Abstract
Background
To date, multiple cases of adverse reactions to COVID-19 vaccines have been reported worldwide. Alopecia areata (AA) is an uncommon type of adverse reaction reported in some articles and has a significant social and psychological impact on patients. Our study aimed to review the AA and COVID-19 vaccine literature.
Methods
This systematic review was conducted by searching for articles on AA following COVID-19 vaccines in international databases such as Embase, MEDLINE, PubMed, Web of Knowledge, and Ovid from December 2019 to December 30, 2023. We included studies that provided data for AA patients following COVID-19 vaccination with at least one dose. Data on sex, age, country/region of origin, vaccine type, days between vaccination and symptom presentation, manifestations of AA, trichoscopy and histopathological findings, treatment, and outcomes were included.
Results
In total, 579 explored studies were identified and assessed, and 25 articles with a total of 51 patients were included in the review. Twenty-seven (52.9%) patients developed new-onset AA following receiving the COVID-19 vaccine, and AA recurrence or exacerbation occurred after receiving the COVID-19 vaccine in 24 (47.1%) patients with preexisting disease. Five vaccines were reported to cause AA in all cases. The Pfizer vaccine (45.1%) was the most frequently reported, followed by the ChAdOx1 nCoV-19 vaccine (27.5%), Moderna mRNA-1273 (19.6%), Sinopharm (3.9%) and SinoVac (3.9%). AA occurred most frequently within one month after the 1st dose, and then, the incidence decreased gradually with time. Topical or systemic corticosteroids were used in 38 patients. Eleven patients were treated with a Janus Kinase inhibitor (jakinib) inhibitor, eight with tofacitinib, and three with an unspecified jakinib. However, 3 of the 11 patients experienced exacerbations after treatment.
Conclusion
AA after COVID-19 vaccination is rare, and physicians should be aware of this phenomenon to improve early diagnosis and appropriate treatment.
Similar content being viewed by others
Introduction
With COVID-19 sweeping the globe, many measures have been applied for prevention and treatment. As one of the most effective measures, vaccines have been widely used in many countries. Over 180 vaccine candidates use a variety of technological platforms, including viral vectors, live attenuated virus, inactivated virus, virus-like particles, nucleic acid (DNA and RNA), peptides, and recombinant protein approaches, which have received approval for use or in development [1].
Although the abovementioned vaccines are generally safe, many articles about the side effects of these vaccines have been published. COVID-19 vaccines can induce various side effects, including headache, nausea, vomiting, fever, fatigue, itching, muscle pain, joint pain, local redness or swelling, and, rarely, anaphylactic shock [2]. Studies have also reported the development or recurrence of alopecia areata (AA) after COVID-19 vaccination. AA is an organ-specific autoimmune disorder characterized by nonscarring hair loss involving the scalp, face, or body. It can affect approximately 2% of the general population and cause severe psychological distress [2, 3]. Spontaneous hair regrowth occurs in approximately 80% of patients within one year after the onset of AA [4]. However, a small number of individuals can develop alopecia universalis (AU), which cannot be recovered in the short term and has a significant social and psychological impact on patients.
Three [5,6,7] systematic reviews, all published in 2022, explored the subject of COVID-19 vaccine-induced AA. The latest review included 13 studies. Since then, numerous new studies have been published in the literature on this subject. Hence, an updated review of the literature is warranted. The aim of our study was to provide a systematic review of this subject.
Materials and methods 材料与方法
The present study was conducted based on the PRISMA (Preferred Reported Items for Systematic Review and Meta-analysis) guidelines. The review protocol was not registered with any groups such as Cochrane or Prospero. A completed copy of the PRISMA checklist (PRISMA 2020) has been added as an appendix file 1. The overall procedure can be divided into four-step selection process of identification, screening, eligibility, and inclusion. The literature screening, data extraction and quality assessment were done independently by two authors. Any disagreements were resolved by discussion or by a third author.
Search strategy
A systematic search was conducted in the Embase, MEDLINE, PubMed, Web of Knowledge, and Ovid databases from December 2019 to December 30, 2023. No restrictions regarding study design, geographic region, or language were applied. A manual search of references cited in the selected articles and published reviews was also used for undetected studies. The search strategy is provided in detail Appendix file 2.
