Abstract
Purpose
This meta-analysis of randomized controlled trials (RCTs) aims to evaluate the efficacy and safety in cervical disc arthroplasty (CDA) and anterior cervical discectomy and fusion (ACDF) for treating cervical degenerative disc diseases (CDDDs).
Methods
The authors searched RCTs in the electronic databases (Cochrane Central Register of Controlled Trials, PubMed, EMBASE, Medline, Embase, Springer Link, Web of Knowledge, OVID and Google Scholar) from their establishment to march 2016 without language restrictions. We also manually searched the reference lists of articles and reviews for possible relevant studies. Researches on CDA versus ACDF in CDDDs were selected in this meta-analysis. The quality of all studies was assessed and effective data was pooled for this meta-analysis. Outcome measurements were surgical parameters (operative time, blood loss, and length of hospital stay), clinical indexes [neck disability index (NDI), neurological success, range of motion (ROM), Visual Analogue Score (VAS)], complications [the number of adverse events, adjacent segment disease (ASD), and reoperation]. Subgroup analysis, sensitivity analysis, and publication bias assessment were also performed, respectively. The meta-analysis was performed with software revman 5.3.
Results
37 articles (20 RCTs) with a total 4004 patients (2212 in the CDA and 1792 in the ACDF) met inclusion criteria. Eight types of disc prostheses were used in the included studies. Patients were followed up for at least 2 years in all the studies. No statistically significant differences were found between CDA and ACDF for blood loss [SMD −0.02; 95 % CI (−0.20, 0.17)], length of hospital stay [MD −0.06; 95 % CI (−0.19, 0.06)]. Statistical differences were found between operative time [MD 14.22; 95 % CI (6.73, 21.71)], NDI [SMD −0.27; 95 % CI (−0.43, −0.10)], neurological success [RR 1.13; 95 % CI (1.08, 1.18)], ROM [MD 6.72; 95 % CI (5.72, 7.71)], VAS of neck [SMD −0.40; 95 % CI (−0.75, −0.04)], VAS of arm [SMD −0.55; 95 % CI (−1.04, −0.06)], the rate of adverse events [RR 0.72 95 % CI (0.53, 0.96)], the rate of ASD [RR 0.62; 95 % CI (0.43, 0.88)], and reoperation [RR 0.50; 95 % CI (0.39, 0.63)]. Subgroup analysis stratified by different types of disc prostheses was also performed.
Conclusions
CDA is associated with higher clinical indexes and fewer complications than ACDF, indicating that it is a safe and effective treatment for CDDDs. However, the operative time of CDA is longer than ACDF. Because of some limitations, these findings should be interpreted with caution. Additional studies are needed. Large, definitive RCTs are needed.
Similar content being viewed by others
Background
Since anterior cervical discectomy and fusion (ACDF) was first described by Smith and Robinson, ACDF is widely accepted as a traditional gold standard surgical procedure for cervical degenerative disc diseases (CDDDs) which included radiculopathy and myelopathy (Bohlman et al. 1993). Clinical studies have reported good outcomes after ACDF (Yue et al. 2005). However, complications of ACDF such as dysphagia, dysphonia, loss of range of motion, pseud-arthrosis and adjacent segment degeneration (ASD) still confuse the spine surgeons.
To avoid complications after as ACDF, the cervical disc arthroplasty (CDA) is designed (DiAngelo et al. 2003). CDA is a treatment option for spine surgeons with the aim of preserving motion at the treated level. During the past decade, the CDA has emerged as an alternative treatment to ACDF and has been shown to provide the pain relief and functional improvements similar or superior to those of ACDF. However, complications of CDA such as instability and heterotopic ossification also confuse the spine surgeons (Zechmeister et al. 2011).
A few previous meta-analyses (Fallah et al. 2012; Gao et al. 2013, 2015; Jiang et al. 2012; Luo et al. 2015a, b; Li et al. 2015; Muheremu et al. 2015; Rao et al. 2015; Ren et al. 2014; Shriver et al. 2015; Verma et al. 2013; Wu et al. 2015; Xing et al. 2013; Yao et al. 2015; Yin et al. 2013; Yang et al. 2012; Yu et al. 2011; Zhu et al. 2016; Zhong et al. 2016) have focused on this problem, but they have different conclusions about whether CDA is superior to CDA in treating CDDDs (Table 1). They used single-site data which is part of a multicenter trial or missed some important data. In the same time, many randomized controlled trials (RCTs) comparing CDA with ACDF for the treatment of CDDDs were performed (Burkus et al. 2010, 2014; Cheng et al. 2009, 2011; Coric et al. 2011; Davis et al. 2013; Delamarter and Zigler 2013; Delamarter et al. 2010; Davis et al. 2015; Hisey et al. 2014, 2015; Heller et al. 2009; Kesman et al. 2012; Kelly et al. 2011; McAfee et al. 2010; Mummaneni et al. 2007; Murrey et al. 2008, 2009; Phillips et al. 2013, 2015; Nabhan et al. 2007a, b, c, 2011; Porchet and Metcalf 2004; Qizhi et al. 2014; Riina et al. 2008; Riew et al. 2008; Rozankovic et al. 2014; Sasso et al. 2007, 2008, 2011; Skeppholm et al. 2015; Vaccaro et al. 2013; Zhang et al. 2012, 2014; Zigler et al. 2013). Therefore, an updated meta-analysis is needed which is based on the latest high quality studies. To solve this problem, we performed an updated meta-analysis to compare the outcomes between CDA and ACDF in treating CDDDs.
