Abstract
Ritonavir is a potent inhibitor of the cytochrome P450 enzyme CYP3A4 and is subject to multiple drug–drug interactions. This becomes especially important when the patient is also taking medications metabolized through CYP3A pathway as increased and potentially toxic drug levels may ensue. Herein we present one such interaction wherein a 57 year old gentleman with human immunodeficiency virus (HIV) infection on highly active antiretroviral therapy that included ritonavir, had addition of fluticasone inhaler to his medication repertoire for treatment of chronic obstructive pulmonary disease. This resulted in severe osteoporosis, iatrogenic Cushing syndrome and adrenal insufficiency due to the potentiated systemic glucocorticoid effect of inhaled fluticasone by ritonavir. This case emphasizes the need for pharmacovigilance when managing patients on complex drug regimens for physicians treating HIV infected patients.
Similar content being viewed by others
Background
Ritonavir is a potent inhibitor of the cytochrome P450 enzyme CYP3A4 that can lead to multiple drug–drug interactions. Systemic complications resulting from inhaled corticosteroids like fluticasone are rare but when used concomitantly with ritonavir can lead to iatrogenic Cushing syndrome and adrenal suppression. It is important to be aware of this interaction to avoid serious and potentially fatal complications. We report a case of iatrogenic Cushing syndrome, adrenal insufficiency and severe osteoporosis due to the potentiated systemic glucocorticoid effect of inhaled fluticasone by ritonavir in a patient with human immunodeficiency virus (HIV).
Case presentation
A 57 year old Caucasian male with HIV infection since 1986 was evaluated in 4/2010 for recurrent rib fractures following trivial stresses such as coughing. His highly active antiretroviral therapy (HAART) since 2006 consisted of lamivudine 150 mg twice daily, zidovudine 300 mg twice daily and lopinavir–ritonavir 400–100 mg twice daily. Fluticasone/salmeterol 250/50 mcg one puff twice daily was introduced in 9/2007 for severe COPD. Other medical conditions included coronary artery disease, dyslipidemia and GERD treated with aspirin, clopidogrel, omeprazole, pravastatin and niacin. Physical examination showed centripetal adiposity, multiple ecchymoses and pronounced pink abdominal and inguinal striae (Fig. 1). Weight had increased by 5 kg from baseline weight. There was palpable rib tenderness. The reminder of the physical examination was unremarkable. Of note his AIDS clinician mistook his phenotypic changes to be those of AIDS related lipodystrophy. These same changes eluded his PCP.
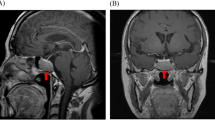
Chest radiographs confirmed numerous rib fractures (Fig. 2). Lumbar and proximal femur bone mineral density (BMD) T-scores were −5.2 and −3.4 respectively. Complete blood count, renal and hepatic function, electrolytes, calcium, phosphate, intact parathyroid hormone, 25-OH-VitD, prolactin, serum and urine protein electrophoresis and serum free light chains were normal. His random morning serum cortisol was 0.5 mcg/dl (normal 4–24 mcg/dL) and 1 h after 250 mcg intravenous cosyntropin stimulation was 7.1 mcg/dL (expected >20 mcg/dL) consistent with adrenal insufficiency. A 24 h urine free cortisol was <7.2 mcg, late night salivary cortisol was <10 ng/dL (<100 ng/dL) and dehydroepiandrosterone sulphate was <30 mcg/dL (40–310 mcg/dL). Serum ACTH was 32 pg/mL (normal 0–46 pg/mL). A 24 h urinary synthetic glucocorticoid screen was only positive for a fluticasone 17-β-carboxylic acid value of 243 pg/mL. Pituitary magnetic resonance imaging (MRI) was normal. Serum and 24-h urine N-telopeptides were 19.2 nmol BCE (5.4–24 nmol BCE) and 23 nmol/mmol BCE (21–66 nmol/mmol BCE) respectively. Total and bone specific alkaline phosphatase were 73 IU/L (20–71 IU/L) and 151 IU/L (40–125 IU/L) respectively.
The patient was diagnosed with iatrogenic Cushing syndrome and adrenal suppression secondary to the potentiated systemic glucocorticoid effect of inhaled fluticasone by ritonavir. Fluticasone was continued as the patient reported significant improvement in his COPD symptoms. Ritonavir boosted protease inhibitor therapy was discontinued and he was placed on Raltegravir which is not known to inhibit CYP3A4. His HIV viral load continues to be undetectable and 2 years after change in antiretroviral therapy his CD4 count is 624. He was placed on physiological doses of hydrocortisone at 20 mg per day and has gradually tapered to 5 mg/day of hydrocortisone 1 year from presentation. His severe osteoporosis was treated with subcutaneous teriparatide, appropriate calcium and vitamin D supplementation and a physical therapy.
