Abstract
Objective
To study the effects of selective serotonin reuptake inhibitors (SSRIs) on cognitive functions, mental improvements, and adverse effects in patients with Alzheimer’s disease (AD).
Methods
Registered in INPLASY (INPLASY202450004), five drugs (citalopram, s-citalopram, quetiapine, olanzapine, and sertraline) were selected as representatives. A comprehensive search was conducted in PubMed, EMBASE, Web of Science, and the Cochrane Library up to May 15, 2024. Search terms were combined using Boolean operators, specifically ‘AND’ between different categories (e.g., ‘Alzheimer’s Disease’ AND ‘SSRIs’) and ‘OR’ within the same category (e.g., ‘citalopram OR s-citalopram OR quetiapine OR olanzapine OR sertraline’), to ensure a thorough retrieval of relevant studies. The selection followed rigorous inclusion and exclusion criteria for meta-analysis.
Results
Fourteen articles from 1118 were selected for meta-analysis. The indicators, including Neuropsychiatric Inventory (NPI), Mini-Mental State Examination (MMSE), Brief Psychiatric Rating Scale (BPRS), and Cornell Scale for Depression in Dementia (CSDD), were used to assess the effects of the drugs on AD treatment. According to the results of NPI, CSDD, BPRS, MMSE, and security assessments, the five antidepressants have significant advantages in AD treatment compared with placebo, while the MMSE of the patient treated with the antidepressants did not show notable changes compared with patients treated only with placebo. Statistical analyses were conducted using Review Manager 5.3, employing random-effects models to account for study heterogeneity and sensitivity analyses to test the robustness of our findings.
Conclusion
This study suggests that SSRI-related antidepressants have great potential values in AD treatment, and further research on the application of SSRI-related antidepressants in AD treatment is necessary.
Similar content being viewed by others
Introduction
Cardiac and neurological diseases remain two main killers worldwide [1,2,3,4,5,6,7,8]. Among them, Alzheimer’s disease (AD) is one of the common neurogenic diseases [9, 10], which has threatened the health of elderly patients [11,12,13]. Depression is a common symptom among AD patients, and several studies have identified a close link between depression and the onset and progression of AD [14,15,16]. Reports indicate that a considerable number of patients have a history of depression at the time of AD diagnosis, and that depression and depressive symptoms increase the risk of transitioning from mild cognitive impairment to AD [17, 18].
At present, even with the current strategies, the treatment effects on AD remain unsatisfactory. However, several studies have indicated that some selective serotonin reuptake inhibitors (SSRIs) have a positive effect on improving AD symptoms [19, 20]. Mental diseases also remain main killer for human beings [21,22,23]. SSRIs, including citalopram, s-citalopram, quetiapine, olanzapine, sertraline, and so on, are characterized by inhibiting 5-hydroxytryptamine of patients and have been generally used in clinical treatment to relieve the symptoms of depression [24, 25]. Recent studies have indicated that even to the patients without depression, SSRIs can delay the conversion from mild cognitive impairment to AD, which suggests that some SSRIs may have the potential to decrease the symptoms of AD [26,27,28]. Moreover, the five SSRIs can inhibit the accumulation of amyloid-β in the brains of the patients via blocking the synthesis process of precursor proteins of amyloid-β. Thus, SSRIs may hold potential therapeutic values on AD treatment that inhibit or delay the progression of AD, especially in patients who are at early stages. SSRIs demonstrate significant benefits in terms of security and lower research costs compared to newer drugs under development, although these advantages should be evaluated in the context of available evidence [29,30,31]. Therefore, the researching the effects of SSRIs in AD treatment is necessary.
In this review, the five SSRIs, including citalopram, s-citalopram, quetiapine, olanzapine, and sertraline were selected as subjects, and the articles which were related with randomized controlled trials (RCTs) of those drugs for AD treatment were included for meta-analysis the effects of SSRIs on AD treatment.
Materials and methods
Search strategy
The related articles were searched from online databases, including PubMed, EMBASE, Web of Science, and the Cochrane Library (from inception to May 15, 2024). The terms, including “citalopram”, “s-citalopram”, “quetiapine”, “olanzapine”, “sertraline”, “Alzheimer’ disease”, “AD” or the combinations of those words were used in the searching of related articles. Initially, we identified 1118 articles that included separate studies for each of the five drugs mentioned. After rigorous screening, 14 articles were ultimately selected for detailed analysis.