Methods
Search strategy
To make an exhaustive search of all relevant literatures, two independent reviewers (LX and ML) conducted a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. We searched RCTs in the online electronic databases (Cochrane Central Register of Controlled Trials, PubMed, EMBASE, Medline, Embase, Springer Link, Web of Knowledge, OVID and Google Scholar) from their establishment to march 2016 without language restrictions. We also manually searched the reference lists of articles and reviews for possible relevant studies. The following Mesh and free text search terms included: “anterior cervical decompression and fusion”, “anterior cervical discectomy and fusion”, “cervical disc replacement”, “disc prostheses” and “cervical arthroplasty” with a limit of “clinical trial”.
Inclusion and exclusion criteria
Studies were eligible for inclusion if they met the following criteria: (1) RCTs comparing CDA with ACDF; (2) a minimum 2-year follow-up. Studies were excluded if they met the following criteria: nonrandomized studies, retrospective studies, reviews, commentaries, meta-analyses, and animal studies; duplicate publications of one trial; and single-site data as part of a multicenter trial. Two reviewers (LX and DZM) independently selected the potentially qualified trials according to the inclusion and exclusion criteria. Any disagreement was resolved by discussion and a conformity was reached.
Data extraction
Study characteristics and secondary surgical outcomes were extracted independently by two reviewers (LX and ML) using a data extraction form, with discrepancies being arbitrated by consensus with a third reviewer (DZM). Informations extracted from studies included studies design, type of prostheses, center, numbers, age, the rate of male, the rate of follow up, surgical parameters (operative time, blood loss, and length of hospital stay), clinical indexes [neck disability index (NDI), neurological success, range of motion (ROM), Visual Analogue Score (VAS)], complications (the number of adverse events, ASD, and reoperation). The time point of clinical indexes and complications is 24 months after operation.
Quality assessment
Quality evaluation of methodology of included studies was performed according to the Cochrane Collaboration’s tool for assessing risk of bias. Reviewers (PL and FD) independently determined random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome data, selective outcome reporting, intend to treat analysis, group similarity at baseline and other sources of bias.
Statistical analysis
All data were performed with Review Manager 5.3 software (The Nordic Cochrane Center, Cochrane Collaboration, Copenhagen, Denmark). The relative risk (RR) and its 95 % confidence interval (CI) were calculated for count data. Standardized mean difference (SMD) or mean difference (MD) and its 95 % CI were calculated for continuous outcomes. P < 0.05 was considered to be statistically significant. Heterogeneity was assessed using Chi squared and I-squared tests. Values of I 2 greater than 50 % with P < 0.05 were considered to be substantial heterogeneity. Subgroup analyses were applied to identify the source of the heterogeneity and random model was applied when significant heterogeneity was observed among the included studies.
Results
Search results
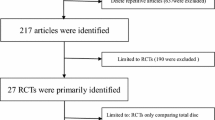
The details of the literature search and selection are displayed in Fig. 1. A total of 1338 relevant researches were identified through PubMed (N = 749), EMBASE (N = 389), CENTRAL (N = 128), and reference lists (N = 72). 1221 trials were remained after excluding the duplicates. After reviewing the titles and abstracts, 1184 trials were excluded because they did not reach the standard of inclusion criteria. A full text review was accessed in the retaining 37 studies, and finally, 20 RCTs with 4004 individuals (CDA = 2212, ACDF = 1792) were included in this meta-analysis (Burkus et al. 2010, 2014; Cheng et al. 2009, 2011; Coric et al. 2011; Davis et al. 2013; Delamarter and Zigler 2013; Delamarter et al. 2010; Davis et al. 2015; Hisey et al. 2014, 2015; Heller et al. 2009; Kesman et al. 2012; Kelly et al. 2011; McAfee et al. 2010; Mummaneni et al. 2007; Murrey et al. 2008, 2009; Phillips et al. 2013, 2015; Nabhan et al. 2007a, b, c, 2011; Porchet and Metcalf 2004; Qizhi et al. 2014; Riina et al. 2008; Riew et al. 2008; Rozankovic et al. 2014; Sasso et al. 2007, 2008, 2011; Skeppholm et al. 2015; Vaccaro et al. 2013; Zhang et al. 2012, 2014; Zigler et al. 2013) (Fig. 2). We recorded the characteristics of 37 included papers in Table 2 (Burkus et al. 2010, 2014; Cheng et al. 2009, 2011; Coric et al. 2011; Davis et al. 2013; Delamarter and Zigler 2013; Delamarter et al. 2010; Davis et al. 2015; Hisey et al. 2014, 2015; Heller et al. 2009; Kesman et al. 2012; Kelly et al. 2011; McAfee et al. 2010; Mummaneni et al. 2007; Murrey et al. 2008, 2009; Phillips et al. 2013; Nabhan et al. 2007a, b, c, 2011; Phillips et al. 2015; Porchet and Metcalf 2004; Qizhi et al. 2014; Riina et al. 2008; Riew et al. 2008; Rozankovic et al. 2014; Sasso et al. 2007, 2008, 2011; Skeppholm et al. 2015; Vaccaro et al. 2013; Zhang et al. 2012, 2014; Zigler et al. 2013).