Eligibility criteria
We included studies that provided data for case reports and case series of AA following COVID-19 vaccination with at least one dose. Review articles, non-peer reviewed sources and abstracts submitted in conferences were not eligible for inclusion. Studies on in vitro and animal models were excluded. Studies without mention of hair involvement after COVID-19 vaccination were excluded [8]. Studies assessing other types of nonscarring alopecia (i.e., telogen effluvium) and scarring alopecia were excluded.
Data extraction and handling
Two independent reviewers screened titles and abstracts, followed by full-text articles. Discussions were used to settle disagreements. The following details of each article were recorded: sex, age, country/region origin, vaccine type, interval days between vaccination and symptom presentation, manifestations of AA, trichoscopy and histopathologic findings, treatment, and outcomes.
Statistical analysis for evidence synthesis
Descriptive statistics were used to detail clinical characteristics of the patients. In case of normally distributed variables means, frequencies and mean ± standard deviation (SD) were used. For categorical data, percentages were displayed.
Causality assessment of AEFI figure
An AEFI CA tool developed by WHO was applied to assess the causality assessment between COVID-19 vaccination and AA reported in the articles. All results were divided into four main categories includes (A) consistent with causal association to immunization; (B) indeterminate; (C) coincidental association; or (D) unclassifiable. Each AEFI report was evaluated separately by two clinicians with specific expertise in vaccinology, and differences in causality assessment’s outcomes were resolved via consensus.世卫组织开发的AEFI CA工具被用于评估文章中报告的COVID-19疫苗接种与AA之间的因果关系评估。所有结果分为四大类,包括:(A)与免疫接种的因果关系一致;(二)不确定的;(三)巧合关联;或 (D) 不可分类。每份AEFI报告均由两名具有疫苗学特定专业知识的临床医生分别评估,因果关系评估结果的差异通过协商一致解决。
Results
Study characteristics
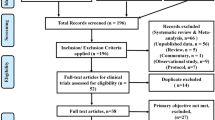
The PRISMA flow diagram is available in Fig. 1. Through the initial searches, we identified a total of 579 potentially relevant articles. After removing 360 duplicates, 219 articles remained. By screening the titles and abstracts, 194 articles were excluded. The full texts of the remaining 25 articles were assessed for eligibility. The studies and clinical characteristics are summarized in Table 1 [3, 4, 6, 7, 9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29]. These studies originated from different countries or regions; eight case reports from Italy, Jamaica, Qatar, Japan, mainland China, Iran, America, Taiwan; four case series from America, Switzerland, California, and Italy; four letters to the editor from Egypt, Italy, and Colombia; three letters from America, Brazil and Taiwan; three correspondences from Italy, Taiwan and Japan; two articles from Iran and Italy; and one commentary from Taiwan.
Patient characteristics
In total, 51 patients, including 31 females (60.7%) and 20 (39.3%) males, were included. The average age of these patients was 37.6 years (37.6 ± 16.5 years). Seven patients (13.7%) were aged older than 60 years, four (7.8%) were aged younger than 20 years, and the remaining forty (78.5%) were aged between 20 and 60 years. 27 patients (52.9%) developed new-onset AA following COVID-19 vaccination, while 24 patients (47.1%) presented with relapsed or aggravated AA. Ten patients (19.6%) complicated with other atopy or autoimmune diseases, including six patients (11.8%) with thyroiditis or elevated thyroid antibody levels, two with atopic dermatitis (AD), one with systemic lupus erythematosus (SLE), and one with asthma. Six patients (11.8%) had a history of COVID-19 infection. Furthermore, two patients (3.9%) had a family history of AA, and two patients (3.9%) had a family history of thyroid dysfunction.
Characteristics of reaction
In our study, Pfizer was the most common AA vaccine, and 23 (45.1%) of the 51 cases were caused by Pfizer, followed by ChAdOx1, nCoV-19 (27.5%), Moderna mRNA-1273 (19.6%), Sinopharm (3.9%) and SinoVac (3.9%).
