel plus carboplatin ± atezolizumab]; planned N = 272) [https://clinicaltrials.gov/ct2/show/NCT02620280]. Similarly, the PD-L1 inhibitor durvalumab combined with nab-paclitaxel is being examined as neoadjuvant therapy for early-stage TNBC in an ongoing phase I/II trial (NCT02489448; planned N = 61) [https://clinicaltrials.gov/ct2/show/NCT02489448]. Results from these trials will provide further rationale for combining nab-paclitaxel with immune therapies as an exciting new treatment approach for early-stage or metastatic breast cancer.
Conclusions
In addition to demonstrated efficacy in the already established setting of MBC, nab-paclitaxel appears to be an effective and well-tolerated neoadjuvant therapy for patients with early-stage breast cancer, particularly the HER2-positive and TNBC subgroups. Ongoing trials are evaluating nab-paclitaxel in all stages and subtypes of breast cancer. One anticipated future role of nab-paclitaxel is as a backbone chemotherapy, and ongoing trials of nab-paclitaxel combined with immune checkpoint inhibitors are particularly exciting, as these may provide more effective treatment regimens for early-stage and metastatic breast cancer.
Abbreviations
- AC:
-
doxorubicin plus cyclophosphamide
- ASCO:
-
American Society for Clinical Oncology
- AUC:
-
area under the curve
- CMF:
-
cyclophosphamide, methotrexate, and fluorouracil
- EC:
-
epirubicin and cyclophosphamide
- ER:
-
estrogen receptor
- FEC:
-
fluorouracil, epirubicin, and cyclophosphamide
- HER2:
-
human epidermal growth factor receptor 2
- HR:
-
hazard ratio
- MBC:
-
metastatic breast cancer
- ITT:
-
intention to treat
- OR:
-
odds ratio
- ORR:
-
overall response rate
- OS:
-
overall survival
- pCR:
-
pathological complete response
- PD-L1:
-
programmed death ligand 1
- PFS:
-
progression-free survival
- PICN:
-
paclitaxel concentrate for nanodispersion
- PRISMA:
-
preferred reporting items for systematic reviews and meta-analyses
- QALY:
-
quality-adjusted life-year
- q2w:
-
every 2 weeks
- q3w:
-
every 3 weeks
- qw:
-
once weekly
- qw 3/4:
-
first 3 of 4 weeks
- RCB:
-
residual cancer burden
- sTIL:
-
stromal tumor-infiltrating lymphocytes
- T-DM1:
-
trastuzumab emtansine
- TNBC:
-
triple-negative breast cancer
References
Clinical practice guidelines in oncology: breast cancer. V2.2016. Fort Washington: National Comprehensive Cancer Network; 2016.
Abraxane for injectable suspension (paclitaxel protein-bound particles for injectable suspension) (albumin-bound) [package insert]. Summit: Celgene Corporation; 2015.
Ibrahim NK, Desai N, Legha S, Soon-Shiong P, Theriault RL, Rivera E, et al. Phase I and pharmacokinetic study of ABI-007, a Cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin Cancer Res. 2002;8:1038–44.
ten Tije AJ, Verweij J, Loos WJ, Sparreboom A. Pharmacological effects of formulation vehicles: implications for cancer chemotherapy. Clin Pharmacokinet. 2003;42:665–85.
Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23:7794–803.
Desai N, Trieu V, Yao Z, Louie L, Ci S, Yang A, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of Cremophor-free, albumin-bound paclitaxel, ABI-007, compared with Cremophor-based paclitaxel. Clin Cancer Res. 2006;12:1317–24.
Chen N, Li Y, Ye Y, Palmisano M, Chopra R, Zhou S. Pharmacokinetics and pharmacodynamics of nab-paclitaxel in patients with solid tumors: disposition kinetics and pharmacology distinct from solvent-based paclitaxel. J Clin Pharmacol. 2014;54:1097–107.