Serial basal and stimulated cortisol levels are reported in Table 1. His cushingoid features improved by 3 months (Fig. 3). One year after initiating osteoporosis treatment lumbar spine BMD improved from 0.530 to 0.932 gm/cm2 (+75.8 %) and total hip BMD from 0.681 to 0.761 gm/cm2 (+11.2 %). These BMD gains are significantly greater than those reported for lumbar spine (11 %) and total hip (5.2 %) in patients with glucocorticoid induced osteoporosis treated with teriparatide (Saag et al. 2009). Follow up BMD at the completion of teriparatide treatment is shown in Table 2 and Fig. 4. The patient has had no further fractures.
Discussion
A search of published English language literature using the keywords ritonavir, protease inhibitors, fluticasone, inhaled corticosteroids, Cushing’s syndrome and adrenal suppression revealed a total of 11 pediatric and 26 adult cases of iatrogenic Cushing’s syndrome and adrenal suppression from concomitant fluticasone and ritonavir therapy. 3 of 24 adult cases were secondary to intranasal fluticasone preparations and rest was from inhaled fluticasone. Fluticasone dose ranged from 500 to 2000 mcg/day in adult patients and 200–1000 mcg/day in pediatric patients. Ritonavir in both low doses “boosted” and in high doses was associated with significant interaction (Table 3).
Combination anti-retroviral therapy, particularly the introduction of protease inhibitors has revolutionized HIV therapy and changed once a fatal disease to a chronic condition (Palella et al. 1998). Ritonavir is a potent inhibitor of cytochrome P450 (CYP) 3A4 isozymes and significantly increases the concentration of drugs primarily eliminated by CYP3A metabolism such as macrolides, azoles, protease inhibitors and corticosteroids (Hsu et al. 1998; Von Moltke et al. 1998). This property of ritonavir is used to therapeutic advantage in ritonavir boosted protease inhibitor regimens and has decreased the pill burden and treatment failures and improved compliance with therapy (Thompson et al. 2010).
With 30 % estimated prevalence of bronchial hyperactivity (Poirier et al. 2001), HIV infected men who smoke are frequently exposed to inhaled corticosteroid therapy. To reduce airway inflammation treatment guidelines for asthma and chronic obstructive airway disease (COPD) recommend the routine use of inhaled corticosteroid alone or in combination with long acting bronchodilators (National Asthma Education and Prevention Program 2002; Vestbo et al. 2013). Fluticasone is a potent glucocorticoid commonly used in reactive airway disease. Compared with other available inhaled steroids, it has high glucocorticoid receptor binding affinity, is highly lipophilic, a large volume of distribution (318 L) (Wuerthwein et al. 1992; Mackie et al. 1996) and a longer elimination half-life (t1/2 7–8 h). Less than 1 % of swallowed fluticasone is bioavailable due to its high first pass metabolism and rapid metabolism in liver by CYP3A4 enzyme system and conversion to inactive 17 B-carboxylic acid derivative (Harding 1990). Concomitant use of fluticasone with potent CYP3A4 inhibitors such as ritonavir can lead to systemic accumulation of fluticasone and suppression of hypothalamic pituitary adrenal (HPA) axis. Ritonavir increased the area under concentration–time curve (AUC) of serum fluticasone by 350-fold in healthy volunteers (Laboratories 2006). For this reason manufactures and the FDA recommend against routine use of combination of ritonavir and fluticasone unless benefits outweigh risks.
Fluticasone has been reported to cause greater dose related adrenal suppression when compared with budesonide, triamcinolone acetonide or beclomethasone dipropionate (Lipworth 1999) even in the absence of CYP3A4 inhibitors like ritonavir. In a recent meta-analysis of 732 subjects with asthma, fluticasone in small to medium doses (50–500 mcg/day) alone showed minimal effect on adrenal function (Masoli et al. 2006). In another prospective, non-randomized, open-label, cross sectional study, investigators found that patients taking high doses of fluticasone (>880 mcg per day) for longer duration had abnormal adrenal function (White et al. 2006).
