Abstract
Background
The goal of this study was to compare the antagonism of elevated dietary Cu (250 mg/kg) from CuSO4 on three different Zn sources (ZnSO4 · H2O; [Zn bis(−2-hydroxy-4-(methylthio)butanoic acid)], Zn(HMTBa)2, a chelated Zn methionine hydroxy analogue; and Zn-Methionine), as measured using multiple indices of animal performance in ROSS 308 broilers.
Methods
Three experiments were conducted in broiler chicks fed a semi-purified diet. All birds were fed a Zn-deficient diet (8.5 mg/kg diet) for 1 wk, and then provided with the experimental diets for 2 wks.
Results
Experiment 1 was a 2 × 2 factorial design with two levels of Cu (8 vs. 250 mg/kg diet from CuSO4) and two Zn sources at 30 mg/kg [ZnSO4 · H2O vs. Zn(HMTBa)2]. Elevated Cu impaired growth performance only in birds fed ZnSO4. Compared to ZnSO4 · H2O, Zn(HMTBa)2 improved feed intake (12 %; P < 0.001) and weight gain (12 %, P < 0.001) and the benefits were more pronounced in the presence of 250 mg/kg diet Cu. Experiment 2 was a dose titration of ZnSO4 · H2O and Zn(HMTBa)2 at 30, 45, 60, and 75 mg/kg diet in the presence of 250 mg/kg CuSO4. Feed:gain was decreased and tibia Zn was increased with increasing Zn levels from 30 to 75 mg/kg. Birds fed Zn(HMTBa)2 consumed more food and gained more weight compared to birds fed ZnSO4, especially at lower supplementation levels (30 and 45 mg/kg; interaction P < 0,05). Experiment 3 compared two organic Zn sources (Zn(HMTBa)2 vs. Zn-Methionine) at 30 mg/kg with or without 250 mg/kg CuSO4. No interactions were observed between Zn sources and Cu levels on performance or tissue mineral concentrations. High dietary Cu decreased weight gain (P < 0.01). Tibia Cu and liver Cu were significantly increased with 250 mg/kg dietary Cu supplementation (P < 0.01). No difference was observed between the two Zn sources.
Conclusions
Dietary 250 mg/kg Cu significantly impaired feed intake and weight gain in birds fed ZnSO4 · H2O, but had less impact in birds fed Zn(HMTBa)2. No difference was observed between the two organic zinc sources. These results are consistent with the hypothesis that chelated organic Zn is better utilized than inorganic zinc in the presence of elevated Cu.
Similar content being viewed by others
Background
Elevated dietary CuSO4 (125–250 mg/kg diet) is often used in broiler and pig diets as a growth promoter and anti-bacterial agent, possibly by modulating the microbial population within the gastrointestinal tract [1, 2]. However, high dietary Cu can reduce the bioavailability of other important nutrients by forming insoluble complexes or by competing for absorption sites. The reciprocal antagonism between Zn and Cu is a prime example of competitive biological interactions between metals due to their similar chemical and physical properties [3, 4]. Excessive Zn supply has been shown to inhibit intestinal absorption, hepatic accumulation, and placental transfer of Cu, as well as to induce clinical and biochemical signs of Cu deficiency [5, 6]. The intestinal absorption of 65Zn was decreased by approximately 20 % when the dietary Cu intake was increased from 3 to 24 mg/kg diet. The mechanism(s) of Zn and Cu interaction is not well understood, and the data are controversial to interpret. They compete for uptake sites in the mucosa, and are regulated by the same metallothionein protein. In addition, they might impact each other’s solubility and binding behavior in gastrointestinal tract. Pang and Applegate indicated that high Cu supplementation (250 mg/kg in diet) increased the percentage of Zn associated with large complexes (>100,000 MW), and decreased the percentage of Zn associated with small complexes (<5,000 MW; P < 0.05), thereby suggesting an antagonism between Cu and Zn [7]. The association of Zn with smaller complexes might enhance the potential for absorption due to the larger surface area.
