Abstract
Superior mesenteric artery syndrome (SMAS) is a rare and unpredictable complication after correction spine surgery for adolescent idiopathic scoliosis (AIS). The management of this condition is poorly investigated, with controversial outcomes. This investigation systematically reviewed current evidence on pathogenesis, risk factors, management, and outcomes of SMAS following correction spine surgery for AIS. The present systematic review was conducted according to the 2020 PRISMA statement. All the included investigations reported SMAS presentation following scoliosis correction surgery in AIS. 29 articles with 61 eligible patients were included in this review. The mean age of the patients was 15.8 ± 7.2 years. The mean weight was 45.3 ± 8.0 kg, the mean height 159.6 ± 13.6 cm, and the mean BMI 16.5 ± 2.9 kg/m2. The mean duration of the treatment for SMAS was 21.6 ± 10.3 days. The mean interval between spine surgery and symptoms of SAMS was 69 days, with high between-studies variability (3 days to 4 years). Prompt identification of risk factors and an early diagnosis are necessary to manage SMAS and reduce the risk of complications. Additional investigations are required to establish risk factors and diagnostic criteria.
Level of evidence Level IV, systematic review.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
The superior mesenteric artery syndrome (SMAS), also known as “Wilkie syndrome”, is a complication of scoliosis surgery [1,2,3]. This clinical condition was first illustrated by Carl von Rokitasky in 1861 and later in greater detail by Wilkie in 1927 [4,5,6,7]. The reported incidence of SMAS after scoliosis surgery is approximately 0.013–4.7% [8,9,10,11]. The aetiology of SMAS is a change of the anatomical relationship between the third part of the duodenum, lumbar spine, and superior mesenteric artery (SMA) [12, 13]. The SMA arises from the anterior aspect of the aorta at the level of L1–L2 vertebral bodies [14, 15]. At its origin, the SMA is encased in fat and lymphatic tissue and descends downwards the mesentery [8, 11, 16]. On the other hand, the duodenum traverses the aorta at the level of the L3 vertebral body and is suspended by the ligament of Treitz between the aorta and the SMA [8, 16, 17]. Any factor which modifies this anatomical relationship could lead to an extrinsic compression of the duodenum [11, 17,18,19]. SMA syndrome presents clinically unspecific simulating other factors and causes of upper gastrointestinal obstruction [4,5,6,7]. The correct diagnosis of SMAS may be challenging, requiring a proper correlation of clinical signs and symptoms with radiographic findings [4,5,6,7]. A timely diagnosis and appropriate management of SMAS is recommended since delayed treatment has a mortality rate of 33% [1, 6, 20]. Its management is conservative initially, with the rationale of weight gain to increase the retro-peritoneal fat and the aortomesenteric angle [13, 21, 22]. If the conservative therapy fails, surgical options are represented by gastrojejunostomy, duodenojejunostomy, or Ladd procedure [13, 21, 22].
Evidence on SMAS is limited. A proper therapeutic algorithm and internationally accepted guidelines are missing. This investigation systematically reviewed current evidence on pathogenesis, risk factors, management, and outcomes of SMAS following correction spine surgery for adolescent idiopathic scoliosis (AIS).
Methods
Eligibility criteria
All the clinical studies investigating SMAS following spine surgery for AIS were accessed. According to the author´s language capabilities, English, German, Italian, and Spanish articles were eligible. According to the Oxford Centre of Evidence-Based Medicine, we included only studies with levels I to IV of evidence [23]. Studies published in grey literature or without full text were not eligible. Opinions, letters, and editorials were not considered. Only clinical studies were eligible. Missing quantitative data under the outcomes of interests warranted the exclusion of the study.
Search strategy
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the 2020 PRISMA statement [24]. The following framework was used to guide the literature search:
-
P (Problem): SMAS following AIS;
-
I (Intervention): scoliosis correction surgery;
-
C (Comparison): height, weight, length of fusion as risk factor for SMAS;
-
T (Time): interval between the surgical treatment and presenting the symptoms for SMAS.
In December 2023, the following databases were accessed: PubMed, Web of Science, and Google Scholar. No time constraint was set for the search. The medical subject headings (MeSH) used in each database for the search are reported in the appendix. Additional filters were not used for the database search.
Selection and data collection
Two authors (G.P. and L.S.) independently executed the database search. All matched titles were screened by hand, and the abstract was examined if suitable. The full text of the abstracts matched the topic and was accessed. Studies without accessible or available full text were not included. A cross-reference of the bibliography of the full-text manuscripts was also performed for the inclusion. Authors debated controversies. A third senior author (F.M.) took the final decision in case of additional differences of opinion.
Data items
Two authors (G.P. and L.S.) separately performed data examination and analysis. The following data at baseline were extracted: author, year and journal of publication, study design, and patient characteristics, including age, gender, weight, height, and BMI. The post-operative day (POD) when symptoms of SMAS started was recorded. Data regarding the type of surgical scoliosis correction, signs and symptoms, diagnosis and management of SMAS were retrieved. Data were extracted in Microsoft Office Excel version 16.72 (Microsoft Corporation, Redmond, USA).
