Abstract
Probiotics are living microorganisms that provide a wide variety of health benefits to the host when ingested in adequate amounts. The bacterial strains most frequently used as probiotic agents are lactic acid bacteria, such as Lactobacillus reuteri, which is one of the few endogenous Lactobacillus species found in the gastrointestinal tract of vertebrates, including humans, rats, pigs and chickens. L. reuteri is one of the most well documented probiotic species and has been widely utilized as a probiotic in humans and animals for many years. Initially, L. reuteri was used in humans to reduce the incidence and the severity of diarrhea, prevent colic and necrotic enterocolitis, and maintain a functional mucosal barrier. As interest in alternatives to in-feed antibiotics has grown in recent years, some evidence has emerged that probiotics may promote growth, improve the efficiency of feed utilization, prevent diarrhea, and regulate the immune system in pigs. In this review, the characteristics of L. reuteri are described, in order to update the evidence on the efficacy of using L. reuteri in pigs.
Similar content being viewed by others
Introduction
Antibiotics are a common additive in livestock feed which have been widely used for growth promotion and prophylaxis purposes in farm animals during the past several decades [1]. However, antibiotic resistance is a looming public health crisis. The use of antibiotics as growth promoters has been forbidden in the European Union, Korea, and Japan. Other countries including the United States and China may ban the feeding of antibiotics within the next few years. As a result, there is increasing interest concerning alternatives to in-feed antibiotics, such as probiotics, prebiotics, plant products and organic acids in the livestock industry [2].
Probiotics are living microorganisms, which, when consumed in adequate amounts, can confer a health benefit to the host [3]. In farm animals, probiotics have been shown to promote growth, improve the efficiency of feed utilization, modulate the gastrointestinal ecosystem, stimulate the immune system and protect the host from gastrointestinal tract (GIT) diseases [4]. Therefore, probiotics provide a potential alternative strategy to in-feed antibiotics [5].
The properties of probiotics are strain-specific, and suitable probiotic strains for pigs are usually selected based on some criteria including pig origin, acid and bile tolerance, their ability to adhere to intestinal cells and to colonise the intestinal tract, production of antimicrobial substances, antibiotic resistance patterns, demonstrable efficacy and safety, and stability to the conditions used in industrial processes [6-8]. The organisms most frequently used as probiotic agents are lactic acid bacteria (LAB) [9], such as Lactobacillus, which are a normal inhabitant of the GIT [10]. Lactobacillus reuteri is one of the dominant species in the GIT of vertebrates such as humans, rats, pigs and chickens [11]. It is one of the most well documented probiotic species and has been widely utilized as a probiotic in humans and animals [12-15].
In recent years, numerous probiotic strains have been used in pig production. The application of probiotics provide a potential alternative strategy to the use of antibiotics. The aim of this review is to systematically review and update the evidence on the efficacy of using L. reuteri in pigs.
Characteristics of Lactobacillus reuteri
L. reuteri is a heterofermentative bacterium, and is considered to be one of the few true autochthonous Lactobacillus species in humans and animals. Many researchers have already selected some specific L. reuteri strains isolated from human feces, breast milk, the human vagina, the human oral cavity, guinea pigs, rats, pigs, broilers and sourdough. There is now mounting evidence to show that selected L. reuteri strains have probiotic characteristics, and can provide health benefits to their hosts. We have constructed a summary table (Table 1), in order to provide an overview of the reported L. reuteri strains used as probiotics.
Probiotic properties
Probiotic bacteria encounter various stresses after ingestion by the host, including exposure to a low pH in the stomach and contact with bile in the small intestine. L. reuteri I5007, initially known as Lactobacillus fermentum I5007, was selected from over 7,000 native Lactobacilli colonies according to criteria including resistance to heat, low pH, copper, and bile salts, as well as storage stability and antagonism to pathogenic agents [16]. Other L. reuteri strains also show resistance to low pH and bile salts [12,17-19].
Adhesion of a probiotic strain to the host GIT is important for bacterial colonization, pathogen exclusion, and interaction with host cells for the protection of epithelial cells or immune modulation [20]. Several studies have demonstrated that L. reuteri have the capacity to colonize, and can adhere to mucin and intestinal epithelial cells [17,21-23]. L. reuteri I5007 shows strong adhesion to Caco-2 cells, IEC-6 cells, IPEC-J2 cells, and porcine intestinal mucus [15,21]. The possible mechanism for L. reuteri adherence and colonization involved in adhesion, has been linked to mucus-binding protein [24], surface protein [22], D-alanyl-LTA [25], exopolysaccharide [26], glucosyltransferase A and inulosucrase [27].