Most previous studies of dietary Zn-Cu antagonism have evaluated inorganic Zn and inorganic Cu. The availability of chelated organic trace minerals (OTM) in the marketplace now offers a potential solution to the inherent limitations associated with the widespread commercial practice of employing inorganic dietary CuSO4, by reducing and possibly avoiding the antagonism between Cu and Zn. Results from recent studies indicate that OTM might be more available for absorption, likely due to reduced incidence of antagonistic reactions with other dietary constituents in the gastrointestinal tract [8–10]. The relative bioavailability of a chelated Zn methionine hydroxy analogue [Zn bis(−2-hydroxy-4-(methylthio)butanoic acid; Zn(HMTBa)2] (compared to ZnSO4) in broilers was reported to be 161 % based on tibia Zn, and 248 % based on metallothionein mRNA expression [11]. When dietary Ca and P were increased, a common practice in diets of layers and pets, the relative bioavailability of chelated Zn increased to 441 % (based on μg total tibia Zn). The authors suggested that the difference in relative bioavailability might be attributed to the fact that chelated Zn possesses stronger chemical (coordinate-covalent) bonds relative to ZnSO4 and, thus, is more resistant to antagonisms in gastrointestinal tract. However, published literature on relative bioavailability of different sources of Zn is mixed. The inconsistencies could be due to differences in the chemical characteristics (and thus bioavailability) between different organic Zn forms. Adding to the complexity of comparing results from different studies using various forms of Zn is the inconsistency of terminology (organic, chelates, complex, etc.) along with the difficulty of characterizing chemical structures.
The primary objectives of the experiments reported here were to investigate the potential antagonism imposed by high dietary CuSO4 on Zn bioavailability in broilers, and to evaluate whether different Zn sources impact the Cu-mediated antagonism. We hypothesized that chelated Zn is more resistant to high Cu antagonism due to their stable structure and resistance to interactions with other nutrients in the gastrointestinal tract. Three Zn sources were used in the experiments: ZnSO4 · H2O, Zn(HMTBa)2, and Zn-methionine (Zn-MET). Zn(HMTBa)2 is a chelated Zn methionine hydroxy analogue [Zn bis(−2-hydroxy-4-(methylthio)butanoic acid)] at 2:1 ratio. To the best of our knowledge, this is the first report of the evaluation of the impact of high Cu on the utilization of different Zn sources in broilers.
Methods
The animal protocols for all experiments were approved by the Novus International Institutional Animal Care and Use Committee, and complied with all federal and state statutes ensuring the humane and ethical treatment of experimental animals. Diet formulation and in vivo activities were performed at the Novus International Research Farm. ZnSO4 · H2O was purchased from Sigma-Aldrich (Saint Louis, MO USA). Zn(HMTBa)2 (MINTREX® Zn, Novus International Inc, Saint Charles, MO USA) is a chelate of one Zn with two molecules of 2-hydroxy-4-(methylthio)butanoic acid (HMTBa), and was obtained from Novus International. Zinc methionine complex (Zn-MET) is the product resulting from complexing a soluble metal salt with methionine, and was purchased from Zinpro (Eden Prairie, MN USA) [12].
Three consecutive experiments were conducted. Experiment 1 was designed to determine whether 250 mg/kg dietary Cu from CuSO4 antagonizes two different Zn sources [ZnSO4 · H2O vs. Zn(HMTBa)2]. ZnSO4 · H2O and Zn(HMTBa)2 were added at 21.5 mg/kg to achieve 30 mg/kg total Zn in the finished feed (the other 8.5 mg/kg came from feed ingredients). Similar calculations were used in Experiments 2 and 3, such that different Zn sources were added on top of the basal to achieve the targeted Zn concentration in the final feed. A total of 288 ROSS 308 male chicks were randomly divided into four experimental treatments with six replicate pens per treatment and 12 birds per pen. The trial was a 2 × 2 factorial design with two levels of Cu (8 vs. 250 mg/kg diet from CuSO4) and two Zn sources at 30 mg/kg [ZnSO4 · H2O vs Zn(HMTBa)2]. Based on Experiment 1, Experiment 2 was designed to compare ZnSO4 · H2O and Zn(HMTBa)2 in a dose titration in the presence of 250 mg/kg diet CuSO4. A total of 576 ROSS 308 male chicks were allotted to eight experimental treatments with six replicate pens per treatment and 12 birds per pen. The dietary treatments included either ZnSO4 · H2O or Zn(HMTBa)2 added to achieve the following dietary levels: 30, 45, 60, and 75 mg/kg Zn. All diets contained 250 mg/kg diet Cu from CuSO4. Based on the results of the first two experiments, Experiment 3 was designed to compare two different organic Zn sources, Zn(HMTBa)2 and Zn-MET, on performance in the presence of 8 vs. 250 mg/kg CuSO4 dietary supplementation. A total of 480 day-old ROSS 308 male broilers were allotted to four treatments, with 8 replicate pens per treatment and 15 birds per pen. The trial was a 2 × 2 factorial design with two levels of Cu (8 and 250 mg/kg diet as CuSO4) and two Zn sources [Zn(HMTBa)2 vs. Zn-MET] at 30 mg/kg diet.