Assessment of the risk of bias
The risk of bias was examined following the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions [25]. The Nonrandomised Studies of Interventions (ROBINS-I) tool was used for all included non-RCTs [26]. Seven domains of potential bias in non-RCTs were assessed. Possible confounding and the nature of patient selection before the comparative intervention are evaluated by two domains. A further domain is used to evaluate bias in the classification during the intervention. The final four domains assess the methodological quality after the intervention comparison has been implemented and relate to deviations from previously intended interventions, missing data, erroneous measurement of outcomes, and bias in the selection of reported outcomes. The figure of the ROBINS-I was elaborated using the Robvis Software (Risk-of-bias VISualization, Riskofbias.info, Bristol, UK) [27]. Case reports included in this investigation were evaluated using the Joanna Briggs Institute (JBI) criteria appraisal tools for case reports [28]. Possible answers were “yes”, “no”, “unclear”, or “not applicable”. Eight questions were answered to evaluate the methodological quality in accordance with the JBI checklist. The included studies were assessed based on the following bias: low risk of bias: studies with more than six “yes”; moderate risk of bias: studies with five to six “yes”; and high risk of bias: studies with less than five “yes”. Two reviewers (G.P. and L.S.) evaluated the risk of bias in the extracted studies separately. Disagreements were solved by a third senior author (F.M.).
Results
Study selection
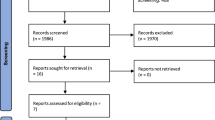
The literature search resulted in 276 articles. Of them, 192 were excluded as they were duplicates. Additionally, 44 studies were excluded for the following reasons: study design (N = 7), low level of evidence (N = 6), full text not available or not in peer-reviewed journals (N = 16), and language limitations (N = 4). An additional 11 studies were excluded because they did not offer quantitative data on the outcomes of interest. Finally, 29 articles were included in the present investigation. Of them, nine had a retrospective study design, and 20 were case reports. The results of the literature search are visible in Fig. 1.
Methodological quality assessment
The ROBINS-I was applied to investigate the risk of bias in 31.0% (9 of 29) of the included studies, as they were non-RCTs. Given the small number of patients studied in the included investigations, the risk of bias based on confounding and the selection of participants was rated in four studies critical because they reported data from only one patient. The residuary studies were rated with a moderate to serious risk of bias in the cited domains. The intervention protocol was well reported in most studies, and no significant deviation from the interventions was identified, showing a primarily low risk of bias in the classification of interventions and deviation from intended interventions. Data were adequately reported in the most included studies, and the measurement of the outcomes was equivalent among the groups. Given the lack of randomisation of the investigators and patients, the measurement of the outcomes was evaluated with a moderate risk of bias in all of the studies. The reported results corresponded to the planned protocol in most included studies. Given the overall poor methodological quality in the included studies, the overall risk of bias was predominantly serious to critical. The assessments of the methodological quality are given in Fig. 2.
The risk of bias using the JBI criteria appraisal tool for case reports was evaluated in 69.0% (20 of 29) of the included studies, as they were case reports. While seven of the included studies received a score of six and five, respectively, indicating moderate quality, 12 received a score of seven or eight, demonstrating good-quality evidence. The case report by Fiorini et al. received a score of three out of eight, suggesting low-quality evidence with a high risk of bias. The detailed assessment steps are shown in Fig. 3.
Study characteristics and results of individual studies
A total of 61 patients were included in the present study. 62% (38 of 61 patients) were women. The mean age was 15.8 ± 7.2 years. The mean weight was 45.3 ± 8.0 kg, the mean height 159.6 ± 13.6 cm, and the mean BMI 16.5 ± 2.9 kg/m2. The generalities and demographics of the included studies are shown in Table 1.
Synthesis of results
The mean interval between spine surgery and symptoms occurrence was 69.1 ± 262.4 days (3 days to 4 years). The mean duration of the treatment for SMAS was 21.6 ± 10.3 days. Table 2 provides an overview of the main findings of the included studies.
Discussion
The systematic review found that the mean interval between spine surgery and symptoms occurrence was 69 days, with high between-studies variability (3 days to 4 years). The mean duration of the management for a diagnosed SMAS was 21.6 days.