L. reuteri has been reported to produce a variety of antimicrobial substances such as lactic acid, hydrogen peroxide [28], reuterin [29-31], and reutericyclin [32], which have beneficial effects for the host organism. L. reuteri strains have been demonstrated to inhibit the in vitro growth of many enteric pathogens, including Escherichia coli, Salmonella Typhimurium, Staphylococcus epidermidis, Staphylococcus aureus, Helicobacter pylori, and rotavirus [12,19,33]. In addition, L. reuteri can produce vitamin B12 [31,34], and has the capacity to de novo synthesize L-lysine and folic acid based on a computer simulation model [15].
L. reuteri exhibited free radical-scavenging capacity in vitro [35], and encoded various antioxidant enzymes [15]. Studies in animals and humans have shown that oral administration of L. reuteri reduced the incidence and the severity of diarrhea, decreased visceral pain, prevented colic and necrotic enterocolitis, maintained a functional mucosal barrier, and induced colonization and immunomodulation [36-39].
Safety and stability aspects
L. reuteri has the most extensive safety assessment record of any probiotic strain. A number of studies conducted both in vivo and in vitro indicate that L. reutei is safe for human consumption, even in large amounts [38,40]. However, as is the case for all other species of LAB, plasmids can be found in some strains of L. reuteri [15,41,42], and some of these plasmids have been shown to encode for antibiotic resistance genes [42]. According to the European Food Safety Authority, probiotics should not contain known antibiotic resistance traits. L. reuteri ATCC 55730 is a commercially available probiotic strain which has been found to carry potentially transferable resistance traits for tetracycline and lincomycin. Therefore, it has been replaced by L. reuteri DSM 17938, a strain where the two resistance plasmids have been removed without losing any probiotic characteristics [42].
Probiotic strains must be able to resist any adverse conditions encountered during industrial production in order to survive [43]. L. reuteri is sensitive to heat, and therefore, freeze-drying is commonly used for maintaining the stability of L. reuteri. Subjecting L. reuteri to a higher fermentation temperature (47°C) or a neutral pH (pH 6.7) has been shown to increase the survival of L. reuteri during subsequent freeze-drying [44].
Strains
Not all L. reuteri strains are the same or provide a beneficial response, and the evolution of L. reuteri with vertebrates resulted in the emergence of host specialization [45]. Probiotic strains need to be carefully chosen, and evaluated for their safety and effectiveness using in vitro assays, animal models, and clinical trials. There are numerous strains of L. reuteri, which have some minor differences that make them unique (Table 1).
L. reuteri DSM 17938, L. reuteri NCIMB 30242, L. reuteri ATCC PTA 6475 which are of human origin are the most commonly used in dietary supplements and have been researched the most. In pigs, L. reuteri I5007, was isolated from the colonic mucosa of healthy weaning piglets, and has been demonstrated in several studies to have probiotic properties [15,21,35,46-49].
Applications of probiotic L. reuteri for pigs
In pigs, the administration of L. reuteri has been shown to have beneficial effects on performance, prevention of diarrhea, stress relief, altered gut microbiota, and immunomodulation. The applications of L. reuteri for pigs are listed in Table 2. Noticeably, L. reuteri is mainly used in neonatal piglets and during the post-weaning period.
Improved performance
In the pig industry, the use of probiotics improves intestinal health which can improve pig performance. Supplementation of L. reuteri has resulted in improved growth and feed efficiency in neonatal and growing pigs. Liu et al. [46] reported that L. reuteri I5007 (6 × 109 CFU/d) supplementation increased average daily gain (ADG) in formula-fed piglets. Wang et al. [47] found that administration of L. reuteri I5007 significantly increased weight gain and feed conversion compared with weaned pigs fed without L. reuteri I5007. Also, Wang et al. [50] reported that weaned piglets supplemented with L. reuteri had faster growth and higher feed intakes than unsupplemented piglets. However, feed conversion was unaffected by L. reuteri supplementation. In addition, Wang et al. [48] showed that dietary supplementation with L. reuteri or aureomycin significantly improved the performance of weanling piglets, and there was no difference between the two feed additives. Other studies using L. reuteri BSA131 tended to show improved ADG and feed conversion in weaned pigs [12]. Wang et al. [51] also reported that supplementation with L. reuteri X-1 increased ADG and feed conversion. Yu et al. [52] determined the influence of different levels of L. reuteri I5007 on performance, nutrient digestibility and immunity of weaned pigs. The results demonstrated that the ideal supplemental concentration of L. reuteri was 5.8 × 107 CFU/g feed.