Birds were housed in stainless steel pens in a thermostatically controlled, electrically heated environment. The dimension of each battery pen was 51 × 69 × 35 cm (width, length, and height, respectively). Each pen was provided with water and an individual feeder. All birds were allowed to consume mash feed and water ad libitum. Room temperature was kept at 32 °C for the first two days, then reduced until a temperature of 23 °C was reached on d 17 of age. The light–dark cycle was as follows: on d 0 through d 7, there were 23 h of light and 1 h of darkness (lights off at 1200 h and on at 0100 h). On d 8 through d 22, there were 20 h of light and 4 h of darkness (lights off at 1200 h and on at 0400 h). All birds were fed a common semi-purified Zn-deficient diet (8.5 mg/kg diet) for the first week to reduce Zn stores and then were fed the experimental diets for about 2 wks (basal diet formula, see Table 1). A common semi-purified basal diet was used and formulated to meet National Research Council (1994) dietary recommendations for all nutrients except for Zn [13]. Each dietary treatment was then made by adding different Zn sources and Cu to the common basal. Methionine activity from Zn(HMTBa)2 (80 % methionine activity) [14], and Zn-MET (20 % methionine activity) was accounted for, and all diets were adjusted to have iso-methionine level.
Body weight and feed intake were recorded at beginning and the end of each experiment. On d 21 (d 19 for Experiment 3), one chick per cage (selected based on body weight approximating the pen mean) was chosen, euthanized with CO2, and the left tibia (without cartilage cap) and liver (Experiment 3)were collected for Zn and Cu (Experiment 3) analyses. Following the careful removal of muscle and connective tissue, the whole tibia was ashed. Tibia Zn and Cu were measured at the Novus International Analytical Services Laboratories (Saint Charles, MO USA) using Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES, Perkin Elmer, Shelton, CT USA) following an internally validated method based on AOAC 985.01 [15]. Tibia Zn and Cu level were reported on a dry ash weight basis, and liver Zn and Cu levels reported on a dry matter basis.
Statistical analyses
In Experiments 1 and 3, data were analyzed with a 2-way ANOVA using the General Linear Models (GLM) procedure of SAS (version 9.1; Cary, NC USA). The model included the main effects of Zn sources, Cu levels (with and without high CuSO4), and their interaction. In Experiment 2, data were analyzed with a 2-way ANOVA using the GLM procedure. The model included the main effect of two Zn sources [Zn(HMTBa)2 vs. ZnSO4 · H2O], and four different supplementation levels, and their 2-way interaction. Means were separated by Fisher’s protected least significant difference method when the F test was significant. Mortality data were transformed to square root of mortality + 1 before analysis. Data are presented in natural numbers. Pen served as the experimental unit. Effects were considered significant at 95 % probability (P ≤ 0.05).
Results
Experiment 1
As shown in Table 2, significant two-way interactions of Cu level and Zn source were observed on body weight (P < 0.05) and weight gain (P < 0.05). Compared to 8 mg/kg CuSO4, weight gain was decreased 14 % with 250 mg/kg Cu supplementation in the ZnSO4 · H2O groups (382 vs. 328 g), but not in the Zn(HMTBa)2 groups (404 vs. 390 g). Similar results were observed on final body weight, where 250 mg/kg dietary Cu reduced final body weight in ZnSO4 · H2O (465 vs. 407 g) but not in the Zn(HMTBa)2 groups (481 vs. 467 g). No interaction was observed on feed intake (P = 0.34, Table 2). For main effect, dietary 250 mg/kg CuSO4 decreased feed intake 10 % compared to the 8 mg/kg CuSO4 control groups regardless of Zn source (P < 0.001, Table 2). Compared to ZnSO4 · H2O, birds fed Zn(HMTBa)2 consumed 12 % more feed (P < 0.001). Feed conversion, mortality, and tibia Zn were not affected by dietary treatments (P > 0.50). In summary, dietary Cu from CuSO4 impaired feed intake across Zn sources, but decreased weight gain only in birds fed ZnSO4 · H2O. Compared to ZnSO4 · H2O, Zn(HMTBa)2 improved weight gain and feed intake, and the benefits were more profound with 250 mg/kg dietary Cu supplementation.