Following spinal surgery in AIS, SMAS is a complication provoked by extrinsic compression of the third part of the duodenum as it crosses between the SMA, which lies anteriorly, and the abdominal aorta and lumbar spine posteriorly [7, 13, 50,51,52]. The SMA supplies blood to the entire small intestine except the duodenal bulb, cecum, ascending, and transverse colon. The vessel arises from the anterior aspect of the aorta at the level of the L1 vertebral body and extends caudally into the small bowel mesentery [53]. The third part of the duodenum passes between the aorta and SMA and is suspended by the ligament of Treitz, also known as the suspensory muscle of the duodenum [54,55,56,57]. The aortomesenteric angle and the aortomesenteric distance usually range from 28° to 65° and 10 to 34 mm, respectively. In SMAS, they are reduced, having values of 6–15° and 2–8 mm, respectively [13, 51, 58,59,60,61]. The third part of the duodenum is generally surrounded by periduodenal fat at the aortomesenteric angle. Modifying this anatomy could lead to vascular compression of the third part of the duodenum, which might lead to SMAS [7, 8, 13, 41, 62]. The risk factors for SMAS can be congenital or acquired, and identifying patients at risk for SMAS is pivotal to avoiding complications. A congenital short ligament of Treitz could pull the duodenum upward into the vascular angle between the SMA and the aorta, causing vascular compression [11, 30]. Acquired causes related to a decrease in intestinal padding include weight loss (weight < 25% percentile, BMI < 25th percentile), eating disorders, cachexia, severely debilitating conditions, and incapacitating illnesses such as malignancies [22, 63,64,65]. A lower BMI, height, or weight than 5%, 50%, or 25% percentile, sagittal kyphosis, and heavy and quick halofemoral traction after anterior spinal release are other important risk factors for SMAS [41]. Acquired and iatrogenic modifications of the aortomesenteric angle due to rapid height gain (> 50% percentile) or postoperative spinal lengthening could also lead to SMAS. Children mostly attend a surgical correction of AIS in their most rapid longitudinal growth phase. Moreover, children or adolescents with scoliosis and thoracic curves also have hyperkyphosis, which leads to a more extended spine and reduces the aortomesenteric angle [8, 11, 18, 19, 29, 30, 41]. Willner et al. [66] showed that a spurt of growth is particularly accelerated in the year before the diagnosis of AIS, and these patients tend to be taller and more slender than their age-matched counterparts. Moreover, there may be an acute increase in vertebral column length after posterior and anterior spinal instrumentation [67, 68]. The traction on the SMA and, consequently, narrowing of the aortomesenteric angle can be caused by the acute increase in vertebral column length following spinal instrumentation. Finally, postoperative weight loss causes retroperitoneal fat reduction that protects the duodenum from compression [8, 11, 18, 19, 29, 30, 41]. During the retroperitoneal dissection in the anterior approach to the thoracolumbar spine, the disruption of the autonomic nerve supply to the small intestine may also trigger the development of the condition [19]. The mean interval between spine surgery and the symptoms of SMAS was 69 days, with high variability between studies (3 days to 4 years). Prompt identification of risk factors and an early diagnosis are necessary to manage SMAS and reduce the risk of complications. Delay in the development of the syndrome might be related to the progressive weight loss occurring in the postoperative period, resulting in gradual loss of the retroperitoneal fat [42, 69, 70]. Symptoms include nausea, anorexia, bilious vomiting, and intestinal constipation, with partial relief of them with postural changes and epigastric pain [71,72,73,74]. During the physical examination, the peristalsis can be normal or hyperkinetic, and the abdomen is soft with occasional tenderness in the epigastrium at deep palpation [19, 41]. Symptoms of SMAS are similar to paralytic ileus [18, 41, 68, 75]. Recurrent vomiting with gastric dilatation could eventually lead to severe hypovolemia, progressive dehydration, oliguria, and electrolyte disorders, such as hypokalaemia and metabolic alkalosis, or even death [68, 76, 77]. Death in SMAS can result from chemical pneumonia by vomitus inhalation or gastric perforation [8, 44, 78].
SMAS is characterised by three criteria: dilated duodenum, reduction of the aortomesenteric angle to less than 25°, and compression of the third part of the duodenum by the SMA [13, 77, 79,80,81]. Barium imaging can assist in the diagnosis, showing the characteristic duodenal dilatation with an abrupt vertical cutoff in the third part of the duodenum and a 4–6-h delay in the gastroduodenal transit [22, 82]. The gold standard radiological methods in SMAS diagnosis is represented by computer tomography angiography (CTA), which reveals compression of the third part of the duodenum by the SMA with subsequent proximal duodenal dilatation and a reduced aortomesenteric angle and aortomesenteric distance [61, 62, 83, 84]. In most patients, conservative management is associated with improving the symptoms, usually occurring after 2–3 days [85]. Oral intake should be restricted, and under radiographic assistance, nasojejunal feeding should be passed distally from the duodenal obstruction to achieve enteral feeding and progressive weight gain with high-caloric nutritional supplements [85]. If the symptomatology persists or enteral feeding is impossible, total parenteral nutrition should be started.
Failure of the conservative treatment can lead to life-threatening conditions such as metabolic alkalosis, electrolyte imbalance, and aspiration pneumonia [13, 20, 86, 87].
If conservative management fails, gastrojejunostomy, duodenojejunostomy, or Ladd procedure is advocated [88,89,90,91]. Although conservative management is the first therapeutic approach in patients with SMAS, the current evidence lacks large clinical trials and recommendations. An internationally accepted therapeutic algorithm for the management of SMAS is necessary. Given the rarity of SMAS, the current evidence is mostly case reports. Therefore, the generalisation of these results is limited, and an estimate of the prevalence of SMAS is unreliable. Moreover, the limited evidence hinders the establishment of reliable risk factors and a proper algorithm for diagnosing and treating SMAS. Additional investigations are required to establish risk factors and diagnostic criteria.