Prevention of diarrhea
Diarrhea is one of the most frequent causes of heavy economic losses in swine operations [53]. The effect of L. reuteri against diarrhea in pigs was confirmed in several reports [16,46,47,54,55]. Diarrhea incidence was lower in piglets fed L. reuteri I5007 compared with a control [46]. Enterotoxigenic E. coli (ETEC) are a major cause of diarrhea in neonatal and weaned pigs [55]. Huang et al. [16] showed that a native Lactobacilli complex preparation (including L. gasseri, L. reuteri, L. acidophilus and L. fermentum) could effectively prevent weaning piglet diarrhea when administered before challenge with an E. coli solution (serovars K99, K88 and 987P at a ratio of 1:1:1). Wang et al. [47] reported that 12, 24, and 48 h after challenge, pigs challenged with E. coli had mild diarrhea and mild fecal scores. Supplementation of L. reuteri I5007 did not alleviate these effects. Only on day 10, did feeding L. reuteri I5007 decrease the occurrence of diarrhea. Chen et al. [54] demonstrated that reuteran produced by L. reuteri may prevent piglet diarrhea by reducing adhesion of ETEC K88.
Alleviate stress
Pigs in industrial farming systems are frequently exposed to oxidative stress, which results in decreased performance and reduced immune function. L. reuteri has been shown to be effective in scavenging free radicals in vitro, and could be used to alleviate oxidative stress [35,37]. Wang et al. [35] reported that supplementation of L. reuteri I5007 improved the antioxidant status of growing-finishing pigs (from 50 to 90 kg) as evidenced by increased levels of antioxidant enzymes such as superoxide dismutase and glutathione peroxidase, and decreased levels of malondialdehyde. Wang et al. [50] determined the anti-oxidative effect of L. reuteri I5007 in weaning piglets using an oxidative stress model induced by diquat. Their results showed that diquat-injection decreased the performance of weaning pigs and increased plasma levels of cortisol, adrenaline, carbonyl and malondialdehyde. L. reuteri supplementation alleviated oxidative stress and enhanced the performance of weanling pigs.
Weaning is one of the most stressful periods that results in gastrointestinal, immunological, and behavioral changes [56]. Wang et al. [48] demonstrated that L. reuteri I5007 alleviated weaning stress syndrome by enhancing the levels of proteins involved in energy metabolism, lipid metabolism, cell structure and mobility, protein synthesis, and immune response, thereby facilitating cellular proliferation and depressing apoptosis.
Modulation of gut microbiota
L. reuteri in neonatal piglets can be used to support the development of a stable microbiota, to stimulate the immune system and to prevent diarrheal diseases. During the weaning and post-weaning periods, L. reuteri is used in pigs to modulate the gastrointestinal microbiota as it aims to prevent post-weaning diarrhea and stimulate growth. Liu et al. [46] reported that L. reuteri I5007 plays a positive role in gut development in neonatal piglets by modulating the microbial composition and intestinal development, Denaturing gradient gel electrophoresis (DGGE) revealed that L. reuteri I5007 affected the colonic microbial communities on day 14 and, in particular, reduced numbers of Clostridium spp. In weaning pigs, administration of L. reuteri BSA131 decreased the number of enterobacteria in the feces [12]. Huang et al. [16] showed that a Lactobacilli compound (including L. gasseri, L. reuteri, L. acidophilus and L. fermentum) significantly decreased E. coli and aerobe counts, and increased Lactobacilli and anaerobe counts in the digesta and mucosa of most sections of the GIT compared with a control group. In addition, oral administration of L. reuteri I5007 not only increased the concentration of butyrate and other branched chain fatty acids but also decreased Clostridium strains accompanied by a lowered pH in the colonic digesta [46]. This indicates that administration of L. reuteri modulates gut microbiota, and thereby affected the microbial metabolites.
Immunomodulation
Probiotics such as L. reuteri may stimulate or suppress innate immune responses via several mechanisms including modulation of pro-inflammatory cytokines. L. reuteri strains can be divided into two subsets, immunosuppressive (ATCC PTA 6475 and ATCC PTA 5289) and immunostimulatory strains (ATCC 55730 and CF48-3A), and each subset has potential therapeutic value [57]. The effects of L. reuteri on immunomodulation were documented in pigs. Wang et al. [47] reported that oral administration of L. reuteri I5007 could enhance T-cell differentiation and induce ileal cytokine expression, which suggests that this probiotic strain could modulate immune function in weaned piglets. Yu et al. [52] showed that L. reuteri I5007 supplementation increased serum specific anti-OVA IgG levels. In neonatal piglets, L. reuteri has been found to decrease the mRNA expression of IL-1β in the ileum [46]. Azevedo et al. [58] found that L. reuteri combined with L. acidophilus could help to maintain immunological homeostasis in neonatal gnotobiotic pigs infected with human rotavirus by regulating TGF-β production.
