Abstract
Transgenesis and genome editing in birds are based on a unique germline transmission system using primordial germ cells (PGCs), which is quite different from the mammalian transgenic and genome editing system. PGCs are progenitor cells of gametes that can deliver genetic information to the next generation. Since avian PGCs were first discovered in nineteenth century, there have been numerous efforts to reveal their origin, specification, and unique migration pattern, and to improve germline transmission efficiency. Recent advances in the isolation and in vitro culture of avian PGCs with genetic manipulation and genome editing tools enable the development of valuable avian models that were unavailable before. However, many challenges remain in the production of transgenic and genome-edited birds, including the precise control of germline transmission, introduction of exogenous genes, and genome editing in PGCs. Therefore, establishing reliable germline-competent PGCs and applying precise genome editing systems are critical current issues in the production of avian models. Here, we introduce a historical overview of avian PGCs and their application, including improved techniques and methodologies in the production of transgenic and genome-edited birds, and we discuss the future potential applications of transgenic and genome-edited birds to provide opportunities and benefits for humans.
Similar content being viewed by others
Background
The advancement of genetic modification tools and precise genome editing technologies has created a new era in which the genotype, phenotype, and traits of animals can be easily modified. Traditionally, animal breeders used selective breeding or artificial breeding strategies to improve productivity, food quality, and other traits of offspring through the selective mating of highly qualified parents [1]. In terms of the genomic DNA sequence of the desired animal, this selective breeding strategy is in line with effect of current genetic modification or genome editing. Thus, it has become possible to more efficiently improve and precisely manipulate the genetic traits of animal via recent genetic modulation technologies in combined with conventional breeding strategy. Currently, the introduction of genome modulation technology to a targeted animal inevitably requires germline modification of that animal, enabling the transmission of modified genetic traits to subsequent generations [2]. Germline modification strategies differ among animal species. In mammalian species, the first transgenic mouse was produced by microinjection of foreign DNA into the pronucleus of a fertilized oocyte [3]. The first genetically modified livestock, including rabbits, sheep, and pigs, were successfully produced in the same manner [4]. Even though the efficiency of developing founder animals is quite low and foreign DNA is randomly integrated into recipient genomes, this strategy is still a major technological method used in animal transgenesis. Another major method in mammalian transgenesis, especially in mice, is the use of germline competent cells like embryonic stem cells (ESCs) for germline modification (Fig. 1a). In mammals, germline chimeras that have a mixture of germ cells originated from both endogenous and exogenous germ cells can be produced via injection of genetically modified ESCs into recipient blastocyst [5, 6]. Through the testcross analysis of germline chimera, genetically modified ESC-mediated transgenic offspring can be generated. However, unlike mammals, birds have a unique transgenesis and genetic modification system (Fig. 1b) due to their oviparity and the physiological properties of the ovum [7]. Since avian zygote shows discoidal meroblastic cleavage with a large amount of yolk and a small germinal disc, it is difficult to introduce foreign DNA into zygote and microinject avian ESCs into blastoderm [8,9,10]. The first transgenic avian specimen was a chicken that was produced via sub-germinal cavity injection of a retroviral vector into an Eyal-Giladi and Kochav (EGK) [11] stage X embryo [12]. Since then, various strategies have been suggested for producing genetically modified transgenic birds, including viral infection into stage X embryos [13,14,15], microinjection of transgenes into fertilized eggs [10, 15], and embryonic stem cells [16]. However, due to low germline transmission efficiency, these methods are not successful in producing genome-modified birds via homologous recombination until recently. To overcome this limitation, much effort has focused on the utilization of primordial germ cells (PGCs) as an alternative strategy comparable to mammalian germline-competent ESCs [17]. Here we present an overview of PGCs and recent progress in transgenesis and genome editing technology, and introduce potential strategies for PGC-mediated genetic modulation in birds.
Transgenic and genome editing system in mammals and birds. a In mammals, transgenic (TG) and genome edited (GE) offspring can be produced via direct introduction of genome editing tool into the zygote or microinjection of genome edited ESCs into the recipient blastocyst. b In birds, TG and GE offspring can be produced via injection of genome edited PGCs into the blood vessel of recipient
Historical overview of avian primordial germ cells
Origin, specification, and development of primordial germ cells
In late nineteenth century, Waldeyer first observed the origin of germ cells in the germinal epithelium of chicken embryos [18]. Thereafter, Swift reported that avian PGCs arose from the endodermal region, the so-called germ wall [19]. Avian PGCs are observed in the epiblast layer and hypoblast in the central region of the area pellucida of EGK stage X blastoderm [11, 20, 21]. During early embryogenesis in chicken (Fig. 2a), PGCs migrate from the central region of the area pellucida toward the germinal crescent region until Hamburger and Hamilton (HH) stage 4 [22,23,24]. After formation of the primitive streak, PGCs are observed in the germinal crescent region of an extraembryonic site at HH stages 4–10 [11, 23, 25]. Subsequently, PGCs located at the anterior region enter the vascular system of extraembryonic blood vessels via the anterior vitelline vein during HH stages 10–12 [26, 27], and they start to settle in the gonadal anlagen at 2.5 d of incubation [28]. On the other hand, mouse PGCs originate from proximal epiblast and specified via bone morphogenetic proteins (BMP) signaling derived from the extraembryonic ectoderm and visceral endoderm [29]. During mouse embryogenesis (Fig. 2b), PGCs move from posterior primitive streak to endoderm, and subsequently migrate from hindgut endoderm to the mesentery, and finally settle in the genital ridge [30, 31]. When compared to mouse PGCs, the unique migratory pathway of avian PGCs enables us to develop PGC-mediated germline transmission and transgenic system in birds.
Schematic representation of the development and migration of PGCs in mouse and chicken. a Mouse PGCs originated from epiblast, and migrate through dorsal mesentery to seettle in the genital ridge. b Chicken PGCs located at the center of area pellucida region, and they migrate through germinal crescent and vascular system to settle in the genital ridge
PGCs have a large amount of cytoplasmic glycogen granules. Therefore, periodic acid-Schiff (PAS) staining is conventionally used to identify PGCs in chick embryos [32], and Eyal-Giladi et al. suggested that PGCs originated from the epiblast around EGK stage X based on PAS staining results [33]. Because there were no specific molecular markers of PGCs or germ plasm, avian species had been assumed to follow the induction mode of PGC specification [34,35,36]. However, after the discovery of the chicken vasa homolog (CVH) gene and the tracing of its expression pattern from the oocyte through all developmental stages, it was revealed that avian germline specification is determined by maternally inherited factors, which strongly suggests that avian PGCs follow the germ plasm model of specification [37]. Moreover, a recent study on tracing chicken deleted in azoospermia-like (DAZL) gene in intrauterine-stage chicken embryos reinforces the evidence for a germ plasm model of avian PGC origin and specification [38].