Conclusion
SMAS is a complication of spinal surgery in AIS caused by extrinsic compression of the third part of the duodenum. The mean interval between spine surgery and symptoms occurrence was 69 days, with high between-studies variability (3 days to 4 years). Prompt identification of risk factors and an early diagnosis are necessary to manage SMAS and reduce the risk of complications. Additional investigations are required to establish risk factors and diagnostic criteria.
Availability of data and materials
The datasets generated during and analysed during the current study are available throughout the manuscript. No datasets were generated or analysed during the current study.
Abbreviations
- ASIF:
-
Anterior spinal instrumentation and fusion
- PSIF:
-
Posterior spinal instrumentation and fusion
- CT:
-
Computer tomography
- CTA:
-
Computed tomography angiography
- D1:
-
First (transversal) part of the duodenum
- D2:
-
Descend part of the duodenum
- D3:
-
Third (horizontal) part of the duodenum
- SMAS:
-
Superior mesenteric artery syndrome
- SMA:
-
Superior mesenteric artery
- MIS:
-
Minimally invasive surgery
- POD:
-
Postoperative day
References
Louie PK, Basques BA, Bitterman A, Shah S, Patel K, Abramchayev I, Lewin J. Superior mesenteric artery syndrome as a complication of scoliosis surgery. Am J Orthop. 2017;46(2):E124–30.
Rai RR, Shah S, Palliyil NS, Dalvie S, Shah R. Superior mesenteric artery syndrome complicating spinal deformity correction surgery: a case report and review of the literature. JBJS Case Connect. 2019;9(4): e0497. https://doi.org/10.2106/JBJS.CC.18.00497.
Baroncini A, Trobisch PD, Migliorini F. Learning curve for vertebral body tethering: analysis on 90 consecutive patients. Spine Deform. 2021;9(1):141–7. https://doi.org/10.1007/s43390-020-00191-5.
Adams JB, Hawkins ML, Ferdinand CH, Medeiros RS. Superior mesenteric artery syndrome in the modern trauma patient. Am Surg. 2007;73(8):803–6.
Ehlers TO, Tsamalaidze L, Pereira L, Stauffer J. Laparoscopic Duodenojejunostomy for the SMA syndrome. Zentralbl Chir. 2018;143(5):461–3. https://doi.org/10.1055/a-0668-1991.
Mathenge N, Osiro S, Rodriguez II, Salib C, Tubbs RS, Loukas M. Superior mesenteric artery syndrome and its associated gastrointestinal implications. Clin Anat. 2014;27(8):1244–52. https://doi.org/10.1002/ca.22249.
Oka A, Awoniyi M, Hasegawa N, Yoshida Y, Tobita H, Ishimura N, Ishihara S. Superior mesenteric artery syndrome: diagnosis and management. World J Clin Cases. 2023;11(15):3369–84. https://doi.org/10.12998/wjcc.v11.i15.3369.
Tsirikos AI, Anakwe RE, Baker AD. Late presentation of superior mesenteric artery syndrome following scoliosis surgery: a case report. J Med Case Rep. 2008;2:9. https://doi.org/10.1186/1752-1947-2-9.
Wilkinson R, Huang CT. Superior mesenteric artery syndrome in traumatic paraplegia: a case report and literature review. Arch Phys Med Rehabil. 2000;81(7):991–4. https://doi.org/10.1053/apmr.2000.3867.
Ylinen P, Kinnunen J, Hockerstedt K. Superior mesenteric artery syndrome: a follow-up study of 16 operated patients. J Clin Gastroenterol. 1989;11(4):386–91.
Hod-Feins R, Copeliovitch L, Abu-Kishk I, Eshel G, Lotan G, Shalmon E, Anekstein Y, Mirovsky Y, Masharawi Y. Superior mesenteric artery syndrome after scoliosis repair surgery: a case study and reassessment of the syndrome’s pathogenesis. J Pediatr Orthop B. 2007;16(5):345–9. https://doi.org/10.1097/BPB.0b013e32826d1d9b.
Gibson D, Hong M Jr, Mehler PS. Superior mesenteric artery syndrome. Mayo Clin Proc. 2021;96(12):2945–6. https://doi.org/10.1016/j.mayocp.2021.09.017.
Merrett ND, Wilson RB, Cosman P, Biankin AV. Superior mesenteric artery syndrome: diagnosis and treatment strategies. J Gastrointest Surg. 2009;13(2):287–92. https://doi.org/10.1007/s11605-008-0695-4.
Negoi I, Beuran M, Hostiuc S, Negoi RI, Inoue Y. Surgical anatomy of the superior mesenteric vessels related to colon and pancreatic surgery: a systematic review and meta-analysis. Sci Rep. 2018;8(1):4184. https://doi.org/10.1038/s41598-018-22641-x.
Zhao YE, Wang ZJ, Zhou CS, Zhu FP, Zhang LJ, Lu GM. Multidetector computed tomography of superior mesenteric artery: anatomy and pathologies. Can Assoc Radiol J. 2014;65(3):267–74. https://doi.org/10.1016/j.carj.2013.10.001.