Conclusions and perspectives
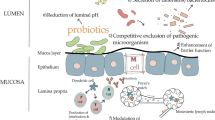
In conclusion, L. reuteri is a probiotic bacteria that is one of the few true autochthonous Lactobacillus species. Numerous studies have demonstrated that they can positively improve performance, prevent diarrhea, alleviate stress, alter gastrointestinal microbiota, regulate the immune system, and thereby improve pig performance and health. The beneficial effects of L. reuteri in pigs have been related to different modes of action. The improvements in pig performance of supplemental L. reuteri are mostly due to the fact that L. reuteri has the ability to colonize the GIT, produce antimicrobial substances and stimulate the intestinal immune system (Figure 1), thereby promoting nutrient metabolism and improve health. However, a clear mode of action has yet to be described. It appears from the data presented that the benefical effects of L. reuteri are strain specific. It will be important to select more powerful or targeted strains. Unfortunately, the viability of L. reuteri is a key criteria for developing L. reuteri products. To expand the probiotic L. reuteri application in pigs, care must be taken during processing techniques such as microencapsulation to maintain bacterial stability.
Mechanisms of L . reuteri modulating in the gut. ① L. reuteri can produce a variety of antimicrobial substances (AMS) such as lactic acid, and reuterin [28-30]. ② L. reuteri has the capacity to colonize, and can adhere to mucin and intestinal epithelial cells [17,21,22] . ③ L. reuteri has been shown to stimulate or suppress innate immune responses by affected the production of cytokines in macrophages (M), monocytes, and dendritic cells (DCs). The modulation of dendritic cells by L. reuteri has been shown to be mediated through dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) and promote development of regulatory T cells producing high amounts of interleukin-10 (IL-10) and transforming growth factor-β (TGF-β) [59,60]. ④ L. reuteri has been reported affected the colonic microbial communities and short chain fatty acid (SCFA) concentration [46]. Please see text for details and references.
Pig husbandry has entered an era when the use of antibiotics is increasingly unwelcomed. Probiotics, which are a potential alternative to in feed antibiotics, can expect a promising future. Besides selection of excellent strains and improved processing techniques, more research, especially in the form of well-designed animal trials, is needed to evaluate the efficacy of L. reuteri. More studies are also needed to explore the mechanisms of action of L. reuteri in pigs. An important fact is that L. reuteri added to pig diets may potentially help improve performance. With evolving knowledge, effective use of L. reuteri will be possible in the future.
References
Thacker PA. Alternatives to antibiotics as growth promoters for use in swine production: a review. J Anim Sci Biotechnol. 2013;4:35.
Wang Z, Zeng X, Mo Y, Smith K, Guo Y, Lin J. Identification and characterization of a bile salt hydrolase from Lactobacillus salivarius for development of novel alternatives to antibiotic growth promoters. Appl Environ Microbiol. 2012;78:8795–802.
Guarner F, Schaafsma GJ. Probiotics. Int J Food Microbiol. 1998;39:237–8.
Gaggia F, Mattarelli P, Biavati B. Probiotics and prebiotics in animal feeding for safe food production. Int J Food Microbiol. 2010;141 Suppl 1:S15–28.
Cho JH, Zhao PY, Kim IH. Probiotics as a dietary additive for pigs: a review. J Anim Vet Adv. 2011;10:2127–34.
Kailasapathy K, Chin J. Survival and therapeutic potential of probiotic organisms with reference to Lactobacillus acidophilus and Bifidobacterium spp. Immunol Cell Biol. 2000;78:80–8.
Tuomola E, Crittenden R, Playne M, Isolauri E, Salminen S. Quality assurance criteria for probiotic bacteria. Am J Clin Nutr. 2001;73:393S–8.
Shokryazdan P, Sieo CC, Kalavathy R, Liang JB, Alitheen NB, Jahromi MF, et al. Probiotic potential of Lactobacillus strains with antimicrobial activity against some human pathogenic strains. Biomed Res Int. 2014;2014:927268.
Land MH, Rouster-Stevens K, Woods CR, Cannon ML, Cnota J, Shetty AK. Lactobacillus sepsis associated with probiotic therapy. Pediatrics. 2005;115:178–81.