Isolation and culture of primordial germ cells
Avian PGCs can usually be isolated at three different developmental stages, including in the germinal crescent of HH stage 4–8 embryos, vascular system of HH stage 14–16 embryos, and gonadal ridge of HH 26–28 embryos. Before the discovery of PGC cell-surface markers, PGCs were isolated using a density gradient-dependent centrifugation method [39, 40]. However, the utility of this method for isolating PGCs was limited due to low yield rates, purity, and viability after isolation. After the identification of PGC-specific surface antigens such as stage-specific embryonic antigen-1 (SSEA1) in chickens and quail germ cell-specific marker (QCR1) in quail, it is possible to collect highly purified avian PGCs using magnetic-activated cell sorting (MACS) or fluorescence-activated cell sorting (FACS) systems via PGC-specific antibodies [41,42,43]. However, it is still difficult to isolate the PGCs of wild or endangered birds using such cell sorting methods, as their PGC-specific surface markers have not yet been identified. Accordingly, Jung et al. recently developed a transwell-mediated size-dependent isolation method for various avian PGCs in HH stage 14–16 embryonic blood, a strategy based on the size of PGCs [44].
Since the in vitro long-term culture of PGCs was successfully established by van der Lavoir in 2006 [45], much effort has been focused on optimizing PGC culture systems and cell signaling mechanisms for the in vitro proliferation of PGCs while maintaining their germline competency. It was subsequently revealed that basic fibroblast growth factor is an essential factor for in vitro proliferation and survival via the MEK/ERK cell signaling pathway [46, 47]. Recently, Whyte et al. [48] demonstrated that the in vitro self-renewal of PGCs requires MEK1, AKT, and SMAD3 cell signaling to maintain germline competency, and Lee et al. [49] found that Wnt/β-catenin signaling is also required for the proliferation of PGCs in vitro. In the near future, PGC culture systems should be developed for multiple bird species and optimized for the application of PGC-mediated avian transgenesis and genome editing.
Production of germline chimeras via primordial germ cells for avian transgenesis
“Germline chimera” usually refers to the presence of mixed gametes from different breeds or species in one individual. For the production of highly efficient transgenic birds, much effort has been focused on improving the efficiency of germline transmission. In 1976, Reynaud observed the colonization of germinal crescent-derived donor turkey PGCs in recipient chicken gonads after intravascular injection and produced a germline chimera chicken that produced functional gametes derived from turkey primordial germ cells [50]. PGCs isolated from quail germinal crescent were later successfully transferred to recipient embryos to produce quail germline chimeras [51]. Subsequently, the first transgenic bird was produced using PGCs isolated from the germinal crescent of HH stage 5 chicken embryos [52]. As shown in Fig. 3, avian germline chimeras and donor-derived progeny have been produced by transferring PGCs isolated from the blood of HH stage 14–16 embryos (bPGCs) [53, 54] and gonads of HH stage 26–28 embryos (gPGCs) [55, 56] in chicken and quail. As previously mentioned, density gradient centrifugation and immunomagnetic cell sorting methods were developed to obtain purified PGCs and efficiently produce germline chimeras [39, 42]. In the meantime, germline chimeras were produced using cryopreserved bPGCs [57] and gPGCs [58]. Cryopreservation of PGCs can enable the preservation of avian genetic resources and restore endangered bird species. Recently, interspecies germline chimera have been produced for the restoration and preservation of birds via transplantation of pheasant PGCs [59] and Houbara Bustard PGCs [60] into chicken or chicken PGCs into guinea fowl. Meanwhile, there are other efforts to produced germline chimera more efficiently through depletion of endogeneous PGCs of recipient embryo. Various methods have been used to eliminate the endogeneous germ cells in birds through exposure to gamma ray [61], administration of busulfan into embryo [62] and removal of blood from recipient embryos at HH stages 14–15 [57]. In 2010, Nakamura et al., reported that the germline chimera efficiency of busulfan-treated founder was about 99%, whereas the efficiency of busulfan-untreated chimera was about 6% [63]. Thus, strategies for depletion of enodogenous PGCs can promote the development of transgenic and genome edited birds efficiently. On the other hand, there have been many effort to develop alternative germline chimera systems without PGCs, using other germline competent cells including blastodermal cells [64], embryonic germ cells [65], germline stem cells, and spermatogonial stem cells [66]. However, their germline transmission efficiency is quite low compared to PGC-mediated germline chimera system. Because germline chimeras and genetically modified chickens can be produced using in vitro cultured PGCs in chickens [45], the in vitro culture system of PGCs has been optimized and the germline competency of in vitro cultured PGCs has subsequently been revealed [46, 47, 67]. Although the germline transmission efficiency was quite variable, from 0% to about 100% for each PGC line, PGCs are still regarded as the most optimal germline-competent cells that can be expanded in vitro without loss of germline competency. To produce more efficiently germline chimeras using PGCs, several effort have been made to enhance the germline competency of PGCs via optimization of culture condition of PGCs [48, 49, 67,68,69]. However, the relationship between in vitro culture of PGC and loss of germline competency is still unclear, and the systems related in vitro long-term culture of competent PGC is inadequate at present. In addition, it may be required to identify best germline competency-associated marker, which contribute to enhance the quality of PGCs. Although there are still challenges to overcome, the PGC-mediated germline transmission system is the most efficient way to produce transgenic and genome-edited birds at present.
Historical contributions to advancemnet of primordial germ cell-mediated production of germline chimeras and genetic modulation in birds. PGC, primordial germ cell; bPGC, embryonic blood-derived PGC; gPGC, embryonic gonad-derived PGC; HR, homologous recombination; TALEN, transcription activator-like effector nuclease; CRISPR/Cas9, clustered regularly interspaced short palindromic repeat (CRISPR)-CRISPR associated protein; HDR, homology-directed repair
Genetic modification and genome editing in birds
Overview of transgenesis in birds
Prior to the establishment of long-term in vitro PGC culture systems, the major transgenic technology used in birds was based on injecting viruses into EGK stage X embryos. In avian species, the first transgenic chicken was produced by microinjection of recombinant avian leukosis viruses into the subgerminal cavity of EGK stage X embryos [12]. Subsequently, Vick et al., successfully produced transgenic chicken using genetically modified PGCs via retrovirus [52] In addition, Mizuarai et al., produced transgenic quail using direct injection of a replication-defective retroviral vector into the blastodermal stage embryos [70]. Because randomly integrated transgene in genome of transgenic animal was frequently silenced [13, 70,71,72], the lentiviral system was introduced to avian transgenesis as an efficient viral transduction system. It successfully produced various transgenic chickens without any gene silencing [73,74,75,76]. Furthermore, Agate et al., produced first green fluorescent protein (GFP)-expressing transgenic finch using microinjection of lentivirus into blastodermal stage embryos [77]. Meanwhile, Shin et al., successfully produced transgenic quails using gPGCs-mediated germline transmission via lentiviral system [78]. Although the efficiency of gPGC-mediated transgenesis was similar to blastoderm-mediated transgenesis in quail, it has been enabled to produce transgenic birds via viral transfection combined with directly purified PGCs without cultivation.