Twenty-six of the 51 (50.9%) patients experienced hair loss within one month after the 1st dose, 13 of the 51 (25.5%) experienced hair loss within one month after the 2nd dose, seven of the 51 (13.7%) experienced hair loss within one month after the 3rd dose, three of the 51 (5.9%) experienced hair loss within two to three months after the 2nd dose, one of the 51 (2%) experienced hair loss within one to two months after the 2nd dose, and one of the 51 (2%) experienced hair loss within three months after the 2nd dose. In general, the incidence decreased gradually over time. Among the 51 patients, 34 patients (66.7%) experienced a patchy AA, while eight patients (15.6%) progressed to alopecia totalis (AT), and nine patients (17.7%) progressed to AU.
Treatment and outcome
Among the 46 patients who received documented treatment, 38 received topical or systemic corticosteroids. Intralesional triamcinolone acetonide was used in 16 patients [4, 12,13,14,15, 24], methylprednisolone pulse steroid therapy in two patients [30], pulses of dexamethasone in two patients [21], and oral prednisolone 25–300 mg daily in five patients [7, 20, 25]; the other patients were treated with various potent topical corticosteroids. In addition, seven patients were treated with 5% minoxidil solution [11, 13, 24], five patients with mesotherapy [21], five with topical growth factors [24], one with squaric acid dibutyleste [11], two with pimecrolimus 1% cream [4, 7], and one with 0.1% tacrolimus topical ointment [13]. Two patients [4, 27] received platelet-rich plasma therapy (PRP). Notably, 11 patients were treated with a jakinib, eight patients [4, 12] were treated with tofacitinib, and three patients were treated [23] with unspecified jakinib for severe AA or refractory (corticosteroid was ineffective) AA [4, 27].
Among the patients who mentioned the outcome of treatment, ten patients [10, 13, 14, 16, 19, 21, 27] had good treatment effects, with reduced hair loss, increased regeneration, or complete regeneration. Interestingly, one patient [25] experienced rapid diffuse hair loss with subsequent depigmentation 2 weeks after the first vaccination but spontaneous total regrowth with snow-white hair in the end. However, the therapeutic efficacy was poor in ten patients. One patient [14] did not respond to hormone therapy and progressed from AT to UT during treatment. Two [30] patients failed to respond to pulsed steroid therapy. One patient [11] showed no improvement after one month of immunotherapy. Many therapies, such as oral prednisone, an excimer lamp, oral cephalothin, and mono ammonium, were used in one [20] patient but still led to little improvement. One [4] patient had little improvement after the treatment of compounded tofacitinib 2%, clobetasol 0.05% ointment, and clobetasol solution. One [4] received ILTAC and platelet-rich plasma therapy, but still leaded to AU. In addition, three patients [23] with moderate-to-severe AA experienced various degrees of exacerbation of hair loss despite therapy with jakinib, one with an increase in total severity of alopecia tool (SALT) of 33%, one with an increase of 25%, and one with an increase of 8%.
Causality assessment
According to the causality assessment, 10/51 (19.6%) were classified as “consistent with causal association to immunization” to the COVID-19 vaccination, 14/51 (27.4%) were “indeterminate”, and 27/51 (53%) were classified as “coincidental association”; no case was considered as “unclassifiable” (Table 1).
Discussion
The present study investigated the current literature on cases of AA following COVID-19 vaccination. Barahmani et al. [31] revealed that women exhibit a higher rate (72%) of AA in a large case–control study. Similarly, our study discovered that women have a comparatively higher prevalence rate (60.7%) of AA. This may be due to women’s higher susceptibility to autoimmune diseases. Moreover, AA typically occurs at a younger age (21–40 years) [32]. Hence, the incidence of AA in elderly individuals is relatively rare. Jang et al. [33] reported that 3.5% of the 1,761 newly diagnosed patients with AA were over 60 years old. However, in our study, 13.7% of the described patients were older than 60 years. Therefore, the relationship between age and the incidence of AA in elderly individuals needs further investigation.