Tannock GW, Tilsala-Timisjarvi A, Rodtong S, Ng J, Munro K, Alatossava T. Identification of Lactobacillus isolates from the gastrointestinal tract, silage, and yoghurt by 16S-23S rRNA gene intergenic spacer region sequence comparisons. Appl Environ Microbiol. 1999;65:4264–7.
Oh PL, Benson AK, Peterson DA, Patil PB, Moriyama EN, Roos S, et al. Diversification of the gut symbiont Lactobacillus reuteri as a result of host-driven evolution. ISME J. 2010;4:377–87.
Chang YH, Kim JK, Kim HJ, Kim WY, Kim YB, Park YH. Selection of a potential probiotic Lactobacillus strain and subsequent in vivo studies. Antonie Van Leeuwenhoek. 2001;80:193–9.
Coccorullo P, Strisciuglio C, Martinelli M, Miele E, Greco L, Staiano A. Lactobacillus reuteri (DSM 17938) in infants with functional chronic constipation: a double-blind, randomized, placebo-controlled study. J Pediatr. 2010;157:598–602.
Francavilla R, Lionetti E, Castellaneta S, Ciruzzi F, Indrio F, Masciale A, et al. Randomised clinical trial: Lactobacillus reuteri DSM 17938 vs. placebo in children with acute diarrhoea-a double-blind study. Aliment Pharmacol Ther. 2012;36:363–9.
Hou C, Wang Q, Zeng X, Yang F, Zhang J, Liu H, et al. Complete genome sequence of Lactobacillus reuteri I5007, a probiotic strain isolated from healthy piglet. J Biotechnol. 2014;179:63–4.
Huang CH, Qiao SY, Li DF, Piao XS, Ren JP. Effects of Lactobacillus on the performance, diarrhea incidence, VFA concentration and gastrointestinal microbial flora of weaning pigs. Asian-Aust J Anim Sci. 2004;17:401–9.
Yu B, Liu JR, Chiou MY, Hsu YR, Chiou PWS. The effects of probiotic Lactobacillus reuteri Pg4 strain on intestinal characteristics and performance in broilers. Asian-Aust J Anim Sci. 2007;20:1243–51.
Whitehead K, Versalovic J, Roos S, Britton RA. Genomic and genetic characterization of the bile stress response of probiotic Lactobacillus reuteri ATCC 55730. Appl Environ Microbiol. 2008;74:1812–9.
Seo BJ, Mun MR, Rejish Kumar J, Kim CJ, Lee I, Chang YH, et al. Bile tolerant Lactobacillus reuteri isolated from pig feces inhibits enteric bacterial pathogens and porcine rotavirus. Vet Res Commun. 2010;34:323–33.
Lebeer S, Vanderleyden J, De Keersmaecker SC. Genes and molecules of lactobacilli supporting probiotic action. Microbiol Mol Biol Rev. 2008;72:728–64.
Li XJ, Yue LY, Guan XF, Qiao SY. The adhesion of putative probiotic lactobacilli to cultured epithelial cells and porcine intestinal mucus. J Appl Microbiol. 2008;104:1082–91.
Wang B, Wei H, Yuan J, Li Q, Li Y, Li N, et al. Identification of a surface protein from Lactobacillus reuteri JCM1081 that adheres to porcine gastric mucin and human enterocyte-like HT-29 cells. Curr Microbiol. 2008;57:33–8.
Miyoshi Y, Okada S, Uchimura T, Satoh E. A mucus adhesion promoting protein, MapA, mediates the adhesion of Lactobacillus reuteri to Caco-2 human intestinal epithelial cells. Biosci Biotechnol Biochem. 2006;70:1622–8.
Mackenzie DA, Jeffers F, Parker ML, Vibert-Vallet A, Bongaerts RJ, Roos S, et al. Strain-specific diversity of mucus-binding proteins in the adhesion and aggregation properties of Lactobacillus reuteri. Microbiology. 2010;156:3368–78.
Walter J, Loach DM, Alqumber M, Rockel C, Hermann C, Pfitzenmaier M, et al. D-alanyl ester depletion of teichoic acids in Lactobacillus reuteri 100–23 results in impaired colonization of the mouse gastrointestinal tract. Environ Microbiol. 2007;9:1750–60.
Wang Y, Gänzle MG, Schwab C. Exopolysaccharide synthesized by Lactobacillus reuteri decreases the ability of enterotoxigenic Escherichia coli to bind to porcine erythrocytes. Appl Environ Microbiol. 2010;76:4863–6.
Walter J, Schwab C, Loach DM, Ganzle MG, Tannock GW. Glucosyltransferase A (GtfA) and inulosucrase (Inu) of Lactobacillus reuteri TMW1.106 contribute to cell aggregation, in vitro biofilm formation, and colonization of the mouse gastrointestinal tract. Microbiology. 2008;154:72–80.