Experiment 2
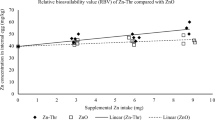
As shown in Table 3, significant two-way interactions of Zn source and level were observed on body weight (P = 0.02), weight gain (P = 0.005), and feed intake (P < 0.001, Table 3). The superiority of Zn(HMTBa)2 over ZnSO4 · H2O was more pronounced at lower levels of Zn supplementation. Birds achieved maximal performance at 45 mg/kg Zn supplementation from Zn(HMTBa)2, and no further improvement was observed at levels above that (Table 3). In contrast, performance was linearly improved in birds fed Zn from ZnSO4 · H2O from 30 to 75 mg/kg, with the best performance at the highest level. Feed:gain was decreased and tibia Zn was increased with increased Zn level regardless of the source (main effect, P < 0.05). No differences were observed on tibia Zn or mortality between Zn sources (P > 0.05). In summary, birds fed Zn(HMTBa)2 ate more and gained more compared to birds fed ZnSO4 · H2O, especially at lower levels of Zn supplementation. Less Zn(HMTBa)2 was needed to achieve similar performance compared to ZnSO4 · H2O. Feed efficiency and tibia Zn increased with increased dietary Zn in the range of 30 to 75 mg/kg.
Experiment 3
No interactions between Cu levels and Zn sources were observed on performance (P > 0.25; Table 4). No differences were observed on feed intake, feed efficiency, and mortality among treatments (P > 0.25, Table 4). Similar to experiment 1, birds fed 250 mg/kg CuSO4 gained less weight and were lighter compared to birds fed 8 mg/kg CuSO4 (main Cu effect, P < 0.05). Tibia Zn and liver Zn were not different among treatments (P > 0.50, Table 5). Dietary Cu supplementation significantly increased bone and hepatic Cu level, regardless of Zn sources (P < 0.001, Table 5). Liver Cu was increased greater than 10 fold with 250 mg/kg Cu supplementation. In summary, 250 mg/kg Cu decreased weight gain and significantly increased liver and tibia Cu concentration. No difference was observed between the two Zn sources.
Discussion
The data reported here are consistent with the hypothesis that chelated organic Zn forms are more efficient, compared to an inorganic zinc salt, in their abilities to overcome the antagonism mediated by elevated dietary Cu. Collectively, the data from our study indicate that a chelated form of Zn [Zn(HMTBa)2] outperforms the inorganic ZnSO4 · H2O, in the context of high dietary Cu-mediated antagonism in broiler chicks. Similarly, Richards et al. recently reported that Zn(HMTBa)2 is more bioavailable than ZnSO4, and the difference is greater under conditions of elevated dietary Ca and P supplementation [11].
To the best of our knowledge, this is the first report demonstrating that high dietary Cu in broilers antagonizes, to differing degrees, different dietary sources of Zn. We used a soy isolate/soy concentrate diet to assess the relative performance differences between zinc sources in chicks. The use of such a diet is an accepted model to assess relative bioavailability or performance differences between Zn sources [8]. The presence of phytate and fiber (similar to corn-soybean meal diets) makes this semi-purified diet more relevant to commercial diets compared to a crystalline amino acid, casein-dextrose, or egg white diet which are devoid of phytate.
The reciprocal antagonisms between dietary Zn and Cu are well known. For example, high dietary Cu (204 mg Cu/kg for 10 d) retarded growth performance in turkey poults [16]. Hall et al. reported a 20 % decrease in 65Zn absorption when dietary Cu was raised from 3 to 24 mg/kg in rats [5]. The mechanism(s) by which Cu antagonizes Zn is not well understood, although it has been postulated that these antagonisms are due to their similar chemical and physical properties [3]. It seems unlikely that Cu-Zn interaction occurs at a systemic level, because a) the absorption of these two metals occurs via different transporters, b) their functions and metabolism are quite different, and c) they do not share common storage proteins except metallothionein [3].