Balcerzak A, Tubbs RS, Wasniewska-Wlodarczyk A, Rapacka E, Olewnik L. Classification of the superior mesenteric artery. Clin Anat. 2022;35(4):501–11. https://doi.org/10.1002/ca.23841.
Shah MA, Albright MB, Vogt MT, Moreland MS. Superior mesenteric artery syndrome in scoliosis surgery: weight percentile for height as an indicator of risk. J Pediatr Orthop. 2003;23(5):665–8. https://doi.org/10.1097/00004694-200309000-00018.
Altiok H, Lubicky JP, DeWald CJ, Herman JE. The superior mesenteric artery syndrome in patients with spinal deformity. Spine. 2005;30(19):2164–70. https://doi.org/10.1097/01.brs.0000181059.83265.b2.
Crowther MA, Webb PJ, Eyre-Brook IA. Superior mesenteric artery syndrome following surgery for scoliosis. Spine. 2002;27(24):E528-533. https://doi.org/10.1097/00007632-200212150-00023.
Chung WH, Anuar AA, Lee KJ, Hasan MS, Chiu CK, Chan CYW, Kwan MK. Superior mesenteric artery syndrome: a rare complication of scoliosis corrective surgery. J Orthop Surg. 2020;28(3):2309499020945014. https://doi.org/10.1177/2309499020945014.
Kirby GC, Faulconer ER, Robinson SJ, Perry A, Downing R. Superior mesenteric artery syndrome: a single centre experience of laparoscopic duodenojejunostomy as the operation of choice. Ann R Coll Surg Engl. 2017;99(6):472–5. https://doi.org/10.1308/rcsann.2017.0063.
Sinagra E, Raimondo D, Albano D, Guarnotta V, Blasco M, Testai S, Marasa M, Mastrella V, Alaimo V, Bova V, Albano G, Sorrentino D, Tomasello G, Cappello F, Leone A, Rossi F, Galia M, Lagalla R, Midiri F, Morreale GC, Amvrosiadis G, Martorana G, Spampinato MG, Virgilio V, Midiri M. Superior mesenteric artery syndrome: clinical, endoscopic, and radiological findings. Gastroenterol Res Pract. 2018;2018:1937416. https://doi.org/10.1155/2018/1937416.
Howick JCI, Glasziou P, Greenhalgh T, Heneghan C, Liberati A, Moschetti I, Phillips B, Thornton H, Goddard O, Hodgkinson M. The 2011 Oxford CEBM levels of evidence. Oxford: Oxford Centre for Evidence-Based Medicine; 2011.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hrobjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71.
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J. Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. 2019;10(10):ED000142.
Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hrobjartsson A, Kirkham J, Juni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schunemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. https://doi.org/10.1136/bmj.i4919.
McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021;12(1):55–61. https://doi.org/10.1002/jrsm.1411.
Munn Z, Barker TH, Moola S, Tufanaru C, Stern C, McArthur A, Stephenson M, Aromataris E. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. 2020;18(10):2127–33. https://doi.org/10.11124/JBISRIR-D-19-00099.
Tsirikos AI, Jeans LA. Superior mesenteric artery syndrome in children and adolescents with spine deformities undergoing corrective surgery. J Spinal Disord Tech. 2005;18(3):263–71.
Zhu ZZ, Qiu Y. Superior mesenteric artery syndrome following scoliosis surgery: its risk indicators and treatment strategy. World J Gastroenterol. 2005;11(21):3307–10. https://doi.org/10.3748/wjg.v11.i21.3307.
Pan CH, Tzeng ST, Chen CS, Chen PQ. Superior mesenteric artery syndrome complicating staged corrective surgery for scoliosis. J Formos Med Assoc. 2007;106(2 Suppl):S37-45. https://doi.org/10.1016/s0929-6646(09)60351-x.
Fiorini S, Saenz Tejeira MM, Tennina C, Tomezzoli S, Requejo N. Superior mesenteric artery syndrome (Wilkie syndrome): case report. Arch Argent Pediatr. 2008;106(6):546–8. https://doi.org/10.1590/S0325-00752008000600015.
Smith BG, Hakim-Zargar M, Thomson JD. Low body mass index: a risk factor for superior mesenteric artery syndrome in adolescents undergoing spinal fusion for scoliosis. J Spinal Disord Tech. 2009;22(2):144–8. https://doi.org/10.1097/BSD.0b013e31816b6b9a.
Keskin M, Akgul T, Bayraktar A, Dikici F, Balik E. Superior mesenteric artery syndrome: an infrequent complication of scoliosis surgery. Case Rep Surg. 2014;2014:263431. https://doi.org/10.1155/2014/263431.
Horn PL, Beeb AC, King DR. A rare cause of postoperative abdominal pain in a spinal fusion patient. Am J Orthop. 2015;44(9):E350-354.
Abol Oyoun N, Kadhim M, Dormans JP. Late-onset superior mesenteric artery syndrome four years following scoliosis surgery - a case report. SICOT J. 2015;1:12. https://doi.org/10.1051/sicotj/2015010.
Bernotavicius G, Saniukas K, Karmonaite I, Zagorskis R. Superior mesenteric artery syndrome. Acta Med Litu. 2016;23(3):155–64. https://doi.org/10.6001/actamedica.v23i3.3379.