Selection criteria
The included papers in this study conformed to the following criteria: (1) the papers published in English and included in authoritative academic journals; (2) the studies selected SSRI-related antidepressants as treatment drugs, and the experiments were double-blind and RCTs; (3) the antidepressants in trials involved in citalopram, s-citalopram, quetiapine, olanzapine, and sertraline. (4) The including criteria for the patients followed the fourth edition of the Mental Disorders Diagnostics and Statistic Manual (DSM-IV) and the National Association of Nervous and Communicative Disorders and Stroke/Alzheimer’s Disease Institute of Standards (NINCDSADRDA) [32, 33].
The exclusion conditions included the following criteria: (1) The papers were not randomized controlled trials, including case reports, reviews, and meta-analysis; (2) the original data of papers were incomplete; (3) the papers involved the experiments of mice or other animal models; (4) the papers were non-English publications.
Data abstraction
Data abstraction was independently finished by two researchers, and any divergence was arbitrated by a nonaligned group. All data were analyzed by RevMan 5.3.5, and the risk bias of the studies was detected by the Risk of Bias tool according to the standard of Cochrane Handbook. Besides, P < 0.10 and I2 > 50% meant that heterogeneity of the papers should be ignored. The information of patients including age, sex, dosing, duration of the trial, baseline cognitive score (Mini-Mental State Examination, MMSE), therapy outcomes, and adverse events were recorded for analysis.
Types of outcome measures
The change of cognition, behavior and global assessment of the patients in included articles were selected and record for meta-analysis. The indicators, including Neuropsychiatric Inventory (NPI), Mini-Mental State Examination (MMSE), Brief Psychiatric Rating Scale (BPRS), and Cornell Scale for Depression in Dementia (CSDD) were selected as primary measurements to assess the effects of the antidepressants on AD patients. Besides, the adverse events of patients with antidepressants or placebo in the treatment process were recorded to reflect the security of the drugs.
Statistical analysis
Statistical analyses were conducted using Review Manager 5.3 [34]. We employed random-effects models to account for significant study heterogeneity, defined by thresholds of I2 > 50% and P < 0.10. These thresholds were selected based on standard guidelines to detect meaningful variability across studies, which could affect the interpretation of the meta-analysis results. To further explore sources of heterogeneity, subgroup analyses were performed based on variables such as drug type, patient demographics, and study duration.
Results
Literature search findings
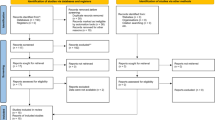
As is shown in Fig. 1, 1118 keyword-related articles were searched from PubMed, EMBASE, Web of Science, and the Cochrane Library of Systematic Reviews. After screening, 495 studies were listed as relevant articles, and 121 of them were determined as eligible by full-text articles assessment. After that, 14 articles were ultimately selected for meta-analysis according to functional outcomes. Besides, three studies compared the effects of citalopram and placebo; one study compared the effects of escitalopram and placebo; four studies compared the effects of olanzapine and placebo; three studies compared the effects of sertraline and placebo; five studies compared the effects of quetiapine and placebo.
Cognition outcome
In Fig. 2, the intervention cycle was in the range of 6–36 weeks. Nine studies (involving 803 participants) assessed changes in cognition by using MMSE [35,36,37,38,39,40,41,42,43]. The results showed that the effects of antidepressants no significant difference between and placebo on AD treatment (P < 0.01), and the mean difference is 0.00 (95% CI − 0.14 to 0.14, 803 participants, I2 = 0%).
Depression outcome
As shown in Fig. 3, the intervention cycle was in the range of 6–24 weeks. Three studies (involving 253 participants) were included to analyze the changes in function by using CSDD [36, 40, 41]. The results showed that there is a significant difference between antidepressants and placebo on AD treatment (P < 0.05), and the mean difference was − 0.58 (95% CI − 0.84 to − 0.33, 253 participants, I2 = 0%).
Behavioral outcome
In Fig. 4, the intervention cycle was in the range of 6–36 weeks. Seven studies (involving 1586 participants) were included to analyze the effects of SSRIs and placebo on NPI of the patients [35, 36, 40,41,42, 44,45,46,47, 49]. The results showed that there was a significant difference between antidepressants and placebo on AD treatment (P < 0.01), and the mean difference was − 0.14 (95% CI − 0.24 to − 0.04, I2 = 0%). In Fig. 5, the intervention cycle was in the range of 6–36 weeks. Five studies (involving 1167 participants) were included to analyze the effects of SSRIs and placebo on the BPRS of the patients [41, 42, 45, 46]. The results showed that there was a significant difference between antidepressants and placebo on AD treatment (P < 0.01), and the mean difference was − 0.15 (95% CI − 0.27 to − 0.03, I2 = 4%).