Of note, our study showed that 24 patients (47.1%) had a history of AA, while six patients (11.8%) had thyroid disease. The incidence of thyroid disease has varied from 8 to 28% in patients with AA [34]. Noso et al. [35] have previously reported an association between thyroid autoimmunity and AA. In their study, they found a positive association of DRB1*15:01-DQB1*06:02 with AA in thyrotropin (TSH) receptor antibody-positive patients, indicating a common etiology and susceptibility between AA and autoimmune thyroiditis. Hence, our study highlights that patients with a history of AA or thyroid dysfunction may have a greater risk of AA following COVID-19 vaccination.
In our study, the most common of the reported vaccines was the Pfizer vaccine, an mRNA vaccine. We deduce that this may be due to the following reasons. Firstly, previous studies have shown that the majority of cutaneous adverse reactions are reported after mRNA-based vaccines. It is believed that polyethylene glycol (PEG) may be one of the causes of allergic reactions in mRNA vaccines [36]. Secondly, it is also possible that Pfizer's vaccine was approved earlier and adopted more widely around the world. In addition, according to our findings, AA occurs most frequently within one month after the 1st dose and then shows a gradual downwards trend over time. This observation is consistent with the report by Qaderi et al. [37] Hence, increased monitoring for AA may be warranted during the first 4 weeks following COVID-19 vaccination.
To date, different treatment options (local and systemic) are available, but none of them can guarantee that patients with AA can fully recover or without relapse [19, 20]. A few patients with severe AT have a poor prognosis, and the condition can persist for a long time. Potent topical glucocorticosteroids or systemic immunosuppressants need to be sustained for more than three months to have a significant therapeutic effect [21]. JAK pathway plays a key role in the pathophysiology of AA and is a potential target for treatment. Even in patients who fail conventional treatment, oral jakinib therapy can promote significant hair regrowth. However, three patients experienced varying degrees of exacerbation after treatment with jakinib. Hence, whether other jakinibs with different selectivity for JAK subtypes may still be beneficial in these patients deserves further investigation in the future.
In eight [4, 6, 11, 16, 24,25,26] of the 51 patients, AA occurred after the first vaccination and became significantly worse after the next dose. Four specific cases worth mentioning are presented below. Three patients [11, 26] developed patchy areas of hair loss after the first vaccination but were still inoculated with the next dose, which eventually resulted in AT. In addition, Abdalla [6] et al. reported the case of a 63-year-old woman who developed patchy hair loss after the first dose of the vaccine. The second dose resulted in increased hair loss, followed by complete hair loss of the entire body hair. We speculate that the first dose may have triggered the autoimmune response, while the second, as performed on an already sensitized immune system, may have boosted the autoimmune attack on the hair bulb, leading to a marked worsening of the condition. This indicates that dermatologists should consider carefully whether to receive a second dose if a patient develops symptoms of AA after the first dose of COVID-19.
The exact pathogenesis by which COVID-19 vaccination induces AA is unclear. Vaccine-associated autoimmunity due to either cross-reactivity between antigens or the effect of adjuvants is a well-known phenomenon. mRNA vaccines such as Pfizer BNT162b2 can lead to the self-production of antigenic proteins that can stimulate the host immune response. As a result, proinflammatory cascades are activated, and numerous cytokines, including interferon (IFN) and interleukin (IL)-6, are released. IL-6 can inhibit the proliferation of hair follicle stem cells and keratinocytes and the transition of hair follicles from telogen to anagen [3, 38]. IFN can cause the collapse of immune privilege in human follicles [39, 40]. In addition, based on an adenoviral vector delivering the gene encoding the spike protein, the AZD1222/ChAdOx1 vaccine shares the same goal of evoking T cell-mediated immune reactions [41]. However, further studies elucidating the genesis of vaccine-associated AA are needed.
Limitations
This study has several limitations. First, a small number of cases had missing data for treatment and outcomes. Furthermore, the current study was subject to possible diagnostic bias. When a particular adverse event such as AA is suggested and publicized to be associated with a vaccine, it can result in preferential identification of cases due to increased awareness. Lastly, these studies mainly consisted of case reports and case series with limited research data. Therefore, future studies with robust designs, such as cohorts, are needed to more accurately confirm these findings.