Quality assessment
the risk of bias of each study was independently assessed by two authors (ML, LX), in accordance with the Cochrane risk of bias tool, which defines nine aspects: (1) random sequence generation (selection bias); (2) allocation concealment (selection bias); (3) blinding of participants (performance bias); (4) blinding of treatment providers (performance bias); (5) blinding of outcome assessors (detection bias); (6) intention to treat (attrition bias); (7) selective reporting (reporting bias); (8) comparable study groups; and (9) other bias. A qualification of risk of bias, including low risk, unclear risk, or high risk, was provided (Fig. 2). The final qualification for each study was determined by consensus among three authors (ML, LX, and DZM).
Study characteristics
All 37 studies included in this meta-analysis were RCTs, 14 RCTs were conducted in the United States, and the other six were done in Asia and Europe. The years of publication ranged from 2004 to 2015. Sample sizes ranged from 19 to 582, and a total of 4004 patients (2212 in the CDA and 1792 in the ACDF) were enrolled in the 37 studies. Disc prostheses types included Bryan (Medtronic Sofamor Danek, Memphis, TN, USA), Discover (DePuy Spine, Raynham, MA, USA), Kineflex (Spinal Motion Inc, Mountain View, CA, USA), Mobi-C (LDR Medical, Troyes, France), PCM (NuVasive Inc, San Diego, CA, USA), Prestige ST (Medtronic Sofamor Danek, Memphis, TN, USA), ProDisc-C (Synthes Inc, West Chester, PA, USA), SECURE-C (Globus Medical, Audubon, PA, USA). Fifteen of the included studies were multi-center trials; Five were a single-center trials (Table 2).
Outcome analysis of surgical parameters
The operation time of the CDA group was significantly longer than that of the ACDF group [MD 14.22; 95 % CI (6.73, 21.71)] (Fig. 3a). However, the amount of blood loss showed no significant difference between two groups [SMD −0.02; 95 % CI (−0.20, 0.17)] (Fig. 3b). Also, there was no significant difference in the length of hospital stay [MD −0.06; 95 % CI (−0.19, 0.06)] (Fig. 3c).
Outcome analysis of clinical indexes
The NDI score [SMD −0.27; 95 % CI (−0.43, −0.10)] (Fig. 4a2), VAS of neck [SMD −0.40; 95 % CI (−0.75, −0.04)] (Fig. 4d) and VAS of arm [SMD −0.55; 95 % CI (−1.04, −0.06)] (Fig. 4d) of the CDA group was significantly lower than that of the ACDF group. The rate of neurological success [RR 1.13; 95 % CI (1.08, 1.18)] (Fig. 4b) and ROM [MD 6.72; 95 % CI (5.72, 7.71)] (Fig. 4c) was significantly higher than that of the ACDF group.
Outcome analysis of complications
The rate of adverse events [RR 0.72 95 % CI (0.53, 0.96)], the rate of ASD [RR 0.62; 95 % CI (0.43, 0.88)], and reoperation [RR 0.50; 95 % CI (0.39, 0.63)] of the CDA group was significantly lower than that of the ACDF group (Fig. 5).
Publication bias
The publication bias was evaluated by a funnel plot. The funnel plot shapes showed no obvious evidence of a symmetry. The results suggested that publication bias was not evident in this meta-analysis.
Sensitivity analysis
Due to the high heterogeneity in the above analysis, we performed subgroup analysis in the meta-analysis based on different disc prostheses types. A sensitivity analysis was also conducted by removing one study at a time. We found that Rozankovic (Rozankovic et al. 2014) influenced the NDI scores in this analysis.
Discussion
CDDDs can result in arm and neck pain, walking instability or a combination of symptoms which included myelopathy and radiculopathy. When symptoms do not respond to conservative treatment, operative treatment is considered. ACDF is an effective treatment for patients with symptomatic CDDDs (Bohlman et al. 1993). It has been performed for about 50 years. However, the loss of motion at the operated level can increase motion at the adjacent levels. ASD emerges gradually as a common complication. The original design purpose of CDA is to maintain the motion of operated level (DiAngelo et al. 2003). The technique is to restore and maintain the original biomechanics of cervical spine, which is attempted to prevent adjacent level degeneration at the operated segments. However, controversy still surrounds regarding whether CDA is better than ACDF.