Safety of drugs
In Fig. 6, the intervention cycle was in the range of 6–36 weeks. Nine studies (involving 1598 adverse events) were included to weight the safety of SSRIs and placebo in the treatments of the patients [35, 36, 38, 40,41,42, 44,45,46]. Although more adverse events were reported by patients in the depressants groups, the total adverse reaction rates of the patients treated with SSRIs did not show any significant difference compared with the patients treated with placebo (OR 0.90, 95% CI 0.60–1.34, 1598 adverse events, I2 = 29%).
Risk of bias and overall quality of evidence
As shown in Fig. 7, the risks of bias items of all included studies were presented as percentages. As could be seen from the graph, bias was detected predominantly in the domains of incomplete data and outcome, limitations applicable to earlier studies. Using the GRADE approach, the overall quality of evidence was judged as moderate indicative.
Discussion
Although SSRIs are associated with cognitive improvements, the direct evidence supporting their role in treating Alzheimer’s disease (AD) remains limited. This study employed multiple approaches to evaluate the efficacy of antidepressants, not only including cognitive and behavioral outcomes but also encompassing drug safety and depression management, to comprehensively assess their potential value in AD treatment.
MMSE score has been used in clinical diagnosis to reflect the situation of AD patients, generally, and one study has indicated that the value of MMSE had a negative relationship with the patients’ progression of hippocampal atrophy [47,48,49,50]. In this study, the MMSE score of the patients with placebo or different antidepressants was compared by meta-analysis, and it was found that SSRIs did not have an effective influence on the MMSE score of the patients. However, the latest studies have indicated that some SSRIs such as quetiapine, olanzapine, and sertraline have potential functions on improving cognitive impairment [51,52,53]. Therefore, the clinical effects of SSRI-related antidepressants should not be drastically ruled out, and more precise RCTs are necessary to further confirm the value of those drugs.
Some SSRIs, including citalopram, S-citalopram, quetiapine, olanzapine, and sertraline, have been applied in clinical for improving the depression of patients. In recent years, more and more studies have found that depression is a key factor that could increase the conversion risk from amnestic mild cognitive impairment to Alzheimer’s disease. For this phenomenon, it may attribute to variants of depression-related genes that induced the accumulation of amyloid-β in patients’ brains [15, 54, 55]. In those studies, most of them have indicated that corresponding antidepressants could improve the symptom of AD to some degree. In this study, CDSSs of patients in included papers were also analyzed by meta-analysis, and the antidepressants showed a significant curative effect on AD patients, which had a significant difference with placebo. Besides, some researches have shown that many AD patients have depression or depression history, thus the improvement of depression may also alleviate the cognitive impairment to some degree [56,57,58].
In this study, the NPI and BPRS assessment of the patients showed that antidepressants expressed significant effects on AD patients compared with placebo, which suggested that SSRI-related antidepressants could alleviate the neurological disorders of elderly patients to further improve the daily behaviors. Citalopram could decrease the accumulation of amyloid β-protein and promote microglial activation to increase the numbers of neurons, and finally improves the deficits in short-term memory, sociability, and depression in mice [59]. Besides, a study has also pointed out that SSRIs, such as fluoxetine, paroxetine, sertraline, and escitalopram, could block the aggregation and fibrillogenesis progressions of Aβ42, and thus inhibit the deterioration of AD via impeding the formation of amyloid plaques in brains of the patients [60].
Safety is one of the most important factors of the drug in clinical research, and it is also the main reason which has restricted the development of drugs of AD [61, 62]. Citalopram, S-citalopram, quetiapine, olanzapine, and sertraline have been commonly used in clinical depression treatment, and their safety has been tested for a long time [63,64,65]. Although total adverse events of AD patients with the five antidepressants are more than the patients with placebo in included articles, the total adverse events rates did not show any significant difference between the patients treated with SSRI-related antidepressants and the patients treated with placebo, which indicated those drugs have more economic advantages in AD treatment compared with the drugs under development.