Cullis PS, Gallagher M, Sabharwal AJ, Hammond P. Minimally invasive surgery for superior mesenteric artery syndrome: a case report and literature review. Scott Med J. 2016;61(1):42–7. https://doi.org/10.1177/0036933015615261.
Grabala P, Grabala M, Kossakowski D, Malinowski P, Larysz D. A case report of a 13-year-old girl diagnosed with superior mesenteric artery syndrome after undergoing spine correction with posterior fusion for rapidly progressed juvenile idiopathic scoliosis. Pol Ann Med. 2016;23(2):165–71.
Traore MM, Leye PA, Bah MD, Kinkpe CV, Ndiaye PI, Daffe M, Toure AO, Kane O. Early form of Wilkie’s syndrome: a rare complication of scoliosis surgery, about a case and review of the literature. Pan Afr Med J. 2016;25:90. https://doi.org/10.11604/pamj.2016.25.90.8773.
Eisenson DL, Shah KN, Cohen EM. Eberson CP (2017) unusual mechanism for superior mesenteric artery syndrome after scoliosis surgery. R I Med J. 2013;100(8):32–5.
Maharajan K, Thambiah JS. Unusual delayed presentation of superior mesenteric artery syndrome following scoliosis correction surgery-a case report and review of literature. J Spine Surg. 2017;3(2):272–7. https://doi.org/10.21037/jss.2017.06.09.
Ovalle-Chao C, Hinojosa-Martinez LM, Gutierrez-Castillo A, Velazco-De La Garza JH, Flores-Villalba E, Diaz-Elizondo JA, Garza-Serna U. Acute-onset of superior mesenteric artery syndrome following surgical correction of scoliosis: case report and review of literature. J Pediatr Surg Case Rep. 2017;19:31–3.
Voleti SPR, Sridhar J. Superior mesenteric artery syndrome after kyphosis correction - a case report. J Orthop Case Rep. 2017;7(5):67–70. https://doi.org/10.13107/jocr.2250-0685.900.
Silva G, Moreira-Silva H, Tavares M. Iatrogenic superior mesenteric artery syndrome. Rev Esp Enferm Dig. 2018;110(11):742–3. https://doi.org/10.17235/reed.2018.5760/2018.
Moyer K, Thompson GH, Poe-Kochert C, Splawski J. Superior mesenteric artery syndrome complicated by gastric mucosal necrosis following congenital scoliosis surgery: a case report. JBJS Case Connect. 2019;9(3): e0380. https://doi.org/10.2106/JBJS.CC.18.00380.
Spinnato P, Aparisi Gomez MP, Giugliano A, Mercatelli D, Bazzocchi A. Superior mesenteric artery syndrome after scoliosis surgery. Pediatr Int. 2019;61(11):1181–2. https://doi.org/10.1111/ped.13992.
Chan CYW, Chung WH, Mihara Y, Lee SY, Ch’ng PY, Hasan MS, Chiu CK, Kwan MK. Perioperative outcome of severe rigid idiopathic scoliosis: single-staged posterior spinal fusion utilizing a dual attending surgeon strategy. A report of 41 patients. J Orthop Surg. 2020;28(3):2309499020936005. https://doi.org/10.1177/2309499020936005.
Araujo AO, Oliveira RG, Arraes AJC, Mamare EM, Rocha ID, Gomes CR. Superior mesenteric artery syndrome—an uncommon complication after surgical corrections of spinal deformities. Rev Bras Ortop. 2021;56(4):523–7. https://doi.org/10.1055/s-0040-1722258.
Baltazar U, Dunn J, Floresguerra C, Schmidt L, Browder W. Superior mesenteric artery syndrome: an uncommon cause of intestinal obstruction. South Med J. 2000;93(6):606–8.
Miyata J, Eshak ES, Yoshioka T, Iso H. Movement of the superior mesenteric artery in patients with superior mesenteric artery syndrome: a case-reference study. Clin Anat. 2022;35(7):891–8. https://doi.org/10.1002/ca.23885.
Schwartz A. Scoliosis, superior mesenteric artery syndrome, and adolescents. Orthop Nurs. 2007;26(1):19–24. https://doi.org/10.1097/00006416-200701000-00007.
Pillay Y. Superior mesenteric artery syndrome: a case report of two surgical options, duodenal derotation and duodenojejunostomy. Case Rep Vasc Med. 2016;2016:8301025. https://doi.org/10.1155/2016/8301025.
Lopez PP, Gogna S, Khorasani-Zadeh A. Anatomy, abdomen and pelvis: duodenum. In: StatPearls. Las Vegas: Treasure Island (FL); 2023.
Lung K, Lui F. Anatomy, abdomen and pelvis: arteries. In: StatPearls. Las Vegas: Treasure Island (FL); 2023.
Shaikh H, Wehrle CJ, Khorasani-Zadeh A. Anatomy, abdomen and pelvis: superior mesenteric artery. In: StatPearls. Las Vegas: Treasure Island (FL); 2023.