There have been a few meta-analyses comparing the safety and efficacy between ACDF and CDA (Fallah et al. 2012; Gao et al. 2013, 2015; Jiang et al. 2012; Luo et al. 2015a, b; Li et al. 2015; Muheremu et al. 2015; Rao et al. 2015; Ren et al. 2014; Shriver et al. 2015; Verma et al. 2013; Wu et al. 2015; Xing et al. 2013; Yao et al. 2015; Yin et al. 2013; Yang et al. 2012; Yu et al. 2011; Zhu et al. 2016; Zhong et al. 2016). However, they have different conclusions (Table 1). To determine the effectiveness and safety of CDA for the treatment of symptomatic cervical disc disease, we performed a meta-analysis of RCTs on this subject. In our meta-analysis, we selected 20 RCTs comparing ACDF with CDA. We compared the surgical parameters (operative time, blood loss, and length of hospital stay), clinical indexes (NDI, neurological success, ROM, VAS), complications (the number of adverse events, ASD, and reoperation). The results of this meta-analysis indicated that CDA was superior to ACDF regarding fewer severe advents, fewer ASDs, fewer reoperations, better neurological success, greater ROM, lower NDI scores and greater neck and arm pain functional recovery. However, the outcomes of operative time are favor to the ACDF group.
Most of previous meta-analysis did not report the surgical parameters (Table 1). In our meta-analysis, the surgical parameters include operative time, blood loss and length of hospital stay. Our meta-analysis indicated that the operation time of the CDA group was significantly longer than that of the ACDF group. However, the amount of blood loss showed no significant difference between two groups. Also, there was no significant difference in the length of hospital stay. The operation time was associated with the different prosthesis types and the level of surgeons. Previous meta-analyses have different conclusions about the clinical indexes between CDA and ACDF (Table 1). In our meta-analysis, the clinical indexes include NDI, neurological success, ROM, VAS. Our study found that the CDA group had significantly better ROM and rate of neurological success, lower NDI scores, significantly lower neck pain scores, and lower arm pain scores than the ACDF group. The clinical indexes are associated with many factors such as decompression technique and ASD. Previous meta-analyses also have different conclusions about the complications between CDA and ACDF (Table 1). In our meta-analysis, the complications include Adverse events, ASD and reoperations. Our results indicated that adverse events, ASD and reoperations in ACDF group were more common than that in CDA group.
There are some strengths in our study. First, this is an updated meta-analysis with a larger sample size and included the latest RCTs to evaluate the efficacy and safety between CDA and ACDF in CDDDs. Second, we used Cochrane risk of bias to assess the quality of evidence.
Although this meta-analysis was performed with the best available evidence presently, some unavoidable weaknesses earned to be noted. First, the follow-up times of all the trials are different. In our paper, we choose 24 months as the time point of most trials, so we combined some articles. Second, many important studies only presented the VAS and NDI scores improvement (include the reductions and improvement) which was not the original data, so only 700–900 patients out of 4004 patients were available. Third, our results are affected by heterogeneity. For example, the results of operation time, blood loss, lengths of the hospital stay, ROM at the operated level, and VAS presented significant heterogeneity. Maybe various surgery interventions, different disc prostheses types and surgical technologies at different centers may influence the results. The results of this meta-analysis should be cautiously accepted. Large, definitive RCTs with longer-term follow-up are needed.
Conclusions
In summary, our updated meta-analysis indicated the CDA was superior to ACDF regarding fewer severe advents, fewer ASDs, fewer reoperations, better neurological success, greater ROM and greater neck and arm pain functional recovery. However, the outcomes of operative time and NDI scores are favor to the ACDF group. More high-quality studies with longer term follow-up are needed to provide a better evaluation of the effectiveness and safety between the two treatments.