Although it suggests that some SSRI-related antidepressants have definite validity in AD treatment in this study, it still lacks sufficient evidence to support the clinical application of these drugs. First, only five common drugs, including citalopram, S-citalopram, quetiapine, olanzapine, and sertraline, were selected as candidates to analyze the effect of SSRI-related antidepressants on AD treatment. It is important to note that quetiapine and olanzapine are, in fact, atypical antipsychotics and not classical SSRIs. Their potential cognitive benefits are considered within the broader context of neuroprotective strategies rather than SSRI-specific mechanisms. This selection may not reflect the overall values of SSRI-related antidepressants and could affect the generalizability of our findings. Second, many patients selected by included articles were not classified by type of symptoms, which might not be strong enough to exclude the relationship between the improvement of AD symptoms and alleviated depression of the patients. This lack of precise symptom categorization could obscure specific drug effects on particular AD symptoms, thereby limiting the extrapolation of our findings to broader patient populations. Third, the data of the meta-analysis just came from published scientific articles; therefore, some negative results, including adverse results and non-statistical results, might not be listed in those articles, which might cause a certain deviation between the studies and actual results. Finally, some of the trials in these studies used flexible strategies for drug dosage and duration of treatment. The dosages of antidepressants ranged from dozens to hundreds of milligrams, and the treatment duration usually lasted for weeks depending on the situations of the patients, which brought some uncertainty to the results.
Limitations
Despite our best efforts, we acknowledge certain limitations in our study design. Firstly, our literature search was limited to databases such as PubMed, Embase, and Web of Science, and did not include other potentially relevant databases such as ClinicalTrials.gov. Secondly, our search was restricted to publications in English, which may have omitted significant non-English research. These factors could affect the comprehensiveness of our review. An I2 value of 29% indicates moderate heterogeneity, which we attribute to a variety of factors including differences in study design, sample sizes, and dosing regimens. Future research should consider these limitations to enhance the universality and accuracy of the findings.
Conclusion
This study suggests that SSRI-related antidepressants have great potential values on AD treatment, and more researches about the application of SSRI-related antidepressants on AD treatment are necessary.
Data availability
The simulation experiment data used to support the findings of this study are available from the corresponding author upon request.
References
Jiang S, Yang Y, Li T, Ma Z, Hu W, Deng C, et al. An overview of the mechanisms and novel roles of Nrf2 in cardiovascular diseases. Expert Opin Ther Targets. 2016;20(12):1413–24. https://doi.org/10.1080/14728222.2016.1250887.
Li T, Providencia R, Mu N, Yin Y, Chen M, Wang Y, et al. Association of metformin monotherapy or combined therapy with cardiovascular risks in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2021;20(1):30. https://doi.org/10.1186/s12933-020-01202-5.
Li T, Mu N, Yin Y, Yu L, Ma H. Targeting AMP-activated protein kinase in aging-related cardiovascular diseases. Aging Dis. 2020;11(4):967–77. https://doi.org/10.14336/ad.2019.0901.
Li X, Liu L, Li T, Liu M, Wang Y, Ma H, et al. SIRT6 in senescence and aging-related cardiovascular diseases. Front Cell Dev Biol. 2021;9: 641315. https://doi.org/10.3389/fcell.2021.641315.
Du MR, Gao QY, Liu CL, Bai LY, Li T, Wei FL. Exploring the pharmacological potential of metformin for neurodegenerative diseases. Front Aging Neurosci. 2022;14: 838173. https://doi.org/10.3389/fnagi.2022.838173.
Shi L, Zhang R, Li T, Han X, Yuan N, Jiang L, et al. Decreased miR-132 plays a crucial role in diabetic encephalopathy by regulating the GSK-3β/Tau pathway. Aging (Albany NY). 2020;13(3):4590–604. https://doi.org/10.18632/aging.202418.
Zhang F, Liu L, Zhang C, Ji S, Mei Z, Li T. Association of metabolic syndrome and its components with risk of stroke recurrence and mortality: a meta-analysis. Neurology. 2021;97(7):e695–705. https://doi.org/10.1212/wnl.0000000000012415.
Wang S, Fan Y, Feng X, Sun C, Shi Z, Li T, et al. Nicorandil alleviates myocardial injury and post-infarction cardiac remodeling by inhibiting Mst1. Biochem Biophys Res Commun. 2018;495(1):292–9. https://doi.org/10.1016/j.bbrc.2017.11.041.
Ning P, Luo A, Mu X, Xu Y, Li T. Exploring the dual character of metformin in Alzheimer’s disease. Neuropharmacology. 2022;207: 108966. https://doi.org/10.1016/j.neuropharm.2022.108966.