On the other hand, there have been many efforts to develop non-viral transgenic systems without PGCs, such as sperm-mediated gene transfection [79, 80] and direct microinjection of transgenes into the fertilized eggs [81]. However, these strategies showed low germline transmission efficiency compared to PGC-mediated transgenesis. Due to the establishment of long-term in vitro culture systems, PGC-mediated transgenesis has become a more optimal method for developing genetically modified birds than the aforementioned methods. Accordingly, a highly efficient non-viral system for stable genomic integration of transgenes into the genome of PGCs was developed using transposable elements, such as piggyBac and Tol2 [82, 83]. The introduction of transgenes into the genomes of cultured PGCs using lipofectin or electroporation showed a remarkably higher efficiency than the conventional methods for producing transgenic chickens. More recently, a piggyBac transposon system with Flipase recombinase recognition sequences was developed for introducing site-specific gene cassette exchange in transgenic chicken genomes via PGCs [84]. Meanwhile, there have been several efforts to develop alternative strategies for transgenesis without the use of PGCs. Although the level of transgenic efficiency is usually lower than PGC-mediated transgenesis, the transgenic birds were produced via direct injection of transfection reagents into circulating PGCs at HH stages 14–16 [85,86,87]. This strategy can be applied to produce genetically modified birds, of which PGCs are difficult to manipulate in vitro.
Precise genome editing technology
In recent years, investigators have successfully developed efficient systems for precise genome editing using programmable nucleases, including zinc-finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and clustered regularly interspaced short palindromic repeat (CRISPR)-CRISPR associated protein (CRISPR/Cas). Compared to conventional genetic modification technology based on homologous recombination events, which have extremely low frequency in eukaryotic cells [88], these programmable nucleases yield a much higher frequency of homologous recombination events [89] and also induce targeted mutagenesis through error-prone non-homologous end-joining (NHEJ) [90]. Because these programmable nucleases share common features with conventional genetic engineering tools, including DNA double-strand break repair, gene disruption, gene insertion, gene correction, and point mutagenesis [91], programmable nucleases are innovative genome editing tools. ZFNs were first discovered in 1996 and consist of a zinc finger-based DNA binding domain for DNA recognition and a FokI nuclease for DNA cleavage [92]. ZFNs have been used in several organisms for gene editing, including mouse, rat, and zebrafish [93, 94], but there are no reports of generating ZFN-mediated gene-edited birds. As a second generation programmable nuclease system, TALENs have a similar protein structure to ZFNs, consisting of a FokI endonuclease and a DNA-binding domain, but they have different DNA-binding domains known as transcription activator-like effectors (TALEs), which can be programmed to bind targeted DNA sequences [95]. Although TALEN-targeted DNA sequences must start with a thymine base [96], the TALEN system is much more convenient for determining target sites than ZFNs. Accordingly, TALENs have been more widely utilized in various species due to easy construction, widely applicable possibilities [97, 98], and lower cytotoxicity than ZFNs [99]. A third generation programmable nuclease system is based on a CRISPR with a Cas endonuclease derived from the RNA-based immune system of prokaryotes against bacteriophages, viruses, or foreign nucleic acids [100]. In 2012, Jinek et al. reported that a dual RNA, called a guide RNA (gRNA), consisting of a 20-bp CRISPR RNA (crRNA) and universal trans-activating crRNA (tracrRNA), together with Streptococcus pyogenes type II Cas9 protein (Cas9), induced cleavage of specific target DNA sequences [101]. Thus, Cas9 coupled with dual RNAs has become a powerful tool for gene editing due to its target-specific cleavage capacity. In the CRISPR/Cas system, the target site selection depends on the protospacer adjacent motif (PAM) sequence NGG, which has an important role in the initiation of Cas9 nuclease activity [102, 103]. Compared to TALEN, CRISPR/Cas9 is simpler, easier to use for constructing chimeric single-guide RNA [104], and has lower cytotoxicity and higher targeting efficiency [105]. To enhance target specificity, avoid breakage of double-stranded DNA, reduce off-target effects, and increase homology directed repair (HDR) events or base conversion, various Cas9 variants such as Cas9n [106], Cas9dn [85], and Cas9 D10A [107] have been developed. In addition to the Cas9 endonuclease, a class 2-type V CRIPSR effector endonuclease called CRISPR from Prevotella and Francisella 1(Cpf1) was recently identified [108] which lacks tracrRNA and utilizes a thymidine-rich PAM recognition sequence, in contrast to the guanine-rich PAM sequence of the class 2-type II effector nuclease Cas9. Although it is difficult to directly compare the effectiveness of Cpf1 and Cas9 because of their different PAM sequences, genome-wide analysis shows that Cpf1 has higher accuracy and specificity and has relatively fewer off-target effects than Cas9 [109, 110]. Researchers should choose and use programmable nucleases appropriately for their own purposes, optimizing for factors such as no dsDNA breaks, higher HDR, lower off-target effects, or precise base conversion.
Generation of genome-edited birds: analysis from the germline transmission perspective
Despite the importance of avian species as an ideal animal model of early embryogenesis and organogenesis in developmental biology [111], it had been difficult to investigate loss or gain of function in specific genes in birds due to the lack of precise gene targeting system. Unlike mammalian species, specific gene-targeted birds could not be successfully produced until an in vitro culture system for PGCs and efficient gene editing technologies were developed (Fig. 3). In 2013, the immunoglobulin gene knockout chicken was first produced via homologous recombination in chicken PGCs [112]. The total germline transmission rate of targeted PGCs is approximately 0.1% because the homologous recombination event occurs at a very low frequency, as previously discussed. However, with recent advances in gene editing technology using programmable nucleases, the ovalbumin gene-targeted chicken was generated with TALEN in 2014 [113]. Although 8% of the chicks of the donor PGC-derived offspring were mutants from the transplantation of an average of 33.3% mutant PGCs, TALEN-mediated gene knockout showed higher germline transmission efficiency in mutant progeny than the conventional homologous recombination-mediated gene knockout system. This is because TALEN-induced NHEJ occurs much more frequently than homologous recombination in eukaryotic cells [91]. Subsequently, the CRISPR/Cas9 system-mediated ovomucoid (OVM) gene-targeted chicken was efficiently produced by transplanting transient puromycin-selected PGCs into endogenous PGC-ablated recipient embryos with gamma-ray irradiation [114]. In that report, the two G0 founders, with the exception of one founder, had on average 93% mutant semen, indicating that the CRISPR/Cas9 system induced OVM mutation was highly efficient in almost all of the donor PGCs. Furthermore, from the testcross analysis of two G0 founders, the donor PGC-derived offspring were 72%, of which 53% were OVM gene mutant offspring. Concurrently, Dimitrov et al. successfully produced CRISPR/Cas9-mediated precise genome-edited chickens via HDR insertion of an additional loxP site into the variable region segment segment (VH) of a loxP previously inserted into the joining gene segment (JH) of chicken immunoglobulin heavy chain (IgH) locus [112, 115]. Through Cre recombination of the loxP site inserted at the IgH locus, an approximately 28-kb genomic DNA sequence at the IgH locus was deleted. From their results, germline transmission rates were highly variable for each PGC line; even a founder from the same PGC line showed 0–90% efficiency. It is therefore important to use reliable germline-competent PGC lines for germline transmission of genetically modified or precisely edited genes. More recently, Tayler et al. successfully produced a CVH gene-targeted chicken via the TALEN-mediated HDR system, which induced GFP transgene integration into the CVH locus on the Z chromosome [116]. The efficiency of HDR-mediated GFP transgene knock-in in the CVH locus was 8.1% in two-week recovered PGCs after two days of puromycin selection. Although the percentage of GFP-integrated PGCs used to generate the G0 founder was not reported, they established stable GFP-knock-in PGCs using puromycin selection for two weeks. They produced 6% CVH-targeted offspring from one G0 male founder that had 10% genomic equivalents in its semen. From the TALEN and CRISPR-mediated genome editing results, the germline transmission efficiency of G0 founders vary among each genome edited PGC lines. In this regards, it is also important to optimize the conditions for stable PGC lines while maintaining their germline competency even after genetic modification and gene editing, because PGC lines seem to have different germline competencies for each established cell line and lose their germline competency during long-term in vitro cultivation and genetic modification [67, 68, 117].