Skinner D, Wehrle CJ, Van Fossen K. Anatomy, abdomen and pelvis: inferior mesenteric artery. In: StatPearls. Las Vegas: Treasure Island (FL); 2023.
Bhagirath Desai A, Sandeep Shah D, Jagat Bhatt C, Umesh Vaishnav K, Salvi B. Measurement of the distance and angle between the aorta and superior mesenteric artery on CT scan: values in indian population in different BMI categories. Ind J Surg. 2015;77(Suppl 2):614–7. https://doi.org/10.1007/s12262-013-0941-1.
Kane KE, Koons AL. The aortomesenteric angle as an aid in diagnosing superior mesenteric artery syndrome. Clin Pract Cases Emerg Med. 2017;1(2):140–1. https://doi.org/10.5811/cpcem.2016.12.30676.
Salem A, Al Ozaibi L, Nassif SMM, Osman R, Al Abed NM, Badri FM. Superior mesenteric artery syndrome: a diagnosis to be kept in mind (Case report and literature review). Int J Surg Case Rep. 2017;34:84–6. https://doi.org/10.1016/j.ijscr.2017.03.018.
Warncke ES, Gursahaney DL, Mascolo M, Dee E. Superior mesenteric artery syndrome: a radiographic review. Abdom Radiol. 2019;44(9):3188–94. https://doi.org/10.1007/s00261-019-02066-4.
Payawal JH, Cohen AJ, Stamos MJ. Superior mesenteric artery syndrome involving the duodenum and jejunum. Emerg Radiol. 2004;10(5):273–5. https://doi.org/10.1007/s10140-003-0322-3.
Heidbreder R. Co-occurring superior mesenteric artery syndrome and nutcracker syndrome requiring Roux-en-Y duodenojejunostomy and left renal vein transposition: a case report and review of the literature. J Med Case Rep. 2018;12(1):214. https://doi.org/10.1186/s13256-018-1743-7.
Lam DJ, Lee JZ, Chua JH, Lee YT, Lim KB. Superior mesenteric artery syndrome following surgery for adolescent idiopathic scoliosis: a case series, review of the literature, and an algorithm for management. J Pediatr Orthop B. 2014;23(4):312–8. https://doi.org/10.1097/BPB.0000000000000050.
Zaraket V, Deeb L. Wilkie’s syndrome or superior mesenteric artery syndrome: fact or fantasy? Case Rep Gastroenterol. 2015;9(2):194–9. https://doi.org/10.1159/000431307.
Willner S. A study of growth in girls with adolescent idiopathic structural scoliosis. Clin Orthop Relat Res. 1974;101:129–35.
Hutchinson DT, Bassett GS. Superior mesenteric artery syndrome in pediatric orthopedic patients. Clin Orthop Relat Res. 1990;250:250–7.
Kim JY, Kim HS, Moon ES, Park JO, Shin DE, Lee GK, Ha JW, Jung YS. Incidence and risk factors associated with superior mesenteric artery syndrome following surgical correction of scoliosis. Asian Spine J. 2008;2(1):27–33. https://doi.org/10.4184/asj.2008.2.1.27.
Derincek A, Wood KB, Muench CA. Superior mesenteric artery syndrome following correction of kyphosis in an adult. J Spinal Disord Tech. 2004;17(6):549–53. https://doi.org/10.1097/01.bsd.0000117543.88865.3e.
Marecek GS, Barsness KA, Sarwark JF. Relief of superior mesenteric artery syndrome with correction of multiplanar spinal deformity by posterior spinal fusion. Orthopedics. 2010;33(7):519. https://doi.org/10.3928/01477447-20100526-26.
Le Moigne F, Lamboley JL, Vitry T, Stoltz A, Galoo E, Salamand P, Michel P, Farthouat P. Superior mesenteric artery syndrome: a rare etiology of upper intestinal obstruction in adults. Gastroenterol Clin Biol. 2010;34(6–7):403–6. https://doi.org/10.1016/j.gcb.2010.04.012.
Ren PLJ, Gupta A. Adolescent with superior mesenteric artery syndrome. J Radiol Case Rep. 2020;14(3):14–23. https://doi.org/10.3941/jrcr.v14i3.3830.
Stamatakos M, Kontzoglou K, Stefanaki C, Tsaknaki S, Iannescu R, Manta A, Safioleas M. Wilkie syndrome. What is this? Chirurgia (Bucur). 2009;104(1):11–5.
Vikman Ya E, Nagulin IuA, Nosova LV. Transmission-emission lung scintigraphy in the diagnosis of lung diseases. Med Radiol. 1986;31(4):3–5.
Lee NJ, Fields MW, Boddapati V, Cerpa M, Dansby J, Lin JD, Sardar ZM, Lehman R, Lenke L. The risks, reasons, and costs for 30- and 90-day readmissions after fusion surgery for adolescent idiopathic scoliosis. J Neurosurg Spine. 2020;34(2):245–53. https://doi.org/10.3171/2020.6.SPINE20197.
Forsyth JM, Muhammad K, Mahmood K. Superior mesenteric artery syndrome as a cause for recurrent abdominal pain and vomiting in a 9-year-old girl. BMJ Case Rep. 2015. https://doi.org/10.1136/bcr-2014-209270.