Martinez RC, Seney SL, Summers KL, Nomizo A, De Martinis EC, Reid G. Effect of Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 on the ability of Candida albicans to infect cells and induce inflammation. Microbiol Immunol. 2009;53:487–95.
Bian L. An in vitro antimicrobial and safety study of Lactobacillus reuteri DPC16 for validation of probiotic concept. Master thesis: Massey University; 2008.
Amin HM, Hashem AM, Ashour MS, Hatti-Kaul R. 1,2 Propanediol utilization by Lactobacillus reuteri DSM 20016, role in bioconversion of glycerol to 1,3 propanediol, 3-hydroxypropionaldehyde and 3-hydroxypropionic acid. J Genet Eng Biotechnol. 2013;11:53–9.
Morita H, Toh H, Fukuda S, Horikawa H, Oshima K, Suzuki T, et al. Comparative genome analysis of Lactobacillus reuteri and Lactobacillus fermentum reveal a genomic island for reuterin and cobalamin production. DNA Res. 2008;15:151–61.
Gänzle MG, Höltzel A, Walter J, Jung G, Hammes WP. Characterization of reutericyclin produced by Lactobacillus reuteri LTH2584. Appl Environ Microbiol. 2000;66:4325–33.
Mukai T, Asasaka T, Sato E, Mori K, Matsumoto M, Ohori H. Inhibition of binding of Helicobacter pylori to the glycolipid receptors by probiotic Lactobacillus reuteri. FEMS Immunol Med Microbiol. 2002;32:105–10.
Taranto MP, Vera JL, Hugenholtz J, De Valdez GF, Sesma F. Lactobacillus reuteri CRL1098 produces cobalamin. J Bacteriol. 2003;185:5643–7.
Wang AN, Yi XW, Yu HF, Dong B, Qiao SY. Free radical scavenging activity of Lactobacillus fermentum in vitro and its antioxidative effect on growing-finishing pigs. J Appl Microbiol. 2009;107:1140–8.
Hoffmann M, Rath E, Holzlwimmer G, Quintanilla-Martinez L, Loach D, Tannock G, et al. Lactobacillus reuteri 100–23 transiently activates intestinal epithelial cells of mice that have a complex microbiota during early stages of colonization. J Nutr. 2008;138:1684–91.
Atkins HL, Geier MS, Prisciandaro LD, Pattanaik AK, Forder RE, Turner MS, et al. Effects of a Lactobacillus reuteri BR11 mutant deficient in the cystine-transport system in a rat model of inflammatory bowel disease. Dig Dis Sci. 2012;57:713–9.
Urbanska M, Szajewska H. The efficacy of Lactobacillus reuteri DSM 17938 in infants and children: a review of the current evidence. Eur J Pediatr. 2014;173:1327–37.
Dicksved J, Schreiber O, Willing B, Petersson J, Rang S, Phillipson M, et al. Lactobacillus reuteri maintains a functional mucosal barrier during DSS treatment despite mucus layer dysfunction. PLoS One. 2012;7, e46399.
Lee DY, Seo YS, Rayamajhi N, Kang ML, Lee SI, Yoo HS. Isolation, characterization, and evaluation of wild isolates of Lactobacillus reuteri from pig feces. J Microbiol. 2009;47:663–72.
Heavens D, Tailford LE, Crossman L, Jeffers F, Mackenzie DA, Caccamo M, et al. Genome sequence of the vertebrate gut symbiont Lactobacillus reuteri ATCC 53608. J Bacteriol. 2011;193:4015–6.
Rosander A, Connolly E, Roos S. Removal of antibiotic resistance gene-carrying plasmids from Lactobacillus reuteri ATCC 55730 and characterization of the resulting daughter strain, L. reuteri DSM 17938. Appl Environ Microbiol. 2008;74:6032–40.
van de Guchte M, Serror P, Chervaux C, Smokvina T, Ehrlich SD, Maguin E. Stress responses in lactic acid bacteria. Antonie Van Leeuwenhoek. 2002;82:187–216.
Liu XT, Hou CL, Zhang J, Zeng XF, Qiao SY. Fermentation conditions influence the fatty acid composition of the membranes of Lactobacillus reuteri I5007 and its survival following freeze-drying. Lett Appl Microbiol. 2014;59:398–403.
Frese SA, Benson AK, Tannock GW, Loach DM, Kim J, Zhang M, et al. The evolution of host specialization in the vertebrate gut symbiont Lactobacillus reuteri. PLoS Genet. 2011;7, e1001314.