Liao W, Xu J, Li B, Ruan Y, Li T, Liu J. Deciphering the roles of metformin in Alzheimer’s disease: a snapshot. Front Pharmacol. 2021;12: 728315. https://doi.org/10.3389/fphar.2021.728315.
Möllers T, Perna L, Stocker H, Ihle P, Schubert I, Schöttker B, et al. Alzheimer’s disease medication and outcomes of hospitalisation among patients with dementia. Epidemiol Psychiatr Sci. 2019;29: e73. https://doi.org/10.1017/s2045796019000702.
Younan D, Petkus AJ, Widaman KF, Wang X, Casanova R, Espeland MA, et al. Particulate matter and episodic memory decline mediated by early neuroanatomic biomarkers of Alzheimer’s disease. Brain. 2020;143(1):289–302. https://doi.org/10.1093/brain/awz348.
Nicoll JAR, Buckland GR, Harrison CH, Page A, Harris S, Love S, et al. Persistent neuropathological effects 14 years following amyloid-β immunization in Alzheimer’s disease. Brain. 2019;142(7):2113–26. https://doi.org/10.1093/brain/awz142.
Herbert J, Lucassen PJ. Depression as a risk factor for Alzheimer’s disease: genes, steroids, cytokines and neurogenesis—what do we need to know? Front Neuroendocrinol. 2016;41:153–71. https://doi.org/10.1016/j.yfrne.2015.12.001.
Xu J, Li Q, Qin W, Jun Li M, Zhuo C, Liu H, et al. Neurobiological substrates underlying the effect of genomic risk for depression on the conversion of amnestic mild cognitive impairment. Brain. 2018;141(12):3457–71. https://doi.org/10.1093/brain/awy277.
Qiu Y, Jacobs DM, Messer K, Salmon DP, Feldman HH. Cognitive heterogeneity in probable Alzheimer disease: clinical and neuropathologic features. Neurology. 2019;93(8):e778–90. https://doi.org/10.1212/wnl.0000000000007967.
Peineau S, Rabiant K, Pierrefiche O, Potier B. Synaptic plasticity modulation by circulating peptides and metaplasticity: involvement in Alzheimer’s disease. Pharmacol Res. 2018;130:385–401. https://doi.org/10.1016/j.phrs.2018.01.018.
Mackin RS, Insel PS, Landau S, Bickford D, Morin R, Rhodes E, et al. Late-life depression is associated with reduced cortical amyloid burden: findings from the Alzheimer’s disease neuroimaging initiative depression project. Biol Psychiatry. 2021;89(8):757–65. https://doi.org/10.1016/j.biopsych.2020.06.017.
Bartels C, Wagner M, Wolfsgruber S, Ehrenreich H, Schneider A. Impact of SSRI therapy on risk of conversion from mild cognitive impairment to Alzheimer’s dementia in individuals with previous depression. Am J Psychiatry. 2018;175(3):232–41. https://doi.org/10.1176/appi.ajp.2017.17040404.
Ai PH, Chen S, Liu XD, Zhu XN, Pan YB, Feng DF, et al. Paroxetine ameliorates prodromal emotional dysfunction and late-onset memory deficit in Alzheimer’s disease mice. Transl Neurodegener. 2020;9(1):18. https://doi.org/10.1186/s40035-020-00194-2.
He S, Li Y, Li T, Xu F, Zeng D, Shi Y, et al. Sex differences between serum total bilirubin levels and cognition in patients with schizophrenia. BMC Psychiatry. 2021;21(1):396. https://doi.org/10.1186/s12888-021-03407-8.
Gao K, Ma WZ, Huck S, Li BL, Zhang L, Zhu J, et al. Association between sarcopenia and depressive symptoms in Chinese older adults: evidence from the China Health and retirement longitudinal study. Front Med (Lausanne). 2021;8: 755705. https://doi.org/10.3389/fmed.2021.755705.
He S, Deng Z, Li Z, Gao W, Zeng D, Shi Y, et al. Signatures of 4 autophagy-related genes as diagnostic markers of MDD and their correlation with immune infiltration. J Affect Disord. 2021;295:11–20. https://doi.org/10.1016/j.jad.2021.08.005.
von Linstow CU, Waider J, Grebing M, Metaxas A, Lesch KP, Finsen B. Serotonin augmentation therapy by escitalopram has minimal effects on amyloid-β levels in early-stage Alzheimer’s-like disease in mice. Alzheimers Res Ther. 2017;9(1):74. https://doi.org/10.1186/s13195-017-0298-y.