Waheed KB, Shah WJ, Jamal A, Mohammed HR, Altaf B, Amjad M, Bassam MA, Almutawa DH, Arulanantham ZJ. Superior mesenteric artery syndrome: an often overlooked cause of abdominal pain! Saudi Med J. 2021;42(10):1145–8. https://doi.org/10.15537/smj.2021.42.10.20210509.
Sato H, Tanaka T. Acute gastric dilatation due to a superior mesenteric artery syndrome: an autopsy case. BMC Gastroenterol. 2014;14:37. https://doi.org/10.1186/1471-230X-14-37.
Amir Ali AA, Hameed K, Nawaz A, Khan I, Perveen S. Superior mesenteric artery syndrome. J Coll Physicians Surg Pak. 2022;32(12):SS100–1. https://doi.org/10.29271/jcpsp.2022.Supp0.SS100.
Biswas A, Babu AA, Neelakantan S, Sarkar PS. Superior mesenteric artery syndrome: CT findings. BMJ Case Rep. 2016. https://doi.org/10.1136/bcr-2016-215885.
Li X, Tian M, Yang H, Liu Y, Chen J, Tian H. Superior mesenteric artery syndrome after colectomy: a case report and literature review. Medicine. 2022;101(35): e30427. https://doi.org/10.1097/MD.0000000000030427.
Rabie ME, Ogunbiyi O, Al Qahtani AS, Taha SB, El Hadad A, El Hakeem I. Superior mesenteric artery syndrome: clinical and radiological considerations. Surg Res Pract. 2015;2015:628705. https://doi.org/10.1155/2015/628705.
Agrawal GA, Johnson PT, Fishman EK. Multidetector row CT of superior mesenteric artery syndrome. J Clin Gastroenterol. 2007;41(1):62–5. https://doi.org/10.1097/MCG.0b013e31802dee64.
Raman SP, Neyman EG, Horton KM, Eckhauser FE, Fishman EK. Superior mesenteric artery syndrome: spectrum of CT findings with multiplanar reconstructions and 3-D imaging. Abdom Imaging. 2012;37(6):1079–88. https://doi.org/10.1007/s00261-012-9852-z.
Shin MS, Kim JY. Optimal duration of medical treatment in superior mesenteric artery syndrome in children. J Korean Med Sci. 2013;28(8):1220–5. https://doi.org/10.3346/jkms.2013.28.8.1220.
Farina R, Foti PV, Cocuzza G, Costanzo V, Costanzo G, Conti A, Torcitto A, Pennisi M. Wilkie’s syndrome. J Ultrasound. 2017;20(4):339–42. https://doi.org/10.1007/s40477-017-0257-2.
Gebhart T. Superior mesenteric artery syndrome. Gastroenterol Nurs. 2015;38(3):189–93. https://doi.org/10.1097/SGA.0000000000000107.
Alsulaimy M, Tashiro J, Perez EA, Sola JE. Laparoscopic Ladd’s procedure for superior mesenteric artery syndrome. J Pediatr Surg. 2014;49(10):1533–5. https://doi.org/10.1016/j.jpedsurg.2014.07.008.
Bing L, Shun-Lin X, Ji-Hua O, Wei-Bing C, Ye-Bo W. Laparascopic Ladd’s procedure as treatment alternative, when parenteral or prolonged hospital nutrition is not an option for superior mesenteric artery syndrome. J Pediatr Surg. 2020;55(3):554–7. https://doi.org/10.1016/j.jpedsurg.2017.07.004.
Dekonenko C, Hill JA, Sobrino JA, Snyder CL, St Peter SD, Oyetunji TA. Ligament of treitz release with duodenal lowering for pediatric superior mesenteric artery syndrome. J Surg Res. 2020;254:91–5. https://doi.org/10.1016/j.jss.2020.04.006.
Sun Z, Rodriguez J, McMichael J, Walsh RM, Chalikonda S, Rosenthal RJ, Kroh MD, El-Hayek K. Minimally invasive duodenojejunostomy for superior mesenteric artery syndrome: a case series and review of the literature. Surg Endosc. 2015;29(5):1137–44. https://doi.org/10.1007/s00464-014-3775-4.
Acknowledgements
None
Registration and protocol
The present review was not registered.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
Author information
Authors and Affiliations
Contributions
Filippo Migliorini: conception, design, drafting; Luise Schäfer: literature search, data extraction, risk of bias assessment; Frank Hildebrand: supervision, revision; Jörg Eschweiler: writing; Gaetano Pappalardo: literature search, data extraction, risk of bias assessment; Enrico Pola: supervision; Luigi Aurelio Nasto: drafting. All authors have agreed to the final version to be published and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study complies with ethical standards.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have any competing interests in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pappalardo, G., Pola, E., Bertini, F.A. et al. Superior mesenteric artery syndrome following spine surgery in idiopathic adolescent scoliosis: a systematic review. Eur J Med Res 29, 410 (2024). https://doi.org/10.1186/s40001-024-02002-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-024-02002-3