Gardner ER, Dahut WL, Scripture CD, Jones J, Aragon-Ching JB, Desai N, et al. Randomized crossover pharmacokinetic study of solvent-based paclitaxel and nab-paclitaxel. Clin Cancer Res. 2008;14:4200–5.
Huang L, Chen S, Yao L, Liu G, Wu J, Shao Z. Phase II trial of weekly nab-paclitaxel and carboplatin treatment with or without trastuzumab as nonanthracycline neoadjuvant chemotherapy for locally advanced breast cancer. Int J Nanomed. 2015;10:1969–75.
Shigematsu H, Kadoya T, Masumoto N, Sasada T, Emi A, Ohara M, et al. The efficacy and safety of preoperative chemotherapy with triweekly abraxane and cyclophosphamide followed by 5-fluorouracil, epirubicin, and cyclophosphamide therapy for resectable breast cancer: a multicenter clinical trial. Clin Breast Cancer. 2015;15:110–6.
Untch M, Jackisch C, Schneeweiss A, Conrad B, Aktas B, Denkert C, et al. Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto-GBG 69): a randomised, phase 3 trial. Lancet Oncol. 2016;17:345–56.
Khan QJ, O’Dea A, Fabian CJ, Connor CS, McGinness M, Varghise Mammen JM, et al. Neoadjuvant chemotherapy plus trastuzumab in stage II/III breast cancer with low HER2 expression. J Clin Oncol. 2015;33(suppl). Abstract 1039.
Martin M, Antolín S, Anton A, Plazaola A, García-Martínez E, Seguí MA, et al. Nabrax: neoadjuvant therapy of breast cancer with weekly single-agent nab-paclitaxel—final efficacy and biomarkers analysis of GEICAM 2011-02 trial. J Clin Oncol. 2014;32(suppl). Abstract 1051.
Nahleh ZA, Barlow WE, Hayes DF, Schott AF, Gralow JR, Perez EA, et al. S0800: nab-paclitaxel, doxorubicin, cyclophosphamide, and pegfilgrastim with or without bevacizumab in treating women with inflammatory or locally advanced breast cancer. Poster presented at: 37th annual San Antonio breast cancer symposium. San Antonio; 2014. Abstract P3-11-16.
Connolly RM, Leal JP, Goetz MP, Zhang Z, Zhou XC, Jacobs LK, et al. TBCRC 008: early change in 18F-FDG uptake on PET predicts response to preoperative systemic therapy in human epidermal growth factor receptor 2-negative primary operable breast cancer. J Nucl Med. 2015;56:31–7.
Hofmann D, Nitz U, Gluz O, Kates RE, Schinkoethe T, Staib P, et al. WSG ADAPT—adjuvant dynamic marker-adjusted personalized therapy trial optimizing risk assessment and therapy response prediction in early breast cancer: study protocol for a prospective, multi-center, controlled, non-blinded, randomized, investigator initiated phase II/III trial. Trials. 2013;14:261.
Gluz O, Nitz U, Christgen M, Grischke E, Forstbauer H, Braun MW, et al. Efficacy of 12 weeks neoadjuvant nab-paclitaxel combined with carboplatinum vs. gemcitabine in triple-negative breast cancer: WSG-ADAPT TN randomized phase II trial. J Clin Oncol. 2015;33(suppl). Abstract 1032.
Gluz O, Nitz U, Liedtke C, Christgen M, Sotlar K, Grischke EM, et al. Comparison of 12 weeks neoadjuvant nab-paclitaxel combined with carboplatinum vs. gemcitabine in triple- negative breast cancer: WSG-ADAPT TN randomized phase II trial. Presented at: 38th annual San Antonio breast cancer symposium. San Antonio; 2015. Abstract S6-07.
Kuwayama T, Yamauchi H, Takano T, Tsugawa K, Sato T, Kitani A, et al. Primary analysis of a randomized phase II, multicenter trial: Neoadjuvant weekly nab-paclitaxel 100 mg/m2 followed by FE100C compared with docetaxel 75 mg/m2 followed by FE100C for early breast cancer in Japan. Presented at: 2015 breast cancer symposium. San Francisco; 2015. Abstract 136.