Conclusions
Our study highlights that patients with a history of AA or thyroid dysfunction may be at higher risk of developing AA after receiving the COVID-19 vaccine. Furthermore, it was found that AA most commonly occurred within one month after the first dose and then gradually decreased over time. We hope to provide a reference for observing the timeframe within which AA may occur following COVID-19 vaccination. Moreover, some patients in our study experienced alopecia after the first dose but proceeded with the second dose, leading to more severe alopecia. Therefore, we recommend that dermatologists carefully assess whether to administer the second dose if alopecia symptoms arise after the initial COVID-19 vaccine dose, to prevent irreversible AA.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Pormohammad A, Zarei M, Ghorbani S, et al. Efficacy and safety of COVID-19 vaccines: a systematic review and meta-analysis of randomized clinical trials. Vaccines (Basel). 2021;9(5):467. https://doi.org/10.3390/vaccines9050467.
Meo SA, Bukhari IA, Akram J, Meo AS, Klonoff DC. COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur Rev Med Pharmacol Sci. 2021;25(3):1663–9. https://doi.org/10.26355/eurrev_202102_24877.
Rossi A, Magri F, Michelini S, et al. Recurrence of alopecia areata after covid-19 vaccination: a report of three cases in Italy. J Cosmetic Dermatol. 2021. https://doi.org/10.1111/jocd.14581.
Scollan ME, Breneman A, Kinariwalla N, et al. Alopecia areata after SARS-CoV-2 vaccination. JAAD Case Rep. 2022;20:1–5. https://doi.org/10.1016/j.jdcr.2021.11.023.
Pastukhova E, Li HOY, Brandts-Longtin O, Kirchhof MG. Alopecia areata as a sequela of COVID-19 vaccination: a systematic review. J Cutan Med Surg. 2023;27(1):64–5. https://doi.org/10.1177/12034754221138249.
Abdalla H, Ebrahim E. Alopecia areata universalis precipitated by SARS-CoV-2 vaccine: a case report and narrative review. Cureus J Med Sci. 2022. https://doi.org/10.7759/cureus.27953.
Ganjei Z, Yazdan Panah M, Rahmati R, Zari Meidani F, Mosavi A. COVID-19 vaccination and alopecia areata: a case report and literature review. Clin Case Rep. 2022;10(9): e6039. https://doi.org/10.1002/ccr3.6039.
Cebeci Kahraman F, Savaş Erdoğan S, Aktaş ND, et al. Cutaneous reactions after COVID-19 vaccination in Turkey: a multicenter study. J Cosmetic Dermatol. 2022;21(9):3692–703. https://doi.org/10.1111/jocd.15209.
Essam R, Ehab R, Al-Razzaz R, Khater MW, Moustafa EA. Alopecia areata after ChAdOx1 nCoV-19 vaccine (Oxford/AstraZeneca): a potential triggering factor? J Cosmetic Dermatol. 2021;20(12):3727–9. https://doi.org/10.1111/jocd.14459.
Gallo G, Mastorino L, Tonella L, Ribero S, Quaglino P. Alopecia areata after COVID-19 vaccination. Clin Exp Vaccine Res. 2022;11(1):129. https://doi.org/10.7774/cevr.2022.11.1.129.
May Lee M, Bertolani M, Pierobon E, Lotti T, Feliciani C, Satolli F. Alopecia areata following COVID-19 vaccination: vaccine-induced autoimmunity? Int J Dermatol. 2022;61(5):634–5. https://doi.org/10.1111/ijd.16113.
Ho JD, McNish A, McDonald L, Burrell C, Smith-Matthews S. Alopecia universalis with unusual histopathologic features after vaccination with ChAdOx1 nCoV-19 (AZD1222). JAAD Case Rep. 2022;25:4–8. https://doi.org/10.1016/j.jdcr.2022.05.002.
Lo A, Mir A, Sami N. Letter in Reply: alopecia areata after SARS-CoV-2 vaccination. JAAD Case Rep. 2022;25:25–6. https://doi.org/10.1016/j.jdcr.2022.04.022.
Bardazzi F, Guglielmo A, Abbenante D, Sacchelli L, Sechi A, Starace MVR. New insights into alopecia areata during COVID-19 pandemic: when infection or vaccination could play a role. J Cosmetic Dermatol. 2022;21(5):1796–8. https://doi.org/10.1111/jocd.14864.
