Abstract
Background
Prior observational research has investigated the association between dietary patterns and Alzheimer’s disease (AD) risk. Nevertheless, due to constraints in past observational studies, establishing a causal link between dietary habits and AD remains challenging.
Methods
Methodology involved the utilization of extensive cohorts sourced from publicly accessible genome-wide association study (GWAS) datasets of European descent for conducting Mendelian randomization (MR) analyses. The principal analytical technique utilized was the inverse-variance weighted (IVW) method.
Results
The MR analysis conducted in this study found no statistically significant causal association between 20 dietary habits and the risk of AD (All p > 0.05). These results were consistent across various MR methods employed, including MR-Egger, weighted median, simple mode, and weighted mode approaches. Moreover, there was no evidence of horizontal pleiotropy detected (All p > 0.05).
Conclusion
In this MR analysis, our finding did not provide evidence to support the causal genetic relationships between dietary habits and AD risk.
Similar content being viewed by others
Introduction
The aging global population has led to dementia emerging as a significant public health concern [1, 2]. According to the World Health Organization (WHO), the global prevalence of dementia was approximately 47 million in 2015, and it is projected to exceed 75 million by 2030 [3, 4]. Dementia significantly impacts both the physical and mental well-being of individuals, diminishes their quality of life, and imposes substantial pressure and financial strain on society and families [5]. It is noteworthy that Alzheimer’s disease (AD) stands as the most prevalent neurodegenerative form of dementia [6].
Numerous empirical studies have investigated the potential contributions of immune inflammation, mitochondrial dysfunction, genetic heredity, gut microbiota abnormalities, and cerebrovascular dysfunction to the pathogenesis of AD [7,8,9]. However, the exact etiology of AD remains unclear. Current pharmacological treatments for AD focus on symptom management without altering the disease progression [10]. Consequently, non-pharmacological interventions are being investigated to ameliorate symptoms and associated dysfunctions in AD.
Dietary interventions have emerged as a key area of research aimed at potentially slowing the onset and progression of AD [11, 12]. Specifically, the Mediterranean diet has been associated with a reduced risk of AD development [11], while a pro-inflammatory diet has been linked to an increased risk of AD [13, 14]. It is important to note that observational studies cannot establish direct causation, and a consensus on the influence of dietary habits on AD is lacking. Large-scale population-based studies are needed to provide genetic evidence supporting the potential impact of dietary interventions in reducing AD risk. Recognizing the potential benefits of dietary interventions for AD patients is clinically significant and requires further investigation in this area.
Mendelian randomization (MR) analysis is a statistical technique that employs genetic variants as instrumental variables to explore causal relationships between exposure factors and outcomes [15]. This method effectively utilizes results from genome-wide association studies (GWAS) to investigate the causal link between exposures and outcomes using genetic variants as instrumental variables (IVs) [16, 17]. Currently, there is a lack of comprehensive research on the causal relationship between dietary habits and AD at both national and international levels. Therefore, this study aimed to investigate the causal association between dietary habits and AD using a two-sample MR approach, aiming to offer valuable insights into this relationship and potentially informing new strategies for preventing and intervening in clinical diseases associated with AD.
Materials and methods
Study design and MR assumptions
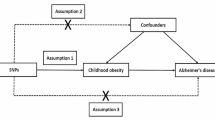
Figure 1 illustrates the study design. We examined bidirectional associations between dietary habits and AD using MR studies. We applied three key assumptions to genetic variants [18]: (1) SNPs are closely linked with exposure; (2) SNPs are not influenced by confounders along the exposure-outcome pathway; and (3) SNPs affect the outcome solely through exposure, without impacting the outcome through other pathways [19].
The central framework of Mendelian randomization analyses of the causal relationship of 20 dietary habits on the risk of AD. Assumption 1: IVs directly affect exposure; Assumption 2: IVs are not associated with confounders; Assumption 3: IVs influence risk of the outcome directly through the exposure
Data sources
The genome-wide association data for the 20 dietary habits analyzed in this study were sourced from the UK Biobank (UKBB) GWAS summary statistics provided by the Benjamin Neale Laboratory (http://www.nealelab.is/uk-biobank/) [20]. The dataset analyzed 361,194 participants and included 13.7 million QC-passing SNPs [21]. Detailed information regarding the assessment questions for dietary habits is provided in Additional file 1: Table S1.