Liu H, Zhang J, Zhang S, Yang F, Thacker PA, Zhang G, et al. Oral administration of Lactobacillus fermentum I5007 favors intestinal development and alters the intestinal microbiota in formula-fed piglets. J Agric Food Chem. 2014;62:860–6.
Wang A, Yu H, Gao X, Li X, Qiao S. Influence of Lactobacillus fermentum I5007 on the intestinal and systemic immune responses of healthy and E. coli challenged piglets. Antonie Van Leeuwenhoek. 2009;96:89–98.
Wang X, Yang F, Liu C, Zhou H, Wu G, Qiao S, et al. Dietary supplementation with the probiotic Lactobacillus fermentum I5007 and the antibiotic aureomycin differentially affects the small intestinal proteomes of weanling piglets. J Nutr. 2012;142:7–13.
Hou CL, Zhang J, Liu XT, Liu H, Zeng XF, Qiao SY. Superoxide dismutase recombinant Lactobacillus fermentum ameliorates intestinal oxidative stress through inhibiting NF-kappaB activation in a trinitrobenzene sulphonic acid-induced colitis mouse model. J Appl Microbiol. 2014;116:1621–31.
Wang AN, Cai CJ, Zeng XF, Zhang FR, Zhang GL, Thacker PA, et al. Dietary supplementation with Lactobacillus fermentum I5007 improves the anti-oxidative activity of weanling piglets challenged with diquat. J Appl Microbiol. 2013;114:1582–91.
Wang SP, Yang LY, Tang XS, Cai LC, Liu G, Kong XF, et al. Dietary supplementation with high-dose Bacillus subtilis or Lactobacillus reuteri modulates cellular and humoral immunities and improves performance in weaned piglets. J Food Agric Environ. 2011;9:181–7.
Yu H, Wang A, Li X, Qiao S. Effect of viable Lactobacillus fermentum on the growth performance, nutrient digestibility and immunity of weaned pigs. J Anim Feed Sci. 2008;17:61–9.
Fairbrother JM, Nadeau E, Gyles CL. Escherichia coli in postweaning diarrhea in pigs: an update on bacterial types, pathogenesis, and prevention strategies. Anim Health Res Rev. 2005;6:17–39.
Chen XY, Woodward A, Zijlstra RT, Ganzle MG. Exopolysaccharides synthesised by Lactobacillus reuteri protect against enterotoxigenic Escherichia coli in piglets. Appl Environ Microbiol. 2014;80:5752–60.
Francis DH. Enterotoxigenic Escherichia coli infection in pigs and its diagnosis. J Swine Health Prod. 2002;10:171–5.
Campbell JM, Crenshaw JD, Polo J. The biological stress of early weaned piglets. J Anim Sci Biotechnol. 2013;4:19.
Jones SE, Versalovic J. Probiotic Lactobacillus reuteri biofilms produce antimicrobial and anti-inflammatory factors. BMC Microbiol. 2009;9:35.
Azevedo MS, Zhang W, Wen K, Gonzalez AM, Saif LJ, Yousef AE, et al. Lactobacillus acidophilus and Lactobacillus reuteri modulate cytokine responses in gnotobiotic pigs infected with human rotavirus. Benef Microbes. 2012;3:33–42.
Walter J, Britton RA, Roos S. Host-microbial symbiosis in the vertebrate gastrointestinal tract and the Lactobacillus reuteri paradigm. Proc Natl Acad Sci U S A. 2011;108 Suppl 1:4645–52.
Liu Y, Fatheree NY, Mangalat N, Rhoads JM. Human-derived probiotic Lactobacillus reuteri strains differentially reduce intestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1087–96.
Jones ML, Martoni CJ, Ganopolsky JG, Sulemankhil I, Ghali P, Prakash S. Improvement of gastrointestinal health status in subjects consuming Lactobacillus reuteri NCIMB 30242 capsules: a post-hoc analysis of a randomized controlled trial. Expert Opin Biol Ther. 2013;13:1643–51.
Jones ML, Martoni CJ, Prakash S. Cholesterol lowering and inhibition of sterol absorption by Lactobacillus reuteri NCIMB 30242: a randomized controlled trial. Eur J Clin Nutr. 2012;66:1234–41.
Jones ML, Martoni CJ, Prakash S. Oral supplementation with probiotic L. reuteri NCIMB 30242 increases mean circulating 25-hydroxyvitamin D: a post hoc analysis of a randomized controlled trial. J Clin Endocrinol Metab. 2013;98:2944–51.