Cirrito JR, Wallace CE, Yan P, Davis TA, Gardiner WD, Doherty BM, et al. Effect of escitalopram on Aβ levels and plaque load in an Alzheimer mouse model. Neurology. 2020;95(19):e2666–74. https://doi.org/10.1212/wnl.0000000000010733.
Sheline YI, West T, Yarasheski K, Swarm R, Jasielec MS, Fisher JR, et al. An antidepressant decreases CSF Aβ production in healthy individuals and in transgenic AD mice. Sci Transl Med. 2014;6(236):23re64. https://doi.org/10.1126/scitranslmed.3008169.
Sheline YI, Snider BJ, Beer JC, Seok D, Fagan AM, Suckow RF, et al. Effect of escitalopram dose and treatment duration on CSF Aβ levels in healthy older adults: a controlled clinical trial. Neurology. 2020;95(19):e2658–65. https://doi.org/10.1212/wnl.0000000000010725.
Cirrito JR, Disabato BM, Restivo JL, Verges DK, Goebel WD, Sathyan A, et al. Serotonin signaling is associated with lower amyloid-β levels and plaques in transgenic mice and humans. Proc Natl Acad Sci USA. 2011;108(36):14968–73. https://doi.org/10.1073/pnas.1107411108.
Locher C, Koechlin H, Zion SR, Werner C, Pine DS, Kirsch I, et al. Efficacy and safety of selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and placebo for common psychiatric disorders among children and adolescents: a systematic review and meta-analysis. JAMA Psychiat. 2017;74(10):1011–20. https://doi.org/10.1001/jamapsychiatry.2017.2432.
Vangala C, Niu J, Montez-Rath ME, Yan J, Navaneethan SD, Winkelmayer WC. Selective serotonin reuptake inhibitor use and hip fracture risk among patients on hemodialysis. Am J Kidney Dis. 2020;75(3):351–60. https://doi.org/10.1053/j.ajkd.2019.07.015.
Laporte S, Chapelle C, Caillet P, Beyens MN, Bellet F, Delavenne X, et al. Bleeding risk under selective serotonin reuptake inhibitor (SSRI) antidepressants: a meta-analysis of observational studies. Pharmacol Res. 2017;118:19–32. https://doi.org/10.1016/j.phrs.2016.08.017.
Guze SB. Diagnostic and statistical manual of mental disorders, 4th ed. (DSM-IV). Am J Psychiatry. 1995;152(8):1228. https://doi.org/10.1176/ajp.152.8.1228.
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34(7):939–44. https://doi.org/10.1212/wnl.34.7.939.
Hou M, Xing H, Li C, Wang X, Deng D, Li J, et al. Short-term efficacy and safety of lasmiditan, a novel 5-HT(1F) receptor agonist, for the acute treatment of migraine: a systematic review and meta-analysis. J Headache Pain. 2020;21(1):66. https://doi.org/10.1186/s10194-020-01138-x.
Zhou T, Wang J, Xin C, Kong L, Wang C. Effect of memantine combined with citalopram on cognition of BPSD and moderate Alzheimer’s disease: a clinical trial. Exp Ther Med. 2019;17(3):1625–30. https://doi.org/10.3892/etm.2018.7124.
An H, Choi B, Park KW, Kim DH, Yang DW, Hong CH, et al. The effect of escitalopram on mood and cognition in depressive Alzheimer’s disease subjects. J Alzheimers Dis. 2017;55(2):727–35. https://doi.org/10.3233/jad-160225.
Moretti R, Torre P, Antonello RM, Cazzato G, Bava A. Depression and Alzheimer’s disease: symptom or comorbidity? Am J Alzheimers Dis Other Demen. 2002;17(6):338–44. https://doi.org/10.1177/153331750201700607.
Finkel SI, Mintzer JE, Dysken M, Krishnan KR, Burt T, McRae T. A randomized, placebo-controlled study of the efficacy and safety of sertraline in the treatment of the behavioral manifestations of Alzheimer’s disease in outpatients treated with donepezil. Int J Geriatr Psychiatry. 2004;19(1):9–18. https://doi.org/10.1002/gps.998.
Paleacu D, Barak Y, Mirecky I, Mazeh D. Quetiapine treatment for behavioural and psychological symptoms of dementia in Alzheimer’s disease patients: a 6-week, double-blind, placebo-controlled study. Int J Geriatr Psychiatry. 2008;23(4):393–400. https://doi.org/10.1002/gps.1892.