Dietary 250 mg/kg Cu significantly impaired feed intake and weight gain in birds fed ZnSO4 · H2O but had little or no impact in birds fed Zn(HMTBa)2. Computer modeling analyses suggest that the interaction between HMTBa and Zn (at a 2:1 molar ratio) is more stable over a range of physiological pH values compared to the interaction between methionine and Zn (at a 1:1 molar ratio) (unpublished observations). In turn, Zn(HMTBa)2 would be predicted to be more stable in solution than Zn-MET, and less susceptible to dietary antagonisms over the variable pH range found in the gastrointestinal tract. Thus, the inherent physicochemical features of Zn(HMTBa)2 might contribute to greater overall bioavailability and superior performance compared to the ZnSO4 · H2O evaluated here. Collectively, the data reported here indicate that Zn(HMTBa)2 was less susceptible to Cu-mediated antagonism compared to ZnSO4 · H2O. Furthermore, bioavailability experiments using tissue zinc levels and zinc-responsive gene expression as indicators of bioavailability have shown that Zn(HMTBa)2 exhibits greater relative bioavailability than these other sources [11, 17, 18].
In experiments such as these, careful consideration must be given to the response range of the outcome measures (eg weight gain, tissue Zn, etc.). Differences between Zn sources can only be determined at low intakes (below the requirement or inflection point). Homeostatic physiological mechanisms preclude the demonstration of differences between Zn sources, once the supplementation level is above the inflection point [8, 11, 19]. At deficient intake, Zn absorption is maximized. Bioavailability is significantly dependent on the degree of absorption. In purified diets, in the absence of phytate, antagonisms, or a challenge (lipopolysaccharides, etc.), there is little difference in utilization between organic and inorganic sources. In commercial diets or phytate-containing diets, absorption of organic sources of minerals are depressed, but to a lesser extent than inorganic sources. For example, it was demonstrated by Wedekind et al. that the inflection point or breakpoint varies among Zn sources [8]. The breakpoint or inflection point for bone Zn was determined to be 54, 60 and 65 mg total Zn per kg/diet for Zn-MET, ZnSO4 and ZnO, respectively for birds fed a corn-SBM diet. Their studies also showed an inflection point for weight gain for ZnSO4 for chicks fed a soy isolate diet to occur at 33 mg Zn/kg diet [20]. Comparison of Zn sources above the inflection point would result in an underestimation of the bioavailability or performance difference that may truly exist between Zn sources. These findings were confirmed in our studies. Marked differences between Zn(HMTBa)2 and ZnSO4 · H2O were observed at 30 and 45 mg Zn/kg diet, but not at higher levels. In the presence of excess Cu (250 mg Cu/kg diet), the requirement or inflection point for weight gain, although not defined in this study, is likely higher than the 33 mg Zn/kg determined by Wedekind et al., wherein no excesses of Ca or Cu were present [8].
Our study had some limitations and strengths. A limitation of this study was the amount of dietary Cu selected for the elevated Cu condition. For environmental reasons, the European Union and China restrict the maximum amount of Cu in animal feed. The 250 mg/kg diet CuSO4 is above the maximum allowed levels in these geographic areas, so the level used in this study might not be relevant for these regions. However, the mineral antagonism demonstrated in our studies is still important, since antagonism could potentially happen at lower levels of Cu supplementation, and 250 mg/kg dietary Cu is a level used commercially in the United States. Organic trace minerals, in general, and Zn(HMTBa)2, specifically, offer an alternative solution to address the challenge of providing required dietary minerals, at lower levels, to meet animal nutritional requirements while avoiding potential antagonism with other nutrients. Furthermore, in Experiment 2, differences between two Zn sources were only observed at 30 and 45 mg/kg, not at 60 and 75 mg/kg. This suggests that the Zn requirement (in the presence of 250 mg/kg Cu) is between 45 and 60 mg/kg. In future studies, dietary Zn supplementation should be chosen below the inflection point, to more sensitively assess bioavailability differences among different Zn sources. Finally, in future studies, biological function parameters should be measured to understand how Cu antagonizes Zn, along with the physiological consequences of that antagonism. For example measurement of metallothionein, collagen, and immune function have served as reliable indices in previous studies. More research is needed to understand the biological consequences of Cu-Zn antagonism in addition to performance and mineral storage in tissues.