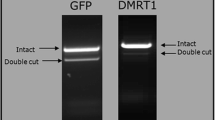
Meanwhile, Cooper et al. reported an ideal method for avian genome editing called sperm transfection-assisted gene editing, which is based on direct delivery of a CRISPR gRNA and Cas9 mRNA mixture into spermatozoa [118]. This method shows a targeting efficiency from 0 to 26.6% mutation in the GFP gene and from 0 to 3% mutation in the doublesex and mab-3 related transcription factor 1 (DMRT1) gene. Although the efficiency of gene editing and germline transmission is still low compared to other current PGC-mediated transgenesis and genome editing methods, this strategy can be utilized as a potential alternative for avian transgenesis and genome editing without culturing PGCs in birds, of which PGCs is difficult to manipulate in vitro.
Application of genome editing technology in birds
The chicken genome sequencing project was completed in 2004, and chicken genomic sequences have been available to the public since that time [119]. Subsequently, the genomic sequences of the zebra finch and turkey have also been made accessible. [120, 121]. Due to recent next generation sequencing technologies, the bird 10K genome sequencing project has been initiated in 2015. Furthermore, the Earth BioGenome Project has recently been proposed to sequence the DNA of all life on Earth, which will covers the genomic information of 1.5 million species [122]. As the genomic information of various avian species has been revealed, it will create infinite possibilities and provide multiple opportunities to access invaluable genetic information from birds [123]. Until recently, there was no way to utilize this valuable avian genetic information in developing genome-edited birds, because there was no efficient genome editing system that could be practically used in birds. The recent progress in genome editing technology in birds via PGCs has ushered in an innovative era of avian genome manipulation for the development of invaluable avian models (Fig. 4). First of all, in chickens, we expect to be able to create an efficient bioreactor system for producing valuable proteins by applying gene editing technology. It is well known that as potential bioreactors chickens have the key benefits that egg white protein is easy to purify and they produce a large amount of egg white protein daily [7, 124]. Although the strategy for developing chickens as bioreactors has focused on the production of target proteins using the ovalbumin promoter, which is the most powerful promoter of egg white proteins [76, 125], it is possible to directly integrate a target protein sequence into the ovalbumin locus via HDR-mediated gene editing. This HDR-mediated target protein insertion into the ovalbumin locus could ultimately be an ideal bioreactor system producing more than one grams of target protein from a single egg with low cost. Genome editing in chickens is also expected to remove or enhance specific nutrients in the meat and eggs of chickens. For example, allergen-free chicken meat and eggs can be developed by knocking out allergen-related genes such as ovalbumin and ovomucoid [113, 114]. In addition, it is possible to make double-muscled and muscle hypertrophy chickens by editing muscle-related genes such as myostatin, as is well-reported in other livestock [126,127,128]. Since conventional genetically modified organism (GMO) has foreign gene or uncontrolled random mutation, there has been public concern about the safety issue of food derived from GMO due to unknown allergen reaction or use of antibiotic resistance genes. On the other hand, genome-edited chickens and other livestock can be produced by controlled precise genome editing technology similar to mutations in intrinsic genomic sequences, like natural mutations, rather than foreign gene insertion as in conventional GMO. Thus, scientists and educators should convince the public that genome edited animals are similar to natural selected or conventional breeding programmed animal via natural mutation [129]. Through the public discussion and social consensus, genome edited animals are expected to be accepted by the consumers in the near future.
Strategies for the production of genome-edited birds. Avian PGCs can be isolated from embryonic blood (HH stages 14–16) and embryonic gonads (HH stage 26–28) by cell-surface antibody-mediated methods, density gradient centrifugation, and size-dependent isolation methods. Genome-edited birds can be produced by transplanting directly isolated or in vitro cultured PGCs into the blood vessels of recipient embryos after the introduction of genome editing tools. Avian genome editing systems can be applied to produce various avian models, such as avian disease resistance models, bioreactor models, and human disease models
Additionally, birds are more likely to develop ovarian cancer than other animal model because they lay a large number of eggs for their lifecycle and have a relatively short ovulation cycle, therefore birds are considered to be one of the best animal model for studying human ovarian cancer [130]. Thus, with precise gene editing in ovarian cancer-related genes, it may be possible to create avian models similar to human ovarian cancer and to reveal the genetic mechanisms of ovarian cancer pathogenesis through gene editing technology. Although avian genome editing research has been conducted mostly in chickens, it will be possible to gradually apply it to various other birds in the near future. Most notably, zebra finches are an exclusive non-human model organism for investigating the biological basis of speech learning, and have been widely used for neurobehavioral research [131]. Zebra finches are also considered as the novel avian models for human diseases that cannot be easily studied in other animal models such as neurological behavior model, Huntington’s disease and vocal learning model [132,133,134,135]. Until recently, transgenic system in zebra finches usually utilize the virus-mediated system that directly injects viruses into the embryos [133]. Gene editing technology can be widely applied to reveal the function and mechanism of invaluable genes in zebra finches through the development of efficient germline transmission systems, including PGC-mediated or sperm-mediated delivery and other reliable strategies. In addition, we expect that it will be possible to control bird-specific diseases and develop avian disease-resistant birds through gene editing of pathogenesis-related genes in birds. In particular, high-risk infectious poultry diseases such as avian influenza and Marek’s disease cause serious problems in various countries and adversely affect the poultry industry. Although it will be necessary to first understand the disease mechanisms and host factors of avian viruses [136, 137], avian gene editing technology is expected to develop avian disease-resistant birds by eliminating host factors or receptors of avian viruses.
Conclusion
Birds are not only important as a food resource, but also an ideal animal model for various disciplines such as behavioral science, immunology and developmental biology. Despite of their importance as an experimental model animal, until a few years ago, there were many challenges and difficulties in transgenesis and gene editing in birds. Recently developed programmable genome editing tools have facilitated a new era of avian models combined with PGC culture systems. It is expected to create innovative genome edited avian models, including specific-gene knockout avian models, allergen-free poultry, human disease model, egg-based bioreactor and avian disease resistance model. Although the establishment of germline-competent cell culture systems has not yet been successful in various birds, and challenges for developing efficient germline transmission strategies still remain, it will be possible to develop such a useful genome-edited avian models in the near future by efficiently introducing gene editing tools into the germline-competent cells of birds. Thus, application of gene editing technology to avian species will provide far more possibilities and benefits to humans.