Shimada H, Ueda S, Saeki T, Shigekawa T, Takeuchi H, Hirokawa E, et al. Neoadjuvant triweekly nanoparticle albumin-bound paclitaxel followed by epirubicin and cyclophosphamide for stage II/III HER2-negative breast cancer: evaluation of efficacy and safety. Jpn J Clin Oncol. 2015;45:642–9.
Sinclair NF, Sakr BJ, Abu-Khalaf MM, Somlo G, Black RC, Chung GG, et al. Multicenter phase II trial of neoadjuvant carboplatin, weekly nab-paclitaxel, and trastuzumab in stage II-III HER2+ breast cancer: a BrUOG study. J Clin Oncol. 2013;31(suppl). Abstract 619.
Tanaka S, Iwamoto M, Kimura K, Matsunami N, Morishima H, Yoshidome K, et al. Phase II study of neoadjuvant anthracycline-based regimens combined with nanoparticle albumin-bound paclitaxel and trastuzumab for human epidermal growth factor receptor 2-positive operable breast cancer. Clin Breast Cancer. 2015;15:191–6.
Zelnak AB, Nikolinakos P, Srinivasiah J, Jonas W, Pippas A, Liu Y, et al. High pathologic complete response in Her2-positive, early-stage breast cancer to a novel nonanthracycline neoadjuvant chemotherapy. Clin Breast Cancer. 2015;15:31–6.
Cheng H, Bai Y, Sikov W, Sinclair N, Bossuyt V, Abu-Khalaf MM, et al. Quantitative measurements of HER2 and phospho-HER2 expression: correlation with pathologic response to neoadjuvant chemotherapy and trastuzumab. BMC Cancer. 2014;14:326.
Varadan V, Kamalakaran S, Gilmore H, Banerjee N, Janevski A, Miskimen KL, et al. Brief-exposure to preoperative bevacizumab reveals a TGF-β signature predictive of response in HER2-negative breast cancers. Int J Cancer. 2015;138:747–57.
Avril S, Varadan V, Miskimen K, Gollamudi J, Abu-Khalaf MM, Somlo G, et al. Association of tumor infiltrating lymphocytes (TILs) with pathologic response in baseline and post-brief exposure HER2+ breast cancer biopsies from BrUOG. J Clin Oncol. 2015;33(suppl). Abstract 619.
von Minckwitz G, Conrad B, Reimer T, Decker T, Eidtmann H, Eiermann W, et al. A randomized phase 2 study comparing EC or CMF versus nab-paclitaxel plus capecitabine as adjuvant chemotherapy for nonfrail elderly patients with moderate to high-risk early breast cancer (ICE II-GBG 52). Cancer. 2015;121:3639–48.
Möbus V, Lück H, Forstbauer H, Wachsmann G, Ober A, Schneeweiss A, et al. GAIN-2: adjuvant phase III trial to compare intense dose-dense (idd) treatment with EnPC to tailored dose-dense (dt) therapy with dtEC-dtD for patients with high-risk early breast cancer: results of the second safety interim analyses. Presented at: 38th annual San Antonio breast cancer symposium. San Antonio; 2015. Abstract P1-13-05.
Loman N, Linderholm B, Joensuu H, Ejlertsen B, Johannsson OT, Geisler J, et al. Nordic trip, a randomized phase 3 study in early triple negative breast cancer. Presented at: 38th annual San Antonio breast cancer symposium. San Antonio; 2015. Abstract OT3-02-10.
Rugo HS, Barry WT, Moreno-Aspitia A, Lyss AP, Cirrincione C, Leung E, et al. Randomized phase III trial of paclitaxel once per week compared with nanoparticle albumin-bound nab-paclitaxel once per week or ixabepilone with bevacizumab as first-line chemotherapy for locally recurrent or metastatic breast cancer: CALGB 40502/NCCTG N063H (Alliance). J Clin Oncol. 2015;33:2361–9.