Abbreviations
- CDA:
-
cervical disc arthroplasy
- ACDF:
-
anterior cervical discectomy and fusion
- CDDDs:
-
cervical degenerative disc diseases
- RCTs:
-
randomized controlled trials
- MD:
-
mean difference
- NDI:
-
neck disability index
- ROM:
-
range of motion
- VAS:
-
Visual Analogue Score
- RR:
-
relative risk
- CI:
-
confidence interval
References
Bohlman HH, Emery SE, Goodfellow DB, Jones PK (1993) Robinson anterior cervical discectomy and arthrodesis for cervical radiculopathy. Long-term follow-up of one hundred and twenty-two patients. J Bone Joint Surg Am 75(9):1298–1307
Burkus JK, Haid RW, Traynelis VC, Mummaneni PV (2010) Long-term clinical and radiographic outcomes of cervical disc replacement with the Prestige disc: results from a prospective randomized controlled clinical trial. J Neurosurg Spine 13(3):308–318. doi:10.3171/2010.3.SPINE09513
Burkus JK, Traynelis VC, Haid RW Jr, Mummaneni PV (2014) Clinical and radiographic analysis of an artificial cervical disc: 7-year follow-up from the Prestige prospective randomized controlled clinical trial: Clinical article. J Neurosurg Spine 21(4):516–528. doi:10.3171/2014.6.SPINE13996
Cheng L, Nie L, Zhang L, Hou Y (2009) Fusion versus Bryan Cervical Disc in two-level cervical disc disease: a prospective, randomised study. Int Orthop 33(5):1347–1351. doi:10.1007/s00264-008-0655-3
Cheng L, Nie L, Li M, Huo Y, Pan X (2011) Superiority of the Bryan((R)) disc prosthesis for cervical myelopathy: a randomized study with 3-year followup. Clin Orthop Relat Res 469(12):3408–3414. doi:10.1007/s11999-011-2039-z
Coric D, Nunley PD, Guyer RD, Musante D, Carmody CN, Gordon CR, Lauryssen C, Ohnmeiss DD, Boltes MO (2011) Prospective, randomized, multicenter study of cervical arthroplasty: 269 patients from the Kineflex|C artificial disc investigational device exemption study with a minimum 2-year follow-up: clinical article. J Neurosurg Spine 15(4):348–358. doi:10.3171/2011.5.SPINE10769
Davis RJ, Kim KD, Hisey MS, Hoffman GA, Bae HW, Gaede SE, Rashbaum RF, Nunley PD, Peterson DL, Stokes JK (2013) Cervical total disc replacement with the Mobi-C cervical artificial disc compared with anterior discectomy and fusion for treatment of 2-level symptomatic degenerative disc disease: a prospective, randomized, controlled multicenter clinical trial: clinical article. J Neurosurg Spine 19(5):532–545. doi:10.3171/2013.6.SPINE12527
Davis RJ, Nunley PD, Kim KD, Hisey MS, Jackson RJ, Bae HW, Hoffman GA, Gaede SE, Danielson GO III, Gordon C, Stone MB (2015) Two-level total disc replacement with Mobi-C cervical artificial disc versus anterior discectomy and fusion: a prospective, randomized, controlled multicenter clinical trial with 4-year follow-up results. J Neurosurg Spine 22(1):15–25. doi:10.3171/2014.7.SPINE13953
Delamarter RB, Zigler J (2013) Five-year reoperation rates, cervical total disc replacement versus fusion, results of a prospective randomized clinical trial. Spine 38(9):711–717. doi:10.1097/BRS.0b013e3182797592
Delamarter RB, Murrey D, Janssen ME, Goldstein JA, Zigler J, Tay BK, Darden B 2nd (2010) Results at 24 months from the prospective, randomized, multicenter Investigational Device Exemption trial of ProDisc-C versus anterior cervical discectomy and fusion with 4-year follow-up and continued access patients. SAS J 4(4):122–128. doi:10.1016/j.esas.2010.09.001
DiAngelo DJ, Roberston JT, Metcalf NH, McVay BJ, Davis RC (2003) Biomechanical testing of an artificial cervical joint and an anterior cervical plate. J Spinal Disord Tech 16(4):314–323
Fallah A, Akl EA, Ebrahim S, Ibrahim GM, Mansouri A, Foote CJ, Zhang Y, Fehlings MG (2012) Anterior cervical discectomy with arthroplasty versus arthrodesis for single-level cervical spondylosis: a systematic review and meta-analysis. PLoS One 7(8):e43407. doi:10.1371/journal.pone.0043407
Gao Y, Liu M, Li T, Huang F, Tang T, Xiang Z (2013) A meta-analysis comparing the results of cervical disc arthroplasty with anterior cervical discectomy and fusion (ACDF) for the treatment of symptomatic cervical disc disease. J Bone Joint Surg Am 95(6):555–561. doi:10.2106/JBJS.K.00599
Gao F, Mao T, Sun W, Guo W, Wang Y, Li Z, Abhinav P (2015) An updated meta-analysis comparing artificial cervical disc arthroplasty (CDA) versus anterior cervical discectomy and fusion (ACDF) for the treatment of cervical degenerative disc disease (CDDD). Spine 40(23):1816–1823. doi:10.1097/BRS.0000000000001138