Abbreviations
- bPGC:
-
Embryonic blood-derived PGC
- Cas9:
-
CRISPR associated protein
- cpf1:
-
CRISPR from Prevotella and Francisella 1
- CRISPR:
-
Clustered regularly interspaced short palindromic repeat
- crRNAs:
-
Clustered regularly interspaced short palindromic repeat RNA
- CVH:
-
Chicken vasa homolog
- DAZL:
-
Deleted in azoospermia-like
- EGK:
-
Eyal-Giladi and Kochav
- ESC:
-
Embryonic stem cell
- GFP:
-
Green fluorescent protein
- GMO:
-
Genetically modified organisms
- gPGC:
-
Embryonic gonad-derived PGC
- gRNA:
-
CRISPR guide RNA
- HDR:
-
Homology directed repair
- HH:
-
Hamburger and Hamilton
- JH:
-
Joining gene segment of immunoglobulin heavy chain
- NHEJ:
-
Non-homologous end joining
- PAM:
-
Protospacer adjacent motif
- PAS:
-
Periodic acid schiff
- PGC:
-
Primordial germ cell
- SSEA1:
-
Stage specific embryonic antigen-1
- TALEN:
-
Transcription activator-like effector nuclease
- tracrRNA:
-
Trans-activating crRNA
- VH:
-
Variable gene segment of immunoglobulin heavy chain
- ZFN:
-
Zinc-finger nuclease
References
Andersson L, Georges M. Domestic-animal genomics: deciphering the genetics of complex traits. Nat Rev Genet. 2004;5:202–12.
Lee HJ, Lee HC, Han JY. Germline modification and engineering in avian species. Mol Cells. 2015;38:743–9.
Gordon JW, Scangos GA, Plotkin DJ, Barbosa JA, Ruddle FH. Genetic transformation of mouse embryos by microinjection of purified DNA. Proc Natl Acad Sci U S A. 1980;77:7380–4.
Hammer RE, Pursel VG, Rexroad CE, Wall RJ, Bolt DJ, Ebert KM, et al. Production of transgenic rabbits, sheep and pigs by microinjection. Nature. 1985;315:680–3.
Thomas KR, Capecchi MR. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem-cells. Cell. 1987;51:503–12.
Smithies O, Gregg RG, Boggs SS, Koralewski MA, Kucherlapati RS. Insertion of DNA sequences into the human chromosomal beta-globin locus by homologous recombination. Nature. 1985;317:230–4.
Han JY. Germ cells and transgenesis in chickens. Comp Immunol Microbiol Infect Dis. 2009;32:61–80.
Lee HC, Choi HJ, Park TS, Lee SI, Kim YM, Rengaraj D, et al. Cleavage events and sperm dynamics in chick intrauterine embryos. PLoS One. 2013;8:e80631.
Bellairs R, Lorenz FW, Dunlap T. Cleavage in the chick embryo. J Embryol Exp Morphol. 1978;43:55–69.
Love J, Gribbin C, Mather C, Sang H. Transgenic birds by DNA microinjection. Biotechnology. 1994;12:60–3.
Eyalgiladi H, Kochav S. From cleavage to primitive streak formation - complementary normal table and a new look at 1st stages of development of Chick .1. General morphology. Dev Biol. 1976;49:321–37.
Salter DW, Smith EJ, Hughes SH, Wright SE, Fadly AM, Witter RL, et al. Gene insertion into the chicken germ line by retroviruses. Poult Sci. 1986;65:1445–58.
Bosselman RA, Hsu RY, Boggs T, Hu S, Bruszewski J, Ou S, et al. Germline transmission of exogenous genes in the chicken. Science. 1989;243:533–5.
Thoraval P, Afanassieff M, Cosset FL, Lasserre F, Verdier G, Coudert F, et al. Germline transmission of exogenous genes in chickens using helper-free ecotropic avian-leukosis virus-based vectors. Transgenic Res. 1995;4:369–77.
Sherman A. Transposition of the drosophila element mariner into the chicken germ line (vol 16, pg 1050, 1998). Nat Biotechnol. 1999;17:81.
Zhu L, van de Lavoir M, Albanese J, Beenhouwer D, Cardarelli P, Cuison S, et al. Production of human monoclonal antibody in eggs of chimeric chickens. Nat Biotechnol. 2005;23:1159–69.
Capecchi MR. Gene targeting in mice: functional analysis of the mammalian genome for the twenty-first century. Nat Rev Genet. 2005;6:507–12.
Waldeyer W. Eirstock und Ei. Eine Beitrag zur Anatomie und Entwicklungsgeschichte der Sexualorgane. Leipzig: Wilhelm Engelmann; 1870.
Swift CH. Origin and early history of the primordial germ cells of the chick. Am J Anat. 1914;18:483–516.
Ginsburg M, Eyalgiladi H. Primordial germ-cells of the young chick blastoderm originate from the central zone of the area pellucida irrespective of the embryo-forming process. Development. 1987;101:209–19.
Hamburger V, Hamilton HL. A series of normal stages in the development of the chick-embryo, (reprinted from journal of morphology, Vol 88, 1951). Dev Dyn. 1992;195:231–72.
Tagami T, Kagami H. Developmental origin of avian primordial germ cells and its unique differentiation in the gonads of mixed-sex chimeras. Mol Reprod Dev. 1998;50:370–6.
Ginsburg M, Eyalgiladi H. Temporal and spatial-aspects of the gradual migration of primordial germ-cells from the epiblast into the germinal crescent in the avian embryo. J Embryol Exp Morphol. 1986;95:53–71.
Kang KS, Lee HC, Kim HJ, Lee HG, Kim YM, Lee HJ, et al. Spatial and temporal action of chicken primordial germ cells during initial migration. Reproduction. 2015;149:179–87.
Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92.
Bernardo AD, Sprenkels K, Rodrigues G, Noce T, Lopes SMCD. Chicken primordial germ cells use the anterior vitelline veins to enter the embryonic circulation. Biol Open. 2012;1:1146–52.
Niewkoop P, Sutasurya L. Primordial germ cells in the chordates. Cambridge: Cambridge University Press; 1979.
Fujimoto T, Ukeshima A, Kiyofuji R. The origin, migration and morphology of the primordial germ cells in the chick embryo. Anat Rec. 1976;185:139–45.
Ying Y, Zhao GQ. Cooperation of endoderm-derived BMP2 and extraembryonic ectoderm-derived BMP4 in primordial germ cell generation in the mouse. Dev Biol. 2001;232:484–92.
Saitou M, Yamaji M. Primordial germ cells in mice. Cold Spring Harb Perspect Biol. 2012;4.
Richardson BE, Lehmann R. Mechanisms guiding primordial germ cell migration: strategies from different organisms. Nat Rev Mol Cell Biol. 2010;11:37–49.
Meyer DB. Migration of primordial germ cells in Chick embryo. Dev Biol. 1964;10:154–90.