Sun S, Tang L, Zhang J, Lv F, Wang Z, Wang L, et al. Cisplatin improves antitumor activity of weekly nab-paclitaxel in patients with metastatic breast cancer. Int J Nanomed. 2014;9:1443–52.
Jain MM, Gupte SU, Patil SG, Pathak AB, Deshmukh CD, Bhatt N, et al. Paclitaxel injection concentrate for nanodispersion versus nab-paclitaxel in women with metastatic breast cancer: a multicenter, randomized, comparative phase II/III study. Breast Cancer Res Treat. 2016;156:125–34.
Lohmann AE, Speers CH, Chia SK. Evaluation of the clinical benefits of nanoparticle albumin-bound paclitaxel in women with metastatic breast cancer in British Columbia. Curr Oncol. 2013;20:97–103.
Jackisch C, Untch M, Dall P, Thomssen C, Albert U-S, Lueck HJ, et al. A national treatment survey on the chemotherapy (CTX) treatment for patients (pts) with metastatic breast cancer (MBC) in Germany. J Clin Oncol. 2013;31(suppl). Abstract e17544.
Force RW, Pugmire BA, Binder G, Duval C, Corzo D, Gluck S. Differences between taxanes in time on therapy and associated discontinuation events in metastatic breast cancer: results from a US claims analysis. Presented at: 2013 breast cancer symposium. San Francisco; 2013. Abstract 158.
Liang C, Li L, Fraser CD, Ko A, Corzo D, Enger C, et al. The treatment patterns, efficacy, and safety of nab-paclitaxel for the treatment of metastatic breast cancer in the United States: results from health insurance claims analysis. BMC Cancer. 2015;15:1019.
Forero-Torres A, Varley KE, Abramson VG, Li Y, Vaklavas C, Lin NU, et al. TBCRC 019: a phase II trial of nanoparticle albumin-bound paclitaxel with or without the anti-death receptor 5 monoclonal antibody tigatuzumab in patients with triple-negative breast cancer. Clin Cancer Res. 2015;21:2722–9.
Palumbo R, Sottotetti F, Trifiro G, Piazza E, Ferzi A, Gambaro A, et al. Nanoparticle albumin-bound paclitaxel (nab-paclitaxel) as second-line chemotherapy in HER2-negative, taxane-pretreated metastatic breast cancer patients: prospective evaluation of activity, safety, and quality of life. Drug Des Dev Ther. 2015;9:2189–99.
Alba E, Ciruelos E, Lopez R, Lopez-Vega JM, Lluch A, Martin M, et al. Cost-utility analysis of nanoparticle albumin-bound paclitaxel versus paclitaxel in monotherapy in pretreated metastatic breast cancer in Spain. Expert Rev Pharmacoecon Outcomes Res. 2013;13:381–91.
Dranitsaris G, Yu B, King J, Kaura S, Zhang A. Nab-paclitaxel, docetaxel, or solvent-based paclitaxel in metastatic breast cancer: a cost-utility analysis from a Chinese health care perspective. Clinicoecon Outcomes Res. 2015;7:249–56.
Lazzaro C, Bordonaro R, Cognetti F, Fabi A, De Placido S, Arpino G, et al. An Italian cost-effectiveness analysis of paclitaxel albumin (nab-paclitaxel) versus conventional paclitaxel for metastatic breast cancer patients: the COSTANza study. Clinicoecon Outcomes Res. 2013;5:125–35.
Nilsson A, Lundkvist J, Lindman H. Time and value of chemotherapy administration burden in a Swedish hospital. Presented at: 38th annual San Antonio breast cancer symposium. San Antonio; 2015. Abstract P6-11-06.
Bachelot TD, Ciruelos E, Peretz-Yablonski T, Puglisi F, Schneeweiss A, Campone M, et al. First-line pertuzumab (P), trastuzumab (H), and taxane therapy for HER2-positive locally recurrent/metastatic breast cancer (LR/mBC): interim safety results (N = 704) from PERUSE. J Clin Oncol. 2014;32(suppl). Abstract 548.