Iatrogenic Cushing syndrome results from prolonged exposure to high doses of glucocorticoids. The vast majority of these cases result from administration of oral or parenteral glucocorticoids (Newell-Price et al. 2006). Typical features include weight gain, central obesity, dorsocervical hump, moon face, facial plethora, thin skin, easy bruising, abdominal striae, hirsutism, proximal myopathy, osteopenia, glucose intolerance, hypertension, nephrolithiasis and psychiatric manifestations such as depression and psychosis (Newell-Price et al. 2006). Osteoporosis is common and tends to involve trabecular bone resulting in an increased risk of fracture within 3 months of daily exposure (van Staa et al. 2000, 2002, 2005).
Pituitary production of corticotropin (ACTH) will be suppressed by exogenous steroids, which leads to atrophy of the adrenal cortex and adrenal insufficiency. Diagnosis is confirmed by low early morning serum cortisol levels and subnormal response to standard ACTH stimulation test. An early morning serum cortisol levels <3 mcg/dL (80 nmol/L) strongly suggests adrenal insufficiency, whereas levels >15 mcg/dL (415 nmol/L) predicts a normal response of serum cortisol to ACTH stimulation test (Hagg et al. 1987; Le Roux et al. 2002). Patients with low or equivocal serum cortisol levels in whom adrenal insufficiency is suspected should undergo standard or low dose synthetic ACTH (Cosyntropin) stimulation test. Suboptimal response to Cosyntropin stimulation test is diagnostic of adrenal insufficiency. Simultaneous measurement of plasma ACTH helps in differentiation of primary from secondary or tertiary adrenal insufficiency. ACTH levels tend to be higher than normal in primary adrenal insufficiency whereas low or low normal in patients with secondary and tertiary adrenal insufficiency. Corticotropin-releasing hormone (CRH) test further differentiate secondary from tertiary adrenal insufficiency (Schulte et al. 1984).
Human immunodeficiency virus associated lipodystrophy shares several morphological features and should be differentiated from Cushing Syndrome. Weight gain, central adiposity, dorsocervical hump, insulin resistance, osteopenia, dyslipidemia are common and are associated with ART therapy (Lichtenstein 2005; Dube et al. 2007; Carr and Cooper 1998). Presence of facial plethora, cutaneous striae, and proximal myopathy differentiates it from Cushing syndrome and should prompt physicians to evaluate for HPA axis suppression. Sudden withdrawal of from steroid therapy has a potential to develop catastrophic adrenal crisis. Replacement with physiological doses of prednisone 5–7.5 mg a day, hydrocortisone 15–20 mg a day or equivalent should be initiated. Measurement of morning cortisol levels every 4–6 weeks serves as screening test for recovery of adrenal function. Morning serum cortisol level less than 3 mcg/dL indicates the need for continued replacement therapy, whereas a value greater than 20 mcg/dL indicates recovered HPA axis. Patients with morning cortisol levels between 3 and 20 mcg/dL will need further studies like Cosyntropin stimulation test or overnight metyrapone test. It may take 9–12 months for the recovery of adrenal function (Hopkins and Leinung 2005).
In patients with iatrogenic Cushing syndrome and adrenal insufficiency secondary to the interaction between ritonavir and fluticasone available options include, replacing ritonavir with another antiretroviral agent, replacing fluticasone with another less potent steroid or leukotriene antagonists or long acting anticholinergic agent such as tiotropium. In the SPIRAL study, Raltegravir demonstrated the non-inferior efficacy and improved lipid profile when ritonavir boosted protease inhibitor therapy was replaced by Raltegravir (Martinez et al. 2010). The latter approach was taken in our patient with favourable outcome.
Conclusions
Despite the recommendations against concomitant use of fluticasone and ritonavir several reports of Cushing syndrome and adrenal suppression are being reported. We believe physicians treating HIV infected patients must be aware of potential interaction of antiretroviral therapy and drugs used to treat associated co-morbidities. When iatrogenic Cushing syndrome is suspected, prompt evaluation and discontinuation of offending medication will prevent potentially fatal complications.
Consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Abbreviations
- HIV:
-
human immunodeficiency virus
- CYP:
-
cytochrome P450
- BMD:
-
bone mineral density
- HAART:
-
highly active antiretroviral therapy
References
Abbott Laboratories (2006) Norvir (ritonavir) package insert. North Chicago, IL
Arrington-Sanders R, Hutton N et al (2006) Ritonavir–fluticasone interaction causing Cushing syndrome in HIV-infected children and adolescents. Pediatr Infect Dis J 25:1044–1048
Bhumbra NA, Sahloff EG et al (2007) Exogenous Cushing syndrome with inhaled fluticasone in a child receiving lopinavir/ritonavir. Ann Pharmacother 41:1306–1309
Carr A, Cooper DA (1998) Lipodystrophy associated with an HIV-protease inhibitor. N Engl J Med 339:1296
Chen F, Kearney T et al (1999) Cushing’s syndrome and severe adrenal suppression in patients treated with ritonavir and inhaled nasal fluticasone. Sex Transm Infect 75:274
Clevenbergh P, Corcostegui M et al (2002) Iatrogenic Cushing’s syndrome in an HIV-infected patient treated with inhaled corticosteroids (fluticasone propionate) and low dose ritonavir enhanced PI containing regimen. J Infect 44:194–195
Dube MP, Komarow L et al (2007) Long-term body fat outcomes in antiretroviral-naive participants randomized to nelfinavir or efavirenz or both plus dual nucleosides. Dual X-ray absorptiometry results from A5005 s, a substudy of Adult Clinical Trials Group 384. J Acquir Immune Defic Syndr 45:508–514
Gillett MJ, Cameron PU et al (2005) Iatrogenic Cushing’s syndrome in an HIV-infected patient treated with ritonavir and inhaled fluticasone. AIDS 19:740–741
Gupta SK, Dubé MP (2002) Exogenous cushing syndrome mimicking human immunodeficiency virus lipodystrophy. Clin Infect Dis 35:E69–E71
Hagg E, Asplund K et al (1987) Value of basal plasma cortisol assays in the assessment of pituitary-adrenal insufficiency. Clin Endocrinol (Oxf) 26:221–226
Harding SM (1990) The human pharmacology of fluticasone propionate. Respir Med 84:25–29
Hillebrand-Haverkort ME, Prummel MF et al (1999) Ritonavir-induced Cushing’s syndrome in a patient treated with nasal fluticasone. AIDS 13:1803
Hopkins RL, Leinung MC (2005) Exogenous Cushing’s syndrome and glucocorticoid withdrawal. Endocrinol Metab Clin N Am 34:371–384
Hsu A, Granneman GR et al (1998) Ritonavir. Clinical pharmacokinetics and interactions with other anti-HIV agents. Clin Pharmacokinet 35:275–291
Jinno S, Goshima C (2008) Progression of Kaposi sarcoma associated with iatrogenic Cushing syndrome in a person with HIV/AIDS. AIDS Read 18:100–104
Johnson SR, Marion AA et al (2006) Cushing syndrome with secondary adrenal insufficiency from concomitant therapy with ritonavir and fluticasone. J Pediatr 148:386–388
Kaviani N, Bukberg P et al (2011) Iatrogenic osteoporosis, bilateral HIP osteonecrosis, and secondary adrenal suppression in an HIV-infected man receiving inhaled corticosteroids and ritonavir-boosted highly active antiretroviral therapy. Endocr Pract 17:74–78
Kedem E, Shahar E et al (2010) Iatrogenic Cushing’s syndrome due to coadministration of ritonavir and inhaled budesonide in an asthmatic human immunodeficiency virus infected patient. J Asthma 47:830–831
le Roux CW, Beckles MA et al (2001) Cushing’s syndrome secondary to inhaled corticosteroids mimicking HIV-associated lipodystrophy. HIV Med 2:133–135
Le Roux CW, Meeran K et al (2002) Is a 0900-h serum cortisol useful prior to a short synacthen test in outpatient assessment? Ann Clin Biochem 39:148–150
Leitman D, Ross K et al (2009) Vertebral compression fractures in an HIV-positive patient with steroid-induced Cushing syndrome: a case report. Cases J 2:7034
Lichtenstein KA (2005) Redefining lipodystrophy syndrome: risks and impact on clinical decision making. J Acquir Immune Defic Syndr 39:395–400
Lipworth BJ (1999) Systemic adverse effects of inhaled corticosteroid therapy: a systematic review and meta-analysis. Arch Intern Med 159:941–955
Mackie A, Ventresca G et al (1996) Pharmacokinetics of intravenous fluticasone propionate in healthy subjects. Br J Clin Pharmacol 41:539–542
Mahlab-Guri K, Asher I (2011) Inhaled fluticasone causes iatrogenic cushing’s syndrome in patients treated with Ritonavir. J Asthma 48:860–863
Martinez E, Larrousse M et al (2010) Substitution of raltegravir for ritonavir-boosted protease inhibitors in HIV-infected patients: the SPIRAL study. AIDS 24:1697–1707