Eyalgiladi H, Ginsburg M, Farbarov A. Avian primordial germ-cells are of epiblastic origin. J Embryol Exp Morphol. 1981;65:139–47.
Ginsburg M, Eyalgiladi H. Primordial germ-cell development in cultures of dispersed central disks of stage-X Chick Blastoderms. Gamete Res. 1989;23:421–7.
Tam PPL, Zhou SX. The allocation of epiblast cells to ectodermal and germ-line lineages is influenced by the position of the cells in the gastrulating mouse embryo. Dev Biol. 1996;178:124–32.
Tsang TE, Khoo PL, Jamieson RV, Zhou SX, Ang SL, Behringer R, et al. The allocation and differentiation of mouse primordial germ cells. Int J Dev Biol. 2001;45:549–55.
Tsunekawa N, Naito M, Sakai Y, Nishida T, Noce T. Isolation of chicken vasa homolog gene and tracing the origin of primordial germ cells. Development. 2000;127:2741–50.
Lee HC, Choi HJ, Lee HG, Lim JM, Ono T, Han JY. DAZL expression explains origin and central formation of primordial germ cells in chickens. Stem Cells Dev. 2016;25:68–79.
Yasuda Y, Tajima A, Fujimoto T, Kuwana T. A method to obtain avian germ-line chimeras using isolated primordial germ-cells. J Reprod Fertil. 1992;96:521–8.
Zhao DF, Kuwana T. Purification of avian circulating primordial germ cells by Nycodenz density gradient centrifugation. Br Poult Sci. 2003;44:30–5.
Chang IK, Tajima A, Yasuda Y, Chikamune T, Ohno T. Simple method for isolation of primordial germ-cell from chick-embryos. Cell Biol Int Rep. 1992;16:853–7.
Ono T, Machida Y. Immunomagnetic purification of viable primordial germ cells of Japanese quail (Coturnix Japonica). Comp Biochem Physiol A Mol Integr Physiol. 1999;122:255–9.
Mozdziak PE, Angerman-Stewart J, Rushton B, Pardue SL, Petitte JN. Isolation of chicken primordial germ cells using fluorescence-activated cell sorting. Poult Sci. 2005;84:594–600.
Jung K, Kim Y, Ono T, Han J. Size-dependent isolation of primordial germ cells from avian species. Mol Reprod Dev. 2017;9999:1–9.
van de Lavoir MC, Diamond JH, Leighton PA, Mather-Love C, Heyer BS, Bradshaw R, et al. Germline transmission of genetically modified primordial germ cells. Nature. 2006;441:766–9.
Choi JW, Kim S, Kim TM, Kim YM, Seo HW, Park TS, et al. Basic fibroblast growth factor activates MEK/ERK cell signaling pathway and stimulates the proliferation of chicken primordial germ cells. PLoS One. 2010;5:e12968.
Macdonald J, Glover JD, Taylor L, Sang HM, McGrew MJ. Characterisation and germline transmission of cultured avian primordial germ cells. PLoS One. 2010;5:e15518.
Whyte J, Glover JD, Woodcock M, Brzeszczynska J, Taylor L, Sherman A, et al. FGF, insulin, and SMAD signaling cooperate for avian primordial germ cell self-renewal. Stem Cell Rep. 2015;5:1171–82.
Lee HC, Lim S, Han JY. Wnt/beta-catenin signaling pathway activation is required for proliferation of chicken primordial germ cells in vitro. Sci Rep. 2016;6:34510.
Reynaud G. Reproductive capacity and offspring of chickens submitted to a transfer of primordial germ cells during embryonic life. Wilehm Roux Arch Dev Biol. 1976;179:85–110.
Wentworth BC, Tsai H, Hallett JH, Gonzales DS, Rajcic-Spasojevic G. Manipulation of avian primordial germ cells and gonadal differentiation. Poult Sci. 1989;68:999–1010.
Vick L, Li Y, Simkiss K. Transgenic birds from transformed primordial germ cells. Proc Biol Sci. 1993;251:179–82.
Tajima A, Naito M, Yasuda Y, Kuwana T. Production of germ-line chimera by transfer of primordial germ-cells in the domestic chicken (Gallus-Domesticus). Theriogenology. 1993;40:509–19.
Ono T, Matsumoto T, Arisawa Y. Production of donor-derived offspring by transfer of primordial germ cells in Japanese quail. Exp Anim. 1998;47:215–9.
Chang IK, Jeong DK, Hong YH, Park TS, Moon YK, Ohno T, et al. Production of germline chimeric chickens by transfer of cultured primordial germ cells. Cell Biol Int. 1997;21:495–9.
Kim MA, Park TS, Kim JN, Park HJ, Lee YM, Ono T, et al. Production of quail (Coturnix Japonica) germline chimeras by transfer of gonadal primordial germ cells into recipient embryos. Theriogenology. 2005;63:774–82.
Naito M, Tajima A, Tagami T, Yasuda Y, Kuwana T. Preservation of chick primordial germ cells in liquid nitrogen and subsequent production of viable offspring. J Reprod Fertil. 1994;102:321–5.
Tajima A, Naito M, Yasuda Y, Kuwana T. Production of germ-line chimeras by transfer of cryopreserved gonadal primordial germ cells (gPGCs) in chicken. J Exp Zool. 1998;280:265–7.
Kang SJ, Choi JW, Kim SY, Park KJ, Kim TM, Lee YM, et al. Reproduction of wild birds via interspecies germ cell transplantation. Biol Reprod. 2008;79:931–7.
Wernery U, Liu C, Baskar V, Guerineche Z, Khazanehdari KA, Saleem S, et al. Primordial germ cell-mediated chimera technology produces viable pure-line Houbara bustard offspring: potential for repopulating an endangered species. PLoS One. 2010;5:e15824.
Carsience RS, Clark ME, Verrinder Gibbins AM, Etches RJ. Germline chimeric chickens from dispersed donor blastodermal cells and compromised recipient embryos. Development. 1993;117:669–75.
Aige-Gil V, Simkiss K. Sterilisation of avian embryos with busulphan. Res Vet Sci. 1991;50:139–44.
Nakamura Y, Usui F, Ono T, Takeda K, Nirasawa K, Kagami H, et al. Germline replacement by transfer of primordial germ cells into partially sterilized embryos in the chicken. Biol Reprod. 2010;83:130–7.
Petitte JN, Clark ME, Liu G, Verrinder Gibbins AM, Etches RJ. Production of somatic and germline chimeras in the chicken by transfer of early blastodermal cells. Development. 1990;108:185–9.
Park TS, Hong YH, Kwon SC, Lim JM, Han JY. Birth of germline chimeras by transfer of chicken embryonic germ (EG) cells into recipient embryos. Mol Reprod Dev. 2003;65:389–95.
Jung JG, Lee YM, Kim JN, Kim TM, Shin JH, Kim TH, et al. The reversible developmental unipotency of germ cells in chicken. Reproduction. 2010;139:113–9.
Song Y, Duraisamy S, Ali J, Kizhakkayil J, Jacob VD, Mohammed MA, et al. Characteristics of long-term cultures of avian primordial germ cells and gonocytes. Biol Reprod. 2014;90:15.