Lyketsos CG, DelCampo L, Steinberg M, Miles Q, Steele CD, Munro C, et al. Treating depression in Alzheimer disease: efficacy and safety of sertraline therapy, and the benefits of depression reduction: the DIADS. Arch Gen Psychiatry. 2003;60(7):737–46. https://doi.org/10.1001/archpsyc.60.7.737.
Kurlan R, Cummings J, Raman R, Thal L. Quetiapine for agitation or psychosis in patients with dementia and Parkinsonism. Neurology. 2007;68(17):1356–63. https://doi.org/10.1212/01.wnl.0000260060.60870.89.
Tariot PN, Schneider L, Katz IR, Mintzer JE, Street J, Copenhaver M, et al. Quetiapine treatment of psychosis associated with dementia: a double-blind, randomized, placebo-controlled clinical trial. Am J Geriatr Psychiatry. 2006;14(9):767–76. https://doi.org/10.1097/01.Jgp.0000196628.12010.35.
Sultzer DL, Davis SM, Tariot PN, Dagerman KS, Lebowitz BD, Lyketsos CG, et al. Clinical symptom responses to atypical antipsychotic medications in Alzheimer’s disease: phase 1 outcomes from the CATIE-AD effectiveness trial. Am J Psychiatry. 2008;165(7):844–54. https://doi.org/10.1176/appi.ajp.2008.07111779.
Porsteinsson AP, Drye LT, Pollock BG, Devanand DP, Frangakis C, Ismail Z, et al. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682–91. https://doi.org/10.1001/jama.2014.93.
Street JS, Clark WS, Gannon KS, Cummings JL, Bymaster FP, Tamura RN, et al. Olanzapine treatment of psychotic and behavioral symptoms in patients with Alzheimer disease in nursing care facilities: a double-blind, randomized, placebo-controlled trial. The HGEU Study Group. Arch Gen Psychiatry. 2000;57(10):968–76. https://doi.org/10.1001/archpsyc.57.10.968.
Deberdt WG, Dysken MW, Rappaport SA, Feldman PD, Young CA, Hay DP, et al. Comparison of olanzapine and risperidone in the treatment of psychosis and associated behavioral disturbances in patients with dementia. Am J Geriatr Psychiatry. 2005;13(8):722–30. https://doi.org/10.1176/appi.ajgp.13.8.722.
Hamelin L, Lagarde J, Dorothée G, Potier MC, Corlier F, Kuhnast B, et al. Distinct dynamic profiles of microglial activation are associated with progression of Alzheimer’s disease. Brain. 2018;141(6):1855–70. https://doi.org/10.1093/brain/awy079.
Dubois B, Epelbaum S, Nyasse F, Bakardjian H, Gagliardi G, Uspenskaya O, et al. Cognitive and neuroimaging features and brain β-amyloidosis in individuals at risk of Alzheimer’s disease (INSIGHT-preAD): a longitudinal observational study. Lancet Neurol. 2018;17(4):335–46. https://doi.org/10.1016/s1474-4422(18)30029-2.
Munro CA, Brandt J, Sheppard JM, Steele CD, Samus QM, Steinberg M, et al. Cognitive response to pharmacological treatment for depression in Alzheimer disease: secondary outcomes from the depression in Alzheimer’s disease study (DIADS). Am J Geriatr Psychiatry. 2004;12(5):491–8. https://doi.org/10.1176/appi.ajgp.12.5.491.
Li DD, Zhang YH, Zhang W, Zhao P. Meta-analysis of randomized controlled trials on the efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer’s disease. Front Neurosci. 2019;13:472. https://doi.org/10.3389/fnins.2019.00472.
Bucki A, Marcinkowska M, Śniecikowska J, Zagórska A, Jamrozik M, Pawłowski M, et al. Multifunctional 6-fluoro-3-[3-(pyrrolidin-1-yl)propyl]-1,2-benzoxazoles targeting behavioral and psychological symptoms of dementia (BPSD). Eur J Med Chem. 2020;191: 112149. https://doi.org/10.1016/j.ejmech.2020.112149.
Geerts H, Spiros A. Simulating the effects of common comedications and genotypes on Alzheimer’s cognitive trajectory using a quantitative systems pharmacology approach. J Alzheimers Dis. 2020;78(1):413–24. https://doi.org/10.3233/jad-200688.