Heller JG, Sasso RC, Papadopoulos SM, Anderson PA, Fessler RG, Hacker RJ, Coric D, Cauthen JC, Riew DK (2009) Comparison of BRYAN cervical disc arthroplasty with anterior cervical decompression and fusion: clinical and radiographic results of a randomized, controlled, clinical trial. Spine 34(2):101–107. doi:10.1097/BRS.0b013e31818ee263
Hisey MS, Bae HW, Davis R, Gaede S, Hoffman G, Kim K, Nunley PD, Peterson D, Rashbaum R, Stokes J (2014) Multi-center, prospective, randomized, controlled investigational device exemption clinical trial comparing Mobi-C cervical artificial disc to anterior discectomy and fusion in the treatment of symptomatic degenerative disc disease in the cervical spine. Int J Spine Surg. doi:10.14444/1007
Hisey MS, Bae HW, Davis RJ, Gaede S, Hoffman G, Kim KD, Nunley PD, Peterson D, Rashbaum RF, Stokes J, Ohnmeiss DD (2015) Prospective, randomized comparison of cervical total disk replacement versus anterior cervical fusion: results at 48 months follow-up. J Spinal Disord Tech 28(4):E237–E243. doi:10.1097/BSD.0000000000000185
Jiang H, Zhu Z, Qiu Y, Qian B, Qiu X, Ji M (2012) Cervical disc arthroplasty versus fusion for single-level symptomatic cervical disc disease: a meta-analysis of randomized controlled trials. Arch Orthop Trauma Surg 132(2):141–151. doi:10.1007/s00402-011-1401-7
Kelly MP, Mok JM, Frisch RF, Tay BK (2011) Adjacent segment motion after anterior cervical discectomy and fusion versus Prodisc-c cervical total disk arthroplasty: analysis from a randomized, controlled trial. Spine 36(15):1171–1179. doi:10.1097/BRS.0b013e3181ec5c7d
Kesman T, Murrey D, Darden B (2012) Single-center results at 7 years of prospective, randomized ProDisc-C total disc arthroplasty versus anterior cervical discectomy and fusion for treatment of one level symptomatic cervical disc disease. Evid Based Spine Care J 3(4):61–62. doi:10.1055/s-0032-1328144
Li GL, Hu JZ, Lu HB, Qu J, Guo LY, Zai FL (2015) Anterior cervical discectomy with arthroplasty versus anterior cervical discectomy and fusion for cervical spondylosis. J Clin Neurosci 22(3):460–467. doi:10.1016/j.jocn.2014.09.010
Luo J, Gong M, Huang S, Yu T, Zou X (2015a) Incidence of adjacent segment degeneration in cervical disc arthroplasty versus anterior cervical decompression and fusion meta-analysis of prospective studies. Arch Orthop Trauma Surg 135(2):155–160. doi:10.1007/s00402-014-2125-2
Luo J, Huang S, Gong M, Dai X, Gao M, Yu T, Zhou Z, Zou X (2015b) Comparison of artificial cervical arthroplasty versus anterior cervical discectomy and fusion for one-level cervical degenerative disc disease: a meta-analysis of randomized controlled trials. Eur J Orthop Surg Traumatol 25(1):S115–S125. doi:10.1007/s00590-014-1510-4
McAfee PC, Cappuccino A, Cunningham BW, Devine JG, Phillips FM, Regan JJ, Albert TJ, Ahrens JE (2010) Lower incidence of dysphagia with cervical arthroplasty compared with ACDF in a prospective randomized clinical trial. J Spinal Disord Tech 23(1):1–8. doi:10.1097/BSD.0b013e31819e2ab8
Muheremu A, Niu X, Wu Z, Muhanmode Y, Tian W (2015) Comparison of the short- and long-term treatment effect of cervical disk replacement and anterior cervical disk fusion: a meta-analysis. Eur J Orthop Surg Traumatol 25(1):S87–S100. doi:10.1007/s00590-014-1469-1
Mummaneni PV, Burkus JK, Haid RW, Traynelis VC, Zdeblick TA (2007) Clinical and radiographic analysis of cervical disc arthroplasty compared with allograft fusion: a randomized controlled clinical trial. J Neurosurg Spine 6(3):198–209. doi:10.3171/spi.2007.6.3.198
Murrey DB, Janssen ME, Odum SM, Gottlieb JR, Spector LR, Darden BV (2008) Two-year results of a randomized controlled clinical trial comparing ProDisc-C and anterior cervical discectomy and fusion. SAS J 2(2):76–85. doi:10.1016/SASJ-2007-0124-RR
Murrey D, Janssen M, Delamarter R, Goldstein J, Zigler J, Tay B, Darden B (2009) Results of the prospective, randomized, controlled multicenter Food and Drug Administration investigational device exemption study of the ProDisc-C total disc replacement versus anterior discectomy and fusion for the treatment of 1-level symptomatic cervical disc disease. Spine J 9(4):275–286. doi:10.1016/j.spinee.2008.05.006
Nabhan A, Ahlhelm F, Pitzen T, Steudel WI, Jung J, Shariat K, Steimer O, Bachelier F, Pape D (2007a) Disc replacement using Pro-Disc C versus fusion: a prospective randomised and controlled radiographic and clinical study. Eur Spine J 16(3):423–430. doi:10.1007/s00586-006-0226-5