To identify genetic variants associated with AD prevalence, we utilized meta-analysis data from the IGAP [22]. This dataset comprised 63,926 subjects, including 21,982 AD cases and 41,944 healthy controls of European origin. Detailed information on all GWAS datasets is provided in Table 1.
Instrumental variable selection
Following the core assumptions of MR studies, SNPs with correlations meeting p < 5 × 10–8 were included as instrumental variables after screening the GWAS data. To minimize the influence of linkage disequilibrium (LD) on analysis outcomes, we imposed the condition of r2 < 0.001 and a window size of 10,000 kb [23, 24]; To ensure robust associations between instrumental and endogenous variables and to prevent weak instrumental variable bias, we calculated R2 [R2 = 2 × EAF × (1 − EAF) × b2], representing the proportion of variation explained by instrumental variable SNPs, and the F statistic [F = R2 × (N − 2)/(1 − R2)], used to evaluate the strength of instrumental variables, for each SNP separately.
Statistical analysis
The IVW analysis, a meta-analysis of the Wald ratios for each SNP using inverse variance weighting, is utilized to obtain an MR estimate [25]. MR-Egger regression, a weighted linear regression of effect estimates for exposure and outcome, differs from the IVW method in that it imposes no restriction on the intercept and permits all SNPs in the MR analysis to be potentially invalid IVs [26]. The Egger intercept enables the detection of horizontal pleiotropy among SNPs in MR analyses [27].
Gene pleiotropy was assessed using the intercept of MR-Egger regression, where larger values indicate a higher likelihood of pleiotropy. If the pleiotropy test yielded a p-value > 0.05, its effect on causal estimation was disregarded. MR-PRESSO examined multiple SNP studies for outliers and pleiotropy, with a p-value > 0.05 indicating no pleiotropy [28]. Lastly, sensitivity analysis was conducted using the Leave-one-out method to assess the individual SNP effects on the results [29]. Differences were considered statistically significant at p < 0.05 in each analysis, and assays were bidirectional and used the Two Sample MR [30], MR-PRESSO [28] and Mendelian Randomization [31] packages in the R software (version 4.0.2).
Results
Causal relationship between dietary habits and AD
In the MR analysis, after excluding palindromic SNPs and SNPs related to confounding factors, the numbers of SNPs that ultimately identified as the IVs for different dietary habits in the MR analysis were 36 (Water intake), 34 (Cereal intake), 86 (Salt added to food), 35 (Dried fruit intake), 34 (Coffee intake), 51 (Cheese intake), 11 (Salad/raw vegetable intake), 49 (Fresh fruit intake), 32 (Tea intake), 15 (Cooked vegetable intake), 10 (Pork intake), 25 (Bread intake), 26 (Lamb/mutton intake), 46 (Oily fish intake), 7(Poultry intake),11 (Non-oily fish intake), 12 (Beef intake),19 (Processed meat intake), 55 (Hot drink temperature), 89 (Alcohol intake frequency), and 30 (Alcoholic drinks per week). The F-statistics were all greater than 10, indicating no weak instrumental bias (Table 1).