Su HA, Juan CK, Chen YC. Alopecia areata following ChAdOx1 nCoV-19 vaccination (Oxford/AstraZeneca). J Formosan Med Assoc. 2022. https://doi.org/10.1016/j.jfma.2022.03.006.
Shakoei S, Kalantari Y, Nasimi M, et al. Cutaneous manifestations following COVID-19 vaccination: a report of 25 cases. Dermatol Ther. 2022. https://doi.org/10.1111/dth.15651.
Gamonal SBL, Marques NCV, Pereira HMB, Gamonal ACC. New-onset systemic lupus erythematosus after ChAdOX1 nCoV-19 and alopecia areata after BNT162b2 vaccination against SARS-CoV-2. Dermatol Ther. 2022;35(9): e15677. https://doi.org/10.1111/dth.15677.
Martora F, Fornaro L, Picone V, et al. Herpes zoster and alopecia areata following mRNA BNT162b2 COVID-19 vaccine: controversial immune effects. J Cosmet Dermatol. 2023;22(1):36–8. https://doi.org/10.1111/jocd.15465.
Matsuda Y, Kawakami Y, Kawamoto M, Hirai Y, Morizane S. A case of extensive alopecia areata following Pfizer-BioNTech BNT162b2 mRNA COVID-19 vaccine with favorable outcome. J Cutaneous Imm & Allergy. 2023;6(3):115–6. https://doi.org/10.1002/cia2.12292.
Iwata K, Kunisada M. Alopecia universalis after injection of messenger RNA COVID-19 vaccine. A case report. IDCases. 2023;33: e01830. https://doi.org/10.1016/j.idcr.2023.e01830.
Hernández Arroyo J, Izquierdo-Condoy JS, Ortiz-Prado E. A case series and literature review of telogen effluvium and alopecia universalis after the administration of a heterologous COVID-19 vaccine scheme. Vaccines (Basel). 2023;11(2):444. https://doi.org/10.3390/vaccines11020444.
Wu Y, Dai Y, Peng J, Song X. Alopecia areata induced by the booster shot of Sinovac COVID-19 vaccination: a case report. Eur J Dermatol. 2022;32(6):804–5. https://doi.org/10.1684/ejd.2022.4363.
Babadjouni A, Phong CH, Nguyen C, Mesinkovska NA. COVID-19 vaccination related exacerbations of hair loss in patients with moderate-to-severe alopecia areata on systemic therapy. JAAD Case Rep. 2022;29:181–5. https://doi.org/10.1016/j.jdcr.2022.08.016.
Genco L, Cantelli M, Noto M, et al. Alopecia Areata after COVID-19 Vaccines. Skin Appendage Disord. 2023;9(2):141–3. https://doi.org/10.1159/000528719.
AlZahrani F, Perlau MJ, Fiorillo L. Alopecia areata and subsequent Marie Antoinette syndrome following COVID-19 infection and vaccination: a case report. SAGE Open Med Case Rep. 2023. https://doi.org/10.1177/2050313X231152065.
Fusano M, Zerbinati N, Bencini PL. Alopecia areata after COVID-19 vaccination: two cases and review of the literature. Dermatol Rep. 2022;14(4):9495. https://doi.org/10.4081/dr.2022.9495.
Teng HC, Chen HH. Platelet-rich plasma in the treatment of alopecia areata after COVID-19 vaccination. Clin Case Rep. 2023;11(5): e7342. https://doi.org/10.1002/ccr3.7342.
Aristizabal M, Hsu JTS, Gold MH. Alopecia areata after CoronaVac vaccination. J Cosmet Dermatol. 2022. https://doi.org/10.1111/jocd.15184.
Chen C, Chen Y, Lan CE. Intractable alopecia areata following the second dose of COVID-19 vaccination: report of two cases. Dermatol Ther. 2022. https://doi.org/10.1111/dth.15689.
Chen CH, Chen YY, Lan CCE. Intractable alopecia areata following the second dose of COVID-19 vaccination: report of two cases. Dermatol Ther. 2022;35(9): e15689. https://doi.org/10.1111/dth.15689.