Nabhan A, Ahlhelm F, Shariat K, Pitzen T, Steimer O, Steudel WI, Pape D (2007b) The ProDisc-C prosthesis: clinical and radiological experience 1 year after surgery. Spine 32(18):1935–1941. doi:10.1097/BRS.0b013e31813162d8
Nabhan A, Steudel WI, Nabhan A, Pape D, Ishak B (2007c) Segmental kinematics and adjacent level degeneration following disc replacement versus fusion: RCT with three years of follow-up. J Long Term Eff Med Implants 17(3):229–236
Nabhan A, Ishak B, Steudel WI, Ramadhan S, Steimer O (2011) Assessment of adjacent-segment mobility after cervical disc replacement versus fusion: RCT with 1 year’s results. Eur Spine J 20(6):934–941. doi:10.1007/s00586-010-1588-2
Phillips FM, Lee JY, Geisler FH, Cappuccino A, Chaput CD, DeVine JG, Reah C, Gilder KM, Howell KM, McAfee PC (2013) A prospective, randomized, controlled clinical investigation comparing PCM cervical disc arthroplasty with anterior cervical discectomy and fusion. 2-year results from the US FDA IDE clinical trial. Spine 38(15):E907–E918. doi:10.1097/BRS.0b013e318296232f
Phillips FM, Geisler FH, Gilder KM, Reah C, Howell KM, McAfee PC (2015) Long-term outcomes of the US FDA IDE prospective, randomized controlled clinical trial comparing PCM cervical disc arthroplasty with anterior cervical discectomy and fusion. Spine 40(10):674–683. doi:10.1097/BRS.0000000000000869
Porchet F, Metcalf NH (2004) Clinical outcomes with the Prestige II cervical disc: preliminary results from a prospective randomized clinical trial. Neurosurg Focus 17(3):E6
Qizhi S, Lei S, Peijia L, Hanping Z, Hongwei H, Junsheng C (2014) A comparison of zero-profile devices and artificial cervical discs in patients with two noncontiguous levels of cervical spondylosis. J Spinal Disord Tech. doi:10.1097/bsd.0000000000000096
Rao MJ, Nie SP, Xiao BW, Zhang GH, Gan XR, Cao SS (2015) Cervical disc arthroplasty versus anterior cervical discectomy and fusion for treatment of symptomatic cervical disc disease: a meta-analysis of randomized controlled trials. Arch Orthop Trauma Surg 135(1):19–28. doi:10.1007/s00402-014-2122-5
Ren C, Song Y, Xue Y, Yang X (2014) Mid- to long-term outcomes after cervical disc arthroplasty compared with anterior discectomy and fusion: a systematic review and meta-analysis of randomized controlled trials. Eur Spine J 23(5):1115–1123. doi:10.1007/s00586-014-3220-3
Riew KD, Buchowski JM, Sasso R, Zdeblick T, Metcalf NH, Anderson PA (2008) Cervical disc arthroplasty compared with arthrodesis for the treatment of myelopathy. J Bone Joint Surg Am 90(11):2354–2364. doi:10.2106/JBJS.G.01608
Riina J, Patel A, Dietz JW, Hoskins JS, Trammell TR, Schwartz DD (2008) Comparison of single-level cervical fusion and a metal-on-metal cervical disc replacement device. Am J Orthop (Belle Mead NJ) 37(4):E71–E77
Rozankovic M, Marasanov SM, Vukic M (2014) Cervical disc replacement with discover versus fusion in a single level cervical disc disease: a prospective single center randomized trial with a minimum two-year follow-up. J Spinal Disord Tech. doi:10.1097/BSD.0000000000000170
Sasso RC, Smucker JD, Hacker RJ, Heller JG (2007) Clinical outcomes of BRYAN cervical disc arthroplasty: a prospective, randomized, controlled, multicenter trial with 24-month follow-up. J Spinal Disord Tech 20(7):481–491. doi:10.1097/BSD.0b013e3180310534
Sasso RC, Best NM, Metcalf NH, Anderson PA (2008) Motion analysis of bryan cervical disc arthroplasty versus anterior discectomy and fusion: results from a prospective, randomized, multicenter, clinical trial. J Spinal Disord Tech 21(6):393–399. doi:10.1097/BSD.0b013e318150d121
Sasso RC, Anderson PA, Riew KD, Heller JG (2011) Results of cervical arthroplasty compared with anterior discectomy and fusion: four-year clinical outcomes in a prospective, randomized controlled trial. J Bone Joint Surg Am 93(18):1684–1692. doi:10.2106/JBJS.J.00476
Shriver MF, Lubelski D, Sharma AM, Steinmetz MP, Benzel EC, Mroz TE (2015) Adjacent segment degeneration and disease following cervical arthroplasty: a systematic review and meta-analysis. Spine J. doi:10.1016/j.spinee.2015.10.032
Skeppholm M, Lindgren L, Henriques T, Vavruch L, Lofgren H, Olerud C (2015) The Discover artificial disc replacement versus fusion in cervical radiculopathy—a randomized controlled outcome trial with 2-year follow-up. Spine J 15(6):1284–1294. doi:10.1016/j.spinee.2015.02.039
Vaccaro A, Beutler W, Peppelman W, Marzluff JM, Highsmith J, Mugglin A, DeMuth G, Gudipally M, Baker KJ (2013) Clinical outcomes with selectively constrained SECURE-C cervical disc arthroplasty: two-year results from a prospective, randomized, controlled, multicenter investigational device exemption study. Spine 38(26):2227–2239. doi:10.1097/BRS.0000000000000031