Masoli M, Weatherall M et al (2006) Inhaled fluticasone propionate and adrenal effects in adult asthma: systematic review and meta-analysis. Eur Respir J 28:960–967
National Asthma Education and Prevention Program (2002) Expert panel report: guidelines for the diagnosis and management of asthma update on selected topics. J Allergy Clin Immunol 110:S141–S219
Newell-Price J, Bertagna X et al (2006) Cushing’s syndrome. Lancet 367:1605–1617
Nocent C, Raherison C et al (2004) Unexpected effects of inhaled fluticasone in an HIV patient with asthma. J Asthma 41:793–795
Palella FJ, Delaney KM et al (1998) Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 338:853–860
Pessanha TM, Campos JM et al (2007) Iatrogenic Cushing’s syndrome in a adolescent with AIDSs on ritonavir and inhaled fluticasone. Case report and literature review. AIDS 21:529–532
Poirier CD, Inhaber N et al (2001) Prevalence of bronchial hyperresponsiveness among HIV-infected men. Am J Respir Crit Care Med 164:542–545
Rouanet I, Peyrière H et al (2003) Cushing’s syndrome in a patient treated by ritonavir/lopinavir and inhaled fluticasone. HIV Med 4:149–150
Saag KG, Zanchetta JR et al (2009) Effects of teriparatide versus alendronate for treating glucocorticoid-induced osteoporosis: thirty-six-month results of a randomized, double-blind, controlled trial. Arthritis Rheum 60:3346–3355
Sagir A, Wettstein M et al (2002) Budesonide-induced acute hepatitis in an HIV-positive patient with ritonavir as a co-medication. AIDS 16:1191–1192
Samaras K, Pett S et al (2005) Iatrogenic Cushing’s syndrome with osteoporosis and secondary adrenal failure in human immunodeficiency virus-infected patients receiving inhaled corticosteroids and ritonavir-boosted protease inhibitors: six cases. J Clin Endocrinol Metab 90:4394–4398
Schulte HM, Chrousos GP et al (1984) The corticotropin-releasing hormone stimulation test: a possible aid in the evaluation of patients with adrenal insufficiency. J Clin Endocrinol Metab 58:1064–1067
Soldatos G, Sztal-Mazer S et al (2005) Exogenous glucocorticoid excess as a result of ritonavir-fluticasone interaction. Intern Med J 35:67–68
St Germain RM, Yigit S et al (2007) Cushing syndrome and severe adrenal suppression caused by fluticasone and protease inhibitor combination in an HIV-infected adolescent. AIDS Patient Care STDS 21:373–377
Thompson MA, Aberg JA et al (2010) Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. JAMA 304:321–333
Valin N, De Castro N et al (2009) Iatrogenic Cushing’s syndrome in HIV-infected patients receiving ritonavir and inhaled fluticasone: description of 4 new cases and review of the literature. J Int Assoc Physicians AIDS Care 8:113–121
van Staa TP, Leufkens HG et al (2000) Use of oral corticosteroids and risk of fractures. J Bone Miner Res 15:993–1000
van Staa TP, Leufkens HG et al (2002) The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int 13:777–787
van Staa TP, Geusens P et al (2005) A simple score for estimating the long-term risk of fracture in patients using oral glucocorticoids. QJM 98:191–198
Vestbo J, Hurd SS et al (2013) Global Strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 187:347–365
Von Moltke LL, Greenblatt DJ et al (1998) Protease inhibitors as inhibitors of human cytochromes P450: high risk associated with ritonavir. J Clin Pharmacol 38:106–111
White M, Crisalida T et al (2006) Effects of long-term inhaled corticosteroids on adrenal function in patients with asthma. Ann Allergy Asthma Immunol 96:437–444
Wuerthwein G, Rehder S et al (1992) Lipophilicity and receptor affinity of glucocorticoids. Pharmazeutische Zeitung Wissenschaft 137:161–167
Authors’ contributions
NE and FM conceived the study design. NE conducted the literature search and drafted the manuscript. FM assisted in the literature search and critical revisions of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Acknowledgments
The authors thank the Department of Radiology for providing the images.
Compliance with ethical guidelines
Competing interests The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Epperla, N., McKiernan, F. Iatrogenic Cushing syndrome and adrenal insufficiency during concomitant therapy with ritonavir and fluticasone. SpringerPlus 4, 455 (2015). https://doi.org/10.1186/s40064-015-1218-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40064-015-1218-x