Woodward N, De Boer RH, Redfern A, Von Neumann-Cosel V, Heath RM, Beith J. An open-label, multicentre, phase IIIb study with intravenous administration of pertuzumab, subcutaneous trastuzumab, and a taxane in patients with HER2-positive metastatic breast cancer (SAPPHIRE). Presented at: 37th annual San Antonio breast cancer symposium. San Antonio; 2014. Abstract OT3-1-03.
Woodward N, De Boer RH, Redfern A, White M, Young J, Truman M, et al. Interim results from the first open-label, multicenter, phase IIIb study investigating the combination of pertuzumab with subcutaneous trastuzumab and a taxane in patients with HER2-positive metastatic breast cancer (SAPPHIRE). Presented at: 38th annual San Antonio breast cancer symposium. San Antonio; 2015. Abstract P4-14-12.
Patel TA, Mejia JA, Rodriguez AA, Chee Ning Chang J. Phase Ib trial of trastuzumab emtansine (TE) in combination with lapatinib (L) plus nab-paclitaxel in metastatic HER2-neu overexpressed breast cancer patients: STELA trial. J Clin Oncol. 2014;32(suppl). Abstract TPS669.
Yardley DA, Brufsky A, Coleman RE, Conte PF, Cortes J, Glück S, et al. Phase II/III weekly nab-paclitaxel plus gemcitabine or carboplatin versus gemcitabine/carboplatin as first-line treatment of patients with metastatic triple-negative breast cancer (the tnAcity study): study protocol for a randomized controlled trial. Trials. 2015;16:575.
Gennari A, Jerusalem G, Maibach R. The SNAP trial: schedules of nab-paclitaxel in metastatic breast cancer, International Breast Cancer Study Group (IBCSG 42-12) and Breast International Group (BIG 2-12). Poster presented at: 36th annual San Antonio breast cancer symposium. San Antonio; 2013. Abstract OT3-1-02.
Avery TP, Jaslow R, Basu-Mallick A, Zibelli A, Fellin F, Cristofanilli M. A phase I study of romidepsin in combination with nab-paclitaxel in patients with metastatic HER-2 negative inflammatory breast cancer (IBC). Presented at: 38th annual San Antonio breast cancer symposium. San Antonio; 2015. Abstract P4-13-18.
Chia SK. Neoadjuvant and adjuvant therapy for HER2 positive disease. Am Soc Clin Oncol Educ Book. 2015;31:e41–8.
Hodge JW, Kwilas A, Ardiani A, Gameiro SR. Attacking malignant cells that survive therapy: exploiting immunogenic modulation. Oncoimmunology. 2013;2:e26937.
Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72.
Winograd R, Byrne KT, Evans RA, Odorizzi PM, Meyer AR, Bajor DL, et al. Induction of T-cell immunity overcomes complete resistance to PD-1 and CTLA-4 blockade and improves survival in pancreatic carcinoma. Cancer Immunol Res. 2015;3:399–411.
Camidge DR, Liu SV, Powderly J, Ready NE, Hodi FS, Gettinger SN, et al. Atezolizumab (MPDL3280A) combined with platinum-based chemotherapy in non-small cell lung cancer (NSCLC): a phase Ib safety and efficacy update. J Thorac Oncol. 2015;10(9 suppl):S176–7.
Adams S, Diamond J, Hamilton E, Pohlmann P, Tolaney S, Molinero L, et al. Safety and clinical activity of atezolizumab (anti-PDL1) in combination with nab-paclitaxel in patients with metastatic triple-negative breast cancer. Presented at: 38th annual San Antonio breast cancer symposium. San Antonio; 2015. Abstract P2-11-06.
Emens L, Adams S, Loi S, Schmid P, Schneeweiss A, Rugo H, et al. A phase III randomized trial of atezolizumab in combination with nab-paclitaxel as first line therapy for patients with metastatic triple-negative breast cancer (mTNBC). Presented at: 38th annual San Antonio breast cancer symposium. San Antonio; 2015. Abstract OT1-01-06.