Verma K, Gandhi SD, Maltenfort M, Albert TJ, Hilibrand AS, Vaccaro AR, Radcliff KE (2013) Rate of adjacent segment disease in cervical disc arthroplasty versus single-level fusion: meta-analysis of prospective studies. Spine 38(26):2253–2257. doi:10.1097/BRS.0000000000000052
Wu AM, Xu H, Mullinix KP, Jin HM, Huang ZY, Lv QB, Wang S, Xu HZ, Chi YL (2015) Minimum 4-year outcomes of cervical total disc arthroplasty versus fusion: a meta-analysis based on prospective randomized controlled trials. Medicine 94(15):e665. doi:10.1097/MD.0000000000000665
Xing D, Ma XL, Ma JX, Wang J, Ma T, Chen Y (2013) A meta-analysis of cervical arthroplasty compared to anterior cervical discectomy and fusion for single-level cervical disc disease. J Clin Neurosci 20(7):970–978. doi:10.1016/j.jocn.2012.03.046
Yang B, Li H, Zhang T, He X, Xu S (2012) The incidence of adjacent segment degeneration after cervical disc arthroplasty (CDA): a meta analysis of randomized controlled trials. PLoS One 7(4):e35032. doi:10.1371/journal.pone.0035032
Yao Q, Liang F, Xia Y, Jia C (2015) A meta-analysis comparing total disc arthroplasty with anterior cervical discectomy and fusion for the treatment of cervical degenerative diseases. Arch Orthop Trauma Surg. doi:10.1007/s00402-015-2337-0
Yin S, Yu X, Zhou S, Yin Z, Qiu Y (2013) Is cervical disc arthroplasty superior to fusion for treatment of symptomatic cervical disc disease? A meta-analysis. Clin Orthop Relat Res 471(6):1904–1919. doi:10.1007/s11999-013-2830-0
Yu L, Song Y, Yang X, Lv C (2011) Systematic review and meta-analysis of randomized controlled trials: comparison of total disk replacement with anterior cervical decompression and fusion. Orthopedics 34(10):e651–e658. doi:10.3928/01477447-20110826-09
Yue WM, Brodner W, Highland TR (2005) Long-term results after anterior cervical discectomy and fusion with allograft and plating: a 5- to 11-year radiologic and clinical follow-up study. Spine 30(19):2138–2144
Zechmeister I, Winkler R, Mad P (2011) Artificial total disc replacement versus fusion for the cervical spine: a systematic review. Eur Spine J 20(2):177–184. doi:10.1007/s00586-010-1583-7
Zhang X, Zhang X, Chen C, Zhang Y, Wang Z, Wang B, Yan W, Li M, Yuan W, Wang Y (2012) Randomized, controlled, multicenter, clinical trial comparing BRYAN cervical disc arthroplasty with anterior cervical decompression and fusion in China. Spine 37(6):433–438. doi:10.1097/BRS.0b013e31822699fa
Zhang HX, Shao YD, Chen Y, Hou Y, Cheng L, Si M, Nie L (2014) A prospective, randomised, controlled multicentre study comparing cervical disc replacement with anterior cervical decompression and fusion. Int Orthop 38(12):2533–2541. doi:10.1007/s00264-014-2497-5
Zhong ZM, Zhu SY, Zhuang JS, Wu Q, Chen JT (2016) Reoperation after cervical disc arthroplasty versus anterior cervical discectomy and fusion: a meta-analysis. Clin Orthop Relat Res. doi:10.1007/s11999-016-4707-5
Zhu Y, Zhang B, Liu H, Wu Y, Zhu Q (2016) Cervical disc arthroplasty versus anterior cervical discectomy and fusion for incidence of symptomatic adjacent segment disease: a meta-analysis of prospective randomized controlled trials. Spine. doi:10.1097/BRS.0000000000001537
Zigler JE, Delamarter R, Murrey D, Spivak J, Janssen M (2013) ProDisc-C and anterior cervical discectomy and fusion as surgical treatment for single-level cervical symptomatic degenerative disc disease: five-year results of a Food and Drug Administration study. Spine 38(3):203–209. doi:10.1097/BRS.0b013e318278eb38
Authors’ contributions
DZM designed the study; LX and ML searched relevant studies and abstracted the data; PL and FD analyzed and interpreted the data; LX and ML wrote the manuscript and DZM approved the final version of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We are very grateful to Dr. Xiao Zhihong for language editing.
Competing interests
The authors declare that they have no competing interests. No organization sponsored the research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Xie, L., Liu, M., Ding, F. et al. Cervical disc arthroplasty (CDA) versus anterior cervical discectomy and fusion (ACDF) in symptomatic cervical degenerative disc diseases (CDDDs): an updated meta-analysis of prospective randomized controlled trials (RCTs). SpringerPlus 5, 1188 (2016). https://doi.org/10.1186/s40064-016-2851-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40064-016-2851-8