Barahmani N, Schabath MB, Duvic M. History of atopy or autoimmunity increases risk of alopecia areata. J Am Acad Dermatol. 2009;61(4):581–91. https://doi.org/10.1016/j.jaad.2009.04.031.
Muller SA, Winkelmann RK. Alopecia areata. An evaluation of 736 patients. Arch Dermatol. 1963;88:290–7. https://doi.org/10.1001/archderm.1963.01590210048007.
Jang YH, Park KH, Kim SL, et al. Alopecia areata in the elderly: a 10-year retrospective study. Ann Dermatol. 2015;27(4):411–6. https://doi.org/10.5021/ad.2015.27.4.411.
Sharma VK, Dawn G, Kumar B. Profile of alopecia areata in northern India. Int J Dermatol. 1996;35(1):22–7. https://doi.org/10.1111/j.1365-4362.1996.tb01610.x.
Noso S, Park C, Babaya N, et al. Organ specificity in autoimmune diseases: thyroid and islet autoimmunity in alopecia areata. J Clin Endocrinol Metab. 2015;100(5):1976–83.
Bogdanov G, Bogdanov I, Kazandjieva J, Tsankov N. Cutaneous adverse effects of the available COVID-19 vaccines. Clin Dermatol. 2021;39(3):523–31. https://doi.org/10.1016/j.clindermatol.2021.04.001.
Qaderi K, Golezar MH, Mardani A, et al. Cutaneous adverse reactions of COVID-19 vaccines: a systematic review. Dermatol Ther. 2022. https://doi.org/10.1111/dth.15391.
Huang WY, Huang YC, Huang KS, et al. Stress-induced premature senescence of dermal papilla cells compromises hair follicle epithelial–mesenchymal interaction. J Dermatol Sci. 2017;86(2):114–22. https://doi.org/10.1016/j.jdermsci.2017.01.003.
Fehrholz M, Bertolini M. Collapse and restoration of hair follicle immune privilege ex vivo: a model for alopecia areata. Methods Mol Biol. 2020;2154:133–41. https://doi.org/10.1007/978-1-0716-0648-3_11.
Kinori M, Bertolini M, Funk W, et al. Calcitonin gene-related peptide (CGRP) may award relative protection from interferon-γ-induced collapse of human hair follicle immune privilege. Exp Dermatol. 2012;21(3):223–6. https://doi.org/10.1111/j.1600-0625.2011.01432.x.
Kanduc D, Shoenfeld Y. Molecular mimicry between SARS-CoV-2 spike glycoprotein and mammalian proteomes: implications for the vaccine. Immunol Res. 2020;68(5):310–3. https://doi.org/10.1007/s12026-020-09152-6.
Acknowledgements
This work was supported by the National Natural Science Foundation of China, the Natural Science Foundation of Jiangxi Province and appreciate a lot for the inimitable care and support of professor Li over the years.
Funding
This research was funded by the National Natural Science Foundation of China (Project No. 81960569), and the Natural Science Foundation of Jiangxi Province (Project No. 20232BAB206126).
Author information
Authors and Affiliations
Contributions
Yunxia Zhu: conceptualization, data curation, formal analysis, investigation, methodology, writing-original draft. Xiaoliang Ouyang: conceptualization, data curation, formal analysis, investigation, methodology. Deng Zhang: conceptualization, data curation, formal analysis, investigation, methodology. Xiuping Wang: conceptualization, data curation, formal analysis, investigation, methodology. Simin Yu: conceptualization, data curation, formal analysis, investigation, methodology. Liang Wu: conceptualization, data curation, formal analysis, investigation, methodology. Yanping Tan: conceptualization, data curation, formal analysis, investigation, methodology. Wei Li: conceptualization, investigation, methodology. Chunming Li: conceptualization, data curation, project administration, supervision, writing review and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no conflict of interest to declare. The article has never been presented anywhere else.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhu, Y., Ouyang, X., Zhang, D. et al. Alopecia areata following COVID-19 vaccine: a systematic review. Eur J Med Res 29, 356 (2024). https://doi.org/10.1186/s40001-024-01956-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-024-01956-8