Dommels YE, Kemperman RA, Zebregs YE, Draaisma RB, Jol A, Wolvers DA, et al. Survival of Lactobacillus reuteri DSM 17938 and Lactobacillus rhamnosus GG in the human gastrointestinal tract with daily consumption of a low-fat probiotic spread. Appl Environ Microbiol. 2009;75:6198–204.
Liu Y, Fatheree NY, Dingle BM, Tran DQ, Rhoads JM. Lactobacillus reuteri DSM 17938 changes the frequency of Foxp3+ regulatory T cells in the intestine and mesenteric lymph node in experimental necrotizing enterocolitis. PLoS One. 2013;8, e56547.
Fåk F, Bäckhed F. Lactobacillus reuteri prevents diet-induced obesity, but not atherosclerosis, in a strain dependent fashion in Apoe−/− mice. PLoS One. 2012;7, e46837.
Eaton KA, Honkala A, Auchtung TA, Britton RA. Probiotic Lactobacillus reuteri ameliorates disease due to enterohemorrhagic Escherichia coli in germfree mice. Infect Immun. 2011;79:185–91.
Iniesta M, Herrera D, Montero E, Zurbriggen M, Matos AR, Marin MJ, et al. Probiotic effects of orally administered Lactobacillus reuteri-containing tablets on the subgingival and salivary microbiota in patients with gingivitis. a randomized clinical trial. J Clin Periodontol. 2012;39:736–44.
Keller MK, Bardow A, Jensdottir T, Lykkeaa J, Twetman S. Effect of chewing gums containing the probiotic bacterium Lactobacillus reuteri on oral malodour. Acta Odontol Scand. 2012;70:246–50.
Kohler GA, Assefa S, Reid G. Probiotic interference of Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 with the opportunistic fungal pathogen Candida albicans. Infect Dis Obstet Gynecol. 2012;2012:636474.
Lin CH, Lin CC, Shibu MA, Liu CS, Kuo CH, Tsai FJ, et al. Oral Lactobacillus reuteri GMN-32 treatment reduces blood glucose concentrations and promotes cardiac function in rats with streptozotocin-induced diabetes mellitus. Br J Nutr. 2014;111:598–605.
Mehling H, Busjahn A. Non-viable Lactobacillus reuteri DSMZ 17648 (Pylopass) as a new approach to Helicobacter pylori control in humans. Nutrients. 2013;5:3062–73.
Hsieh FC, Lee CL, Chai CY, Chen WT, Lu YC, Wu CS. Oral administration of Lactobacillus reuteri GMNL-263 improves insulin resistance and ameliorates hepatic steatosis in high fructose-fed rats. Nutr Metab (Lond). 2013;10:35.
Lu YC, Yin LT, Chang WT, Huang JS. Effect of Lactobacillus reuteri GMNL-263 treatment on renal fibrosis in diabetic rats. J Biosci Bioeng. 2010;110:709–15.
Guo J, Mauch A, Galle S, Murphy P, Arendt EK, Coffey A. Inhibition of growth of Trichophyton tonsurans by Lactobacillus reuteri. J Appl Microbiol. 2011;111:474–83.
Valladares R, Sankar D, Li N, Williams E, Lai KK, Abdelgeliel AS, et al. Lactobacillus johnsonii N6.2 mitigates the development of type 1 diabetes in BB-DP rats. PLoS One. 2010;5, e10507.
Livingston M, Loach D, Wilson M, Tannock GW, Baird M. Gut commensal Lactobacillus reuteri 100–23 stimulates an immunoregulatory response. Immunol Cell Biol. 2010;88:99–102.
Zhao PY, Kim IH. Effect of direct-fed microbial on growth performance, nutrient digestibility, fecal noxious gas emission, fecal microbial flora and diarrhea score in weanling pigs. Anim Feed Sci Technol. 2015;200:86–92.
De Angelis M, Siragusa S, Caputo L, Ragni A, Burzigotti R, Gobbetti M. Survival and persistence of Lactobacillus plantarum 4.1 and Lactobacillus reuteri 3S7 in the gastrointestinal tract of pigs. Vet Microbiol. 2007;123:133–44.
Acknowledgments
This research was financially supported by the National Natural Science Foundation of China (Grant Number 31420103908).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CH and SQ carried out the literature study and drafted the manuscript. XZ, FY and HL critically evaluated the manuscript. All authors read and approved the final manuscript.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Hou, C., Zeng, X., Yang, F. et al. Study and use of the probiotic Lactobacillus reuteri in pigs: a review. J Animal Sci Biotechnol 6, 14 (2015). https://doi.org/10.1186/s40104-015-0014-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40104-015-0014-3