The statistical design of our studies and the use of an experimental basal diet containing phytate were some of the strengths of our studies. A factorial design offers advantages over one-way ANOVA studies. There is more power to measure main effects as well as the ability to measure interactions. Significant interactions or trends were observed in all three experiments which demonstrated the improved performance of chelated Zn(HMTBa)2 vs ZnSO4 · H2O in the presence of elevated Cu. The presence of phytate, common in cereals and grains, is an important antagonist that reduces Zn bioavailability. In the presence of antagonisms (ie, phytate, fiber, elevated Cu, Ca, P, etc.), bioavailability differences between OTM and ITM are increased [20]. The presence of elevated Cu and phytate are conditions that are relevant to diets fed commercially to poultry and livestock. The advantages of the Zn(HMTBa)2 chelate demonstrated in our study under conditions of elevated Cu has also been demonstrated under other antagonistic conditions (eg, elevated Ca and P) [11]. Bioavailability of Zn(HMTBa)2, relative to ZnSO4 · H2O was 161 % (total bone Zn) and 248 % (metallothionein) in the presence of typical Ca and P (0.82 % Ca and 0.47 % available P). However, in the presence of elevated Ca and P (1.2 % Ca; 1 % available P) bioavailability of Zn(HMTBa)2, relative to ZnSO4 · H2O was even greater: 441 % (total bone Zn) and 426 % (metallothionein) [11].
The use of high dietary CuSO4 as a growth promoter is common practice in both the broiler and swine industries in North America and some other geographical areas. The bioavailability of other nutrients (Zn and P) should be considered when high CuSO4 is used. One practical strategy is to increase the level of addition of these other nutrients as is often practiced in today’s broiler industry. However, this could result in additional antagonisms along with excess nutrient excretion to the environment. Organic trace minerals have the potential advantage of providing dietary minerals that are more bioavailable. Consequently, less mineral is required to achieve a similar performance level compared to inorganic trace minerals [21, 22]. However, not all OTMs are equally capable of avoiding these antagonisms, and thus do not always provide equivalent bioavailability. More research is needed to fully decipher the mode of action of OTMs and their benefits, compared to inorganic sources of Zn, along with understanding the differences among different OTMs.
Conclusions
Dietary 250 mg/kg Cu significantly impaired feed intake and weight gain in birds fed ZnSO4 · H2O and had little or no impact in birds fed Zn(HMTBa)2. No significant differences were observed between Zn(HMTBa)2 and Zn-Met.
Abbreviations
- MW:
-
molecular weight
- OTM(s):
-
organic trace mineral(s)
- Zn(HMTBa)2 :
-
[Zn bis(−2-hydroxy-4-(methylthio)butanoic acid)] at 2:1 ratio
- Zn-MET:
-
zinc methionine
References
Apgar GA, Kornegay ET, Lindemann MD, Notter DR. Evaluation of copper sulfate and a copper lysine complex as growth promoters for weanling swine. J Anim Sci. 1995;73:2640–6.
Arias VJ, Koutsos EA. Effects of copper source and level on intestinal physiology and growth of broiler chickens. Poult Sci. 2006;85:999–1007.
Bremner I, Beattie JH. Copper and zinc metabolism in health and disease: speciation and interactions. Proc Nutr Soc. 1995;54:489–99.
Oestreicher P, Cousins RJ. Copper and zinc absorption in the rat: mechanism of mutual antagonism. J Nutr. 1985;115:159–66.
Hall AC, Young BW, Bremner I. Intestinal metallothionein and the mutual antagonism between copper and zinc in the rat. J Inorg Biochem. 1979;11:57–66.
Hill GM, Ku PK, Miller ER, Ullrey DE, Losty TA, O’Dell BL. A copper deficiency in neonatal pigs induced by a high zinc maternal diet. J Nutr. 1983;113:867–72.
Pang Y, Applegate TJ. Effects of dietary copper supplementation and copper source on digesta pH, calcium, zinc, and copper complex size in the gastrointestinal tract of the broiler chicken. Poult Sci. 2007;86:531–7.
Wedekind KJ, Hortin AE, Baker DH. Methodology for assessing zinc bioavailability: efficacy estimates for zinc-methionine, zinc sulfate, and zinc oxide. J Anim Sci. 1992;70:178–87.
Yan F, Waldroup PW. Evaluation of MINTREX® manganese as a source of manganese for young broilers. Int J Poult Sci. 2006;5:708–13.
Wang Z, Cerrate S, Coto C, Yan F, Waldroup PW. Evaluation of MINTREX® copper as source of copper in broiler diets. Int J Poult Sci. 2007;6:308–13.