Seki H, Asamuna F, Morinaga S, Suzuki K, Kaneda M, Kamiya N, et al. Weekly nab-paclitaxel followed by FEC in patients with operable breast cancer: phase II study. J Clin Oncol. 2015;33(suppl). Abstract 138.
Khasraw M, Mukaro VR, West L, Brandt C, Woollett AM, Edwards M, et al. Tailored neoadjuvant epirubicin and cyclophosphamide (EC) and nanoparticle albumin bound (nab)-paclitaxel for newly diagnosed breast cancer (BC). J Clin Oncol. 2015;33(suppl). Abstract e12025.
Tsugawa K, Kawamoto H, Kojima Y, Tsuchiya K, Shimo A, Hayami R, et al. Feasibility study of weekly nanoparticle albumin-bound (nab)-paclitaxel (150 mg/m2) followed by 5FU, epirubicin, plus cyclophosphamide (FEC) as neoadjuvant chemotherapy for HER2-negative breast cancer. Poster presented at: 9th European breast cancer conference. Glasgow; 2014. Abstract 43.
Somlo G, Frankel P, Li M, Treece T, Kruper L, Yim J, et al. Phase II trial of neoadjuvant chemotherapy with carboplatin and nab-paclitaxel in patients with triple negative locally advanced and inflammatory breast cancer (TNBC). Poster presented at: 38th annual San Antonio breast cancer symposium. San Antonio; 2015. Abstract P1 14-10.
Mrozek E, Layman R, Ramaswamy B, Lustberg M, Vecchione A, Knopp MV, et al. Phase II trial of neoadjuvant weekly nanoparticle albumin-bound paclitaxel, carboplatin, and biweekly bevacizumab therapy in women with clinical stage II or III HER2-negative breast cancer. Clin Breast Cancer. 2014;14:228–34.
Matsuda N, Alvarez RH, Krishnamurthy S, Willey JS, Wang X, Lim B, et al. Phase II study of panitumumab, nab-paclitaxel, and carboplatin followed by FEC neoadjuvant chemotherapy for patients with primary HER-2 negative inflammatory breast cancer. J Clin Oncol. 2015;33(suppl). Abstract 1065.
Dent S, Fraser J, Graham N, Campbell M, Hopkins S, Dranitsaris G. Clinical outcomes of women with metastatic breast cancer treated with nab-paclitaxel: experience from a single academic cancer centre. Curr Oncol. 2013;20:24–9.
Aigner J, Marme F, Smetanay K, Schuetz F, Jaeger D, Schneeweiss A. Nab-paclitaxel monotherapy as a treatment of patients with metastatic breast cancer in routine clinical practice. Anticancer Res. 2013;33:3407–13.
Hamilton E, Kimmick G, Hopkins J, Marcom PK, Rocha G, Welch R, et al. Nab-paclitaxel/bevacizumab/carboplatin chemotherapy in first-line triple negative metastatic breast cancer. Clin Breast Cancer. 2013;13:416–20.
Moebus V, Forstbauer H, Wachsmann G, Schneeweiss A, Ober A, von Abel E, et al. Adjuvant phase III trial to compare intense dose-dense adjuvant treatment with EnPC to dose dense, tailored therapy with dtEC-dtD for patients with high-risk early breast cancer (GAIN-2). J Clin Oncol. 2013;31(suppl). Abstract TPS1137.
Acknowledgements
Medical writing assistance was provided by Rita Nahta, Ph.D., of MediTech Media and funded by Celgene Corporation. Adam Brufsky, MD, is fully responsible for the content and editorial decisions for this manuscript.
Competing interests
Dr. Brufsky is a consultant to Celgene Corporation.
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Funding
Celgene Corporation provided funding for medical writing support. Celgene Corporation was not involved in the design of the systematic review, the analysis or interpretation of data, or the writing of the report.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Brufsky, A. nab-Paclitaxel for the treatment of breast cancer: an update across treatment settings. Exp Hematol Oncol 6, 7 (2017). https://doi.org/10.1186/s40164-017-0066-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40164-017-0066-5