Naito M, Harumi T, Kuwana T. Long-term culture of chicken primordial germ cells isolated from embryonic blood and production of germline chimaeric chickens. Anim Reprod Sci. 2015;153:50–61.
Miyahara D, Oishi I, Makino R, Kurumisawa N, Nakaya R, Ono T, et al. Chicken stem cell factor enhances primordial germ cell proliferation cooperatively with fibroblast growth factor 2. J Reprod Dev. 2016;62:143–9.
Mizuarai S, Ono K, Yamaguchi K, Nishijima K, Kamihira M, Iijima S. Production of transgenic quails with high frequency of germ-line transmission using VSV-G pseudotyped retroviral vector. Biochem Biophys Res Commun. 2001;286:456–63.
Jahner D, Stuhlmann H, Stewart CL, Harbers K, Lohler J, Simon I, et al. De novo methylation and expression of retroviral genomes during mouse embryogenesis. Nature. 1982;298:623–8.
Challita PM, Kohn DB. Lack of expression from a retroviral vector after transduction of murine hematopoietic stem cells is associated with methylation in vivo. Proc Natl Acad Sci U S A. 1994;91:2567–71.
McGrew MJ, Sherman A, Ellard FM, Lillico SG, Gilhooley HJ, Kingsman AJ, et al. Efficient production of germline transgenic chickens using lentiviral vectors. EMBO Rep. 2004;5:728–33.
Chapman SC, Lawson A, Macarthur WC, Wiese RJ, Loechel RH, Burgos-Trinidad M, et al. Ubiquitous GFP expression in transgenic chickens using a lentiviral vector. Development. 2005;132:935–40.
Scott BB, Lois C. Generation of tissue-specific transgenic birds with lentiviral vectors. Proc Natl Acad Sci U S A. 2005;102:16443–7.
Lillico SG, Sherman A, McGrew MJ, Robertson CD, Smith J, Haslam C, et al. Oviduct-specific expression of two therapeutic proteins in transgenic hens. Proc Natl Acad Sci U S A. 2007;104:1771–6.
Agate R, Scott B, Haripal B, Lois C, Nottebohm F. Transgenic songbirds offer an opportunity to develop a genetic model for vocal learning. Proc Natl Acad Sci U S A. 2009;106:17963–7.
Shin SS, Kim TM, Kim SY, Kim TW, Seo HW, Lee SK, et al. Generation of transgenic quail through germ cell-mediated germline transmission. FASEB J. 2008;22:2435–44.
Collares T, Campos VF, De Leon PM, Cavalcanti PV, Amaral MG, Dellagostin OA, et al. Transgene transmission in chickens by sperm-mediated gene transfer after seminal plasma removal and exogenous DNA treated with dimethylsulfoxide or N,N-dimethylacetamide. J Biosci. 2011;36:613–20.
Nakanishi A, Iritani A. Gene transfer in the chicken by sperm-mediated methods. Mol Reprod Dev. 1993;36:258–61.
Love J, Gribbin C, Mather C, Sang H. Transgenic birds by DNA microinjection. Biotechnology (N Y). 1994;12:60–3.
Macdonald J, Taylor L, Sherman A, Kawakami K, Takahashi Y, Sang HM, et al. Efficient genetic modification and germ-line transmission of primordial germ cells using piggyBac and Tol2 transposons. Proc Natl Acad Sci U S A. 2012;109:E1466–72.
Park TS, Han JY. piggyBac transposition into primordial germ cells is an efficient tool for transgenesis in chickens. Proc Natl Acad Sci U S A. 2012;109:9337–41.
Lee HJ, Lee HC, Kim YM, Hwang YS, Park YH, Park TS, et al. Site-specific recombination in the chicken genome using Flipase recombinase-mediated cassette exchange. FASEB J. 2016;30:555–63.
Tyack SG, Jenkins KA, O’Neil TE, Wise TG, Morris KR, Bruce MP, et al. A new method for producing transgenic birds via direct in vivo transfection of primordial germ cells. Transgenic Res. 2013;22:1257–64.
Lambeth LS, Morris KR, Wise TG, Cummins DM, O’Neil TE, Cao Y, et al. Transgenic chickens overexpressing aromatase have high estrogen levels but maintain a predominantly male phenotype. Endocrinology. 2016;157:83–90.
Zhang Z, Sun P, Yu F, Yan L, Yuan F, Zhang W, et al. Transgenic quail production by microinjection of lentiviral vector into the early embryo blood vessels. PLoS One. 2012;7:e50817.
Paques F, Haber JE. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 1999;63:349–404.
Rouet P, Smih F, Jasin M. Introduction of double-strand breaks into the genome of mouse cells by expression of a rare-cutting endonuclease. Mol Cell Biol. 1994;14:8096–106.
Bibikova M, Golic M, Golic KG, Carroll D. Targeted chromosomal cleavage and mutagenesis in Drosophila using zinc-finger nucleases. Genetics. 2002;161:1169–75.
Kim H, Kim JS. A guide to genome engineering with programmable nucleases. Nat Rev Genet. 2014;15:321–34.
Kim YG, Cha J, Chandrasegaran S. Hybrid restriction enzymes: zinc finger fusions to Fok I cleavage domain. Proc Natl Acad Sci U S A. 1996;93:1156–60.
Doyon Y, McCammon JM, Miller JC, Faraji F, Ngo C, Katibah GE, et al. Heritable targeted gene disruption in zebrafish using designed zinc-finger nucleases. Nat Biotechnol. 2008;26:702–8.
Geurts AM, Cost GJ, Freyvert Y, Zeitler B, Miller JC, Choi VM, et al. Knockout rats via embryo microinjection of zinc-finger nucleases. Science. 2009;325:433.
Miller JC, Tan S, Qiao G, Barlow KA, Wang J, Xia DF, et al. A TALE nuclease architecture for efficient genome editing. Nat Biotechnol. 2011;29:143–8.
Cox DB, Platt RJ, Zhang F. Therapeutic genome editing: prospects and challenges. Nat Med. 2015;21:121–31.
Reyon D, Tsai SQ, Khayter C, Foden JA, Sander JD, Joung JK. FLASH assembly of TALENs for high-throughput genome editing. Nat Biotechnol. 2012;30:460–5.
Schmid-Burgk JL, Schmidt T, Kaiser V, Honing K, Hornung V. A ligation-independent cloning technique for high-throughput assembly of transcription activator-like effector genes. Nat Biotechnol. 2013;31:76–81.
Mussolino C, Morbitzer R, Lutge F, Dannemann N, Lahaye T, Cathomen T. A novel TALE nuclease scaffold enables high genome editing activity in combination with low toxicity. Nucleic Acids Res. 2011;39:9283–93.
Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007;315:1709–12.
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–21.
Jinek M, Jiang F, Taylor DW, Sternberg SH, Kaya E, Ma E, et al. Structures of Cas9 endonucleases reveal RNA-mediated conformational activation. Science. 2014;343:1247997.