Richards JD, Fisher P, Evans JL, Wedekind KJ. Greater bioavailability of chelated compared to inorganic zinc in broiler chicks in presence of elevated calcium and phosphorus. Open Access Animal Physiol. 2015;7:1–14.
AAFCO Committees. AAFCO 2015 Official Publication. Champaign (IL): Association of American Feed Control Officials (AAFCO); 2015.
National Research Council (U.S.), Subcommittee on Poultry Nutrition. Nutrient requirements of poultry. 9th ed. Washington: National Academy Press; 1994.
Yi GF, Atwell CA, Hume JA, Dibner JJ, Knight CD, Richards JD. Determining the methionine activity of Mintrex organic trace minerals in broiler chicks by using radiolabel tracing or growth assay. Poult Sci. 2007;86:877–87.
International AOAC. Official methods of analysis of AOAC International. 18th ed. Gaithersburg: AOAC International; 2005.
Ward TL, Watkins KL, Southern LL. Interactive effects of dietary copper and water copper level on growth, water intake, and plasma and liver copper concentrations of poults. Poult Sci. 1994;73:1306–11.
Manangi MK, Vazquez-Añon M, Richards JD, Carter S, Buresh RE, Christensen KD. Impact of feeding lower levels of chelated trace minerals vs. industry levels of inorganic trace minerals on broiler performance, yield, foot pad health, and litter mineral concentration. J Appl Poul Res. 2012;21:881–90.
Richards JD, Zhao J, Harrell RJ, Atwell CA, Dibner JJ. Trace mineral nutrition in poultry and swine. Asian-Aust J Anim Sci. 2010;23:1527–34.
Schlegel P, Windisch W. Bioavailability of zinc glycinate in comparison with zinc sulphate in the presence of dietary phytate in an animal model with Zn labelled rats. J Anim Physiol Anim Nutr (Berl). 2006;90:216–22.
Wedekind KJ, Baker DH. Zinc bioavailability in feed-grade sources of zinc. J Anim Sci. 1990;68:684–9.
Zhao J, Shirley RB, Vazquez-Añon M, Dibner JJ, Richards JD, Fisher P, et al. Effects of chelated trace minerals on growth performance, breast meat yeld, amd footpad health in commercial meat broilers. J Appl Poul Res. 2010;19:365–72.
Zhao J, Shirley RB, Hampton TR, Richards JD, Harrell RJ, Dibner JJ, et al. A dose titration comparison of MINTREX® versus ZnSO4 on performance in broilers with high dietary copper supplementation. Poult Sci. 2008;87:51–2.
Acknowledgments
The authors gratefully acknowledge the participation, expertise, and dedication of the entire staff of the Novus Research Farm.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
KJW, FY, PF, JLE, TRH, and MV-A are employees of Novus International, which manufactures and markets organic trace mineral products including zinc chelated to hydroxymethylthiobutyric acid [Zn(HMTBa)2; MINTREX® Zn]. JZ, RBS, and JJD were employees of Novus during the course of the study and analysis of the data; JJD is currently a paid consultant of Novus.
Authors’ contributions
JZ participated in the 1) design of the study, 2) execution of the experiments, 3) analyses of the data, 4) interpretation of the data, and 5) preparation of the manuscript; JZ is the guarantor of the data and other content in this manuscript. RBS participated in the 1) design of the study, 2) execution of the experiments, 3) analyses of the data, 4) interpretation of the data. JJD participated in the 1) design of the study, 2) execution of the experiments, 3) analyses of the data, 4) interpretation of the data, and 5) preparation of the manuscript. KJW participated in the 1) design of the study, 2) execution of the experiments, 3) analyses of the data, 4) interpretation of the data, and 5) preparation of the manuscript. FY participated in the 1) analyses of the data, 2) interpretation of the data, and 3) preparation of the manuscript. PF participated in the 1) execution of the experiments. THR participated in the 1) execution of the experiments. JLE participated in the 1) interpretation of the data and 2) preparation of the manuscript. MV-A participated in the 1) design of the study, 2) analyses of the data, 3) interpretation of the data, and 4) preparation of the manuscript. All authors read and approved the final version of manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhao, J., Shirley, R.B., Dibner, J.J. et al. Superior growth performance in broiler chicks fed chelated compared to inorganic zinc in presence of elevated dietary copper. J Animal Sci Biotechnol 7, 13 (2016). https://doi.org/10.1186/s40104-016-0072-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40104-016-0072-1