Takemoto M, Ohta Y, Hishikawa N, Yamashita T, Nomura E, Tsunoda K, et al. The efficacy of sertraline, escitalopram, and nicergoline in the treatment of depression and apathy in Alzheimer’s disease: the Okayama depression and apathy project (ODAP). J Alzheimers Dis. 2020;76(2):769–72. https://doi.org/10.3233/jad-200247.
Moriguchi S, Takahata K, Shimada H, Kubota M, Kitamura S, Kimura Y, et al. Excess tau PET ligand retention in elderly patients with major depressive disorder. Mol Psychiatry. 2021;26(10):5856–63. https://doi.org/10.1038/s41380-020-0766-9.
Baek SH, Park SJ, Jeong JI, Kim SH, Han J, Kyung JW, et al. Inhibition of Drp1 ameliorates synaptic depression, Aβ deposition, and cognitive impairment in an Alzheimer’s disease model. J Neurosci. 2017;37(20):5099–110. https://doi.org/10.1523/jneurosci.2385-16.2017.
Fišar Z, Hansíková H, Křížová J, Jirák R, Kitzlerová E, Zvěřová M, et al. Activities of mitochondrial respiratory chain complexes in platelets of patients with Alzheimer’s disease and depressive disorder. Mitochondrion. 2019;48:67–77. https://doi.org/10.1016/j.mito.2019.07.013.
Chen B, Zhong X, Mai N, Peng Q, Wu Z, Ouyang C, et al. Cognitive impairment and structural abnormalities in late life depression with olfactory identification impairment: an Alzheimer’s disease-like pattern. Int J Neuropsychopharmacol. 2018;21(7):640–8. https://doi.org/10.1093/ijnp/pyy016.
Paroni G, Panza F, De Cosmo S, Greco A, Seripa D, Mazzoccoli G. Klotho at the edge of Alzheimer’s disease and senile depression. Mol Neurobiol. 2019;56(3):1908–20. https://doi.org/10.1007/s12035-018-1200-z.
Zhang Q, Yang C, Liu T, Liu L, Li F, Cai Y, et al. Citalopram restores short-term memory deficit and non-cognitive behaviors in APP/PS1 mice while halting the advance of Alzheimer’s disease-like pathology. Neuropharmacology. 2018;131:475–86. https://doi.org/10.1016/j.neuropharm.2017.12.021.
Tin G, Mohamed T, Shakeri A, Pham AT, Rao PPN. Interactions of selective serotonin reuptake inhibitors with β-amyloid. ACS Chem Neurosci. 2019;10(1):226–34. https://doi.org/10.1021/acschemneuro.8b00160.
Cummings J. The role of biomarkers in Alzheimer’s disease drug development. Adv Exp Med Biol. 2019;1118:29–61. https://doi.org/10.1007/978-3-030-05542-4_2.
Berman MH, Nichols TW. Treatment of neurodegeneration: integrating photobiomodulation and neurofeedback in Alzheimer’s dementia and Parkinson’s: a review. Photobiomodul Photomed Laser Surg. 2019;37(10):623–34. https://doi.org/10.1089/photob.2019.4685.
Porsteinsson AP, Antonsdottir IM. An update on the advancements in the treatment of agitation in Alzheimer’s disease. Expert Opin Pharmacother. 2017;18(6):611–20. https://doi.org/10.1080/14656566.2017.1307340.
Szabó L, Mile V, Kiss DJ, Kovács K, Földes T, Németh T, et al. Applicability evaluation of advanced processes for elimination of neurophysiological activity of antidepressant fluoxetine. Chemosphere. 2018;193:489–97. https://doi.org/10.1016/j.chemosphere.2017.11.047.
Jacobsen JP, Rudder ML, Roberts W, Royer EL, Robinson TJ, Oh A, et al. SSRI augmentation by 5-hydroxytryptophan slow release: mouse pharmacodynamic proof of concept. Neuropsychopharmacology. 2016;41(9):2324–34. https://doi.org/10.1038/npp.2016.35.
Funding
The authors have no support or funding to report.
Author information
Authors and Affiliations
Contributions
JZ as the chief investigator of the study, conceived the study, and is responsible for the overall conduct of the study. HW, SL, WP, TL, and JZ are responsible for literature screening and responsible for the statistical analysis. WP and JZ supervised the drafting of the manuscript. All the authors have read and approved the final manuscript and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, H., Li, S., Zhang, J. et al. Efficacy of selective serotonin reuptake inhibitors-related antidepressants in Alzheimer’s disease: a meta-analysis. Eur J Med Res 29, 438 (2024). https://doi.org/10.1186/s40001-024-02006-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-024-02006-z