Anders C, Niewoehner O, Duerst A, Jinek M. Structural basis of PAM-dependent target DNA recognition by the Cas9 endonuclease. Nature. 2014;513:569–73.
Cho SW, Kim S, Kim JM, Kim JS. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat Biotechnol. 2013;31:230–2.
Ding Q, Regan SN, Xia Y, Oostrom LA, Cowan CA, Musunuru K. Enhanced efficiency of human pluripotent stem cell genome editing through replacing TALENs with CRISPRs. Cell Stem Cell. 2013;12:393–4.
Shen B, Zhang W, Zhang J, Zhou J, Wang J, Chen L, et al. Efficient genome modification by CRISPR-Cas9 nickase with minimal off-target effects. Nat Methods. 2014;11:399–402.
Komor AC, Kim YB, Packer MS, Zuris JA, Liu DR. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature. 2016;533:420–4.
Zetsche B, Gootenberg JS, Abudayyeh OO, Slaymaker IM, Makarova KS, Essletzbichler P, et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell. 2015;163:759–71.
Kim D, Kim J, Hur JK, Been KW, Yoon SH, Kim JS. Genome-wide analysis reveals specificities of Cpf1 endonucleases in human cells. Nat Biotechnol. 2016;34:863–8.
Kleinstiver BP, Tsai SQ, Prew MS, Nguyen NT, Welch MM, Lopez JM, et al. Genome-wide specificities of CRISPR-Cas Cpf1 nucleases in human cells. Nat Biotechnol. 2016;34:869–74.
Stern CD. The chick; a great model system becomes even greater. Dev Cell. 2005;8:9–17.
Schusser B, Collarini EJ, Yi H, Izquierdo SM, Fesler J, Pedersen D, et al. Immunoglobulin knockout chickens via efficient homologous recombination in primordial germ cells. Proc Natl Acad Sci U S A. 2013;110:20170–5.
Park TS, Lee HJ, Kim KH, Kim JS, Han JY. Targeted gene knockout in chickens mediated by TALENs. Proc Natl Acad Sci U S A. 2014;111:12716–21.
Oishi I, Yoshii K, Miyahara D, Kagami H, Tagami T. Targeted mutagenesis in chicken using CRISPR/Cas9 system. Sci Rep. 2016;6:23980.
Dimitrov L, Pedersen D, Ching KH, Yi H, Collarini EJ, Izquierdo S, et al. Germline gene editing in chickens by efficient CRISPR-mediated homologous recombination in primordial germ cells. PLoS One. 2016;11:e0154303.
Taylor L, Carlson DF, Nandi S, Sherman A, Fahrenkrug SC, McGrew MJ. Efficient TALEN-mediated gene targeting of chicken primordial germ cells. Development. 2017;144:928–34.
van de Lavoir MC, Collarini EJ, Leighton PA, Fesler J, Lu DR, Harriman WD, et al. Interspecific germline transmission of cultured primordial germ cells. PLoS One. 2012;7:e35664.
Cooper CA, Challagulla A, Jenkins KA, Wise TG, O’Neil TE, Morris KR, et al. Generation of gene edited birds in one generation using sperm transfection assisted gene editing (STAGE). Transgenic Res. 2017;26:331–47.
International Chicken Genome Sequencing C. Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature. 2004;432:695–716.
Dalloul RA, Long JA, Zimin AV, Aslam L, Beal K, Blomberg Le A, et al. Multi-platform next-generation sequencing of the domestic turkey (Meleagris gallopavo): genome assembly and analysis. PLoS Biol. 2010;8.
Warren WC, Clayton DF, Ellegren H, Arnold AP, Hillier LW, Kunstner A, et al. The genome of a songbird. Nature. 2010;464:757–62.
Pennisi E. Sequencing all life captivates biologists. Science. 2017;355:894–5.
Zhang G, Rahbek C, Graves GR, Lei F, Jarvis ED, Gilbert MT. Genomics: bird sequencing project takes off. Nature. 2015;522:34.
Lillico SG, McGrew MJ, Sherman A, Sang HM. Transgenic chickens as bioreactors for protein-based drugs. Drug Discov Today. 2005;10:191–6.
Park TS, Lee HG, Moon JK, Lee HJ, Yoon JW, Yun BNR, et al. Deposition of bioactive human epidermal growth factor in the egg white of transgenic hens using an oviduct-specific minisynthetic promoter. FASEB J. 2015;29:2386–96.
Lv Q, Yuan L, Deng J, Chen M, Wang Y, Zeng J, et al. Efficient generation of Myostatin gene mutated rabbit by CRISPR/Cas9. Sci Rep. 2016;6:25029.
Crispo M, Mulet AP, Tesson L, Barrera N, Cuadro F, dos Santos-Neto PC, et al. Efficient generation of Myostatin knock-out sheep using CRISPR/Cas9 technology and microinjection into zygotes. PLoS One. 2015;10:e0136690.
Wang K, Ouyang H, Xie Z, Yao C, Guo N, Li M, et al. Efficient generation of Myostatin mutations in pigs using the CRISPR/Cas9 system. Sci Rep. 2015;5:16623.
Tizard M, Hallerman E, Fahrenkrug S, Newell-McGloughlin M, Gibson J, de Loos F, et al. Strategies to enable the adoption of animal biotechnology to sustainably improve global food safety and security. Transgenic Res. 2016;25:575–95.
Johnson PA, Giles JR. The hen as a model of ovarian cancer. Nat Rev Cancer. 2013;13:432–6.
Petkov CI, Jarvis ED. Birds, primates, and spoken language origins: behavioral phenotypes and neurobiological substrates. Front Evol Neurosci. 2012;4:12.
Spierings MJ, Ten Cate C. Zebra finches as a model species to understand the roots of rhythm. Front Neurosci. 2016;10:345.
Velho TA, Lois C. Generation of transgenic zebra finches with replication-deficient lentiviruses. Cold Spring Harb Protoc. 2014;2014:1284–9.
Liu WC, Kohn J, Szwed SK, Pariser E, Sepe S, Haripal B, et al. Human mutant huntingtin disrupts vocal learning in transgenic songbirds. Nat Neurosci. 2015;18:1617–22.
Abe K, Matsui S, Watanabe D. Transgenic songbirds with suppressed or enhanced activity of CREB transcription factor. Proc Natl Acad Sci U S A. 2015;112:7599–604.
Biggs PM, Nair V. The long view: 40 years of Marek’s disease research and avian pathology. Avian Pathol. 2012;41:3–9.
Long JS, Giotis ES, Moncorge O, Frise R, Mistry B, James J, et al. Species difference in ANP32A underlies influenza a virus polymerase host restriction. Nature. 2016;529:101–4.
Acknowledgements
Not applicable.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2015R1A3A2033826).
Availability of data and materials
Not applicable.
Author information
Authors and Affiliations
Contributions
JYH conceived of the view of manuscript and critically revised the manuscript. YHP wrote and contributed to revising of the manuscript. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Han, J.Y., Park, Y.H. Primordial germ cell-mediated transgenesis and genome editing in birds. J Animal Sci Biotechnol 9, 19 (2018). https://doi.org/10.1186/s40104-018-0234-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40104-018-0234-4