In the MR analysis, we found that water intake (OR = 0.722 95%CI: 0.395–1.322, p = 0.266), Salt added to food (OR = 1.052, 95%CI: 0.689–1.610, p = 0.315), Dried fruit intake (OR = 0.592, 95%CI: 0.316–1.103, p = 0.245), Coffee intake (OR = 0.833, 95%CI: 0.505–1.369, p = 0.449), Salad/raw vegetable intake (OR = 2.237, 95%CI: 0.755–6.627, p = 0.146), Cereal intake (OR = 0.652, 95%CI: 0.381–1.114, p = 0.416), Fresh fruit intake (OR = 1.171, 95%CI: 0.683–2.009, p = 0.659), Tea intake (OR = 1.158, 95%CI: 0.888–1.512, p = 0.321), Cooked vegetable intake (OR = 0.772, 95%CI: 0.332–1.799, p = 0.533), Cheese intake (OR = 0.813, 95%CI: 0.586–1.125, p = 0.207), Bread intake (OR = 1.235, 95%CI: 0.763–1.995, p = 0.457), Lamb/mutton intake (OR = 0.900, 95%CI: 0.383–2.115, p = 0.109), Pork intake(OR = 1.211, 95%CI: 0.505–2.917, p = 0.707), Oily fish intake (OR = 0.808, 95%CI: 0.578–1.128, p = 0.202), Non-oily fish intake (OR = 1.341, 95%CI: 0.679–2.651, p = 0.508), Processed meat intake (OR = 1.342, 95%CI: 0.837–2.156, p = 0.347), Hot drink temperature (OR = 0.547, 95%CI: 0.292–1.024, p = 0.069), Beef intake (OR = 0.793, 95%CI: 0.324–1.937, p = 0.284), Poultry intake(OR = 1.736, 95%CI: 0.626–4.819, p = 0.298), Alcohol intake frequency (OR = 0.923, 95%CI: 0.753–1.134, p = 0.364), Alcoholic drinks per week (OR = 1.162, 95%CI: 0.803–1.678, p = 0.479) were not associated with AD risk. To sum up, there was no significant causal relationship between 20 dietary habits and risk of AD (All p > 0.05). Furthermore, the IVW results of the MR analysis are shown in Fig. 2. And are illustrated as a scatter plot (Additional file 1: Figure S1).
Sensitivity analyses
The Cochran’s Q test identified heterogeneity among the IVs related to dried fruit intake, coffee intake, cereal intake, fresh fruit intake, bread intake, hot drink temperature, alcohol intake frequency, and alcoholic drinks per week (refer to Table 2). Notably, there was no significant evidence of horizontal pleiotropy observed in the correlation between dietary habits and AD, as all p-values exceeded 0.05. This enhances the reliability of the inferred causal relationships based on our findings. Moreover, a leave-one-out sensitivity analysis was performed to evaluate the impact of individual SNPs on the causal effect, confirming that no single SNP was driving the observed effect (see Additional file 1: Figure S2). Additionally, a funnel plot was employed for visualization purposes (refer to Additional file 1: Figure S3).
Discussion
There has been increasing attention to the relationship between healthy eating habits and neurodegenerative diseases, particularly AD [32]. Numerous studies have investigated the impact of nutrients and dietary patterns on AD prevention [33]. Specifically, epidemiological evidence suggests that individuals consuming a pro-inflammatory diet have a higher risk of developing AD [34, 35], while adherence to the Mediterranean diet is associated with a reduced risk of AD [36]. Additionally, a meta-analysis of 11 observational studies has shown that increased fish consumption may benefit AD patients [37]. Conversely, a dietary pattern characterized by relatively high carbohydrate intake has been linked to an increased risk of AD in older adults [38]. Generally, dietary factors are believed to potentially influence the risk of cognitive decline (CD) and AD through endogenous mechanisms triggered by the food metabolome (Additional files 2, 3).
The consistency of findings regarding the relationship between diet and AD risk varies. A prospective cohort study involving 8225 participants without dementia revealed that diet quality during midlife was not significantly associated with subsequent dementia risk [39]. Similarly, another prospective study with 2232 individuals and a mean follow-up of 6.9 years found no significant association between diet quality and the risk of AD and other forms of dementia [40]. Furthermore, a meta-analysis of 5 randomized controlled trials (RCTs) reported significant heterogeneity in the association between dietary habits and AD risk [41]. In our MR study, we did not find any statistically significant causal association between dietary habits and AD, which contradicts some prior research findings [42,43,44]. Therefore, establishing causality necessitates additional controlled trials.
There are various possible explanations for this disparity in the results. While MR can effectively address bias caused by confounding factors, it does not replace RCTs and serves as a valuable complement to them [45, 46]. Therefore, caution must be exercised when interpreting this conclusion. Most findings in this area originate from epidemiological studies, which, despite demonstrating correlations between dietary habits and AD, do not establish causality. Observational studies may be influenced by confounding factors such as socio-economic status, lifestyle habits, and physical health [47]. Moreover, discrepancies in data sources across different MR studies may also contribute to the inconsistency. For instance, previous cohort studies reported no association between coffee intake and AD incidence [48], consistent with earlier MR studies [49, 50]. However, recent research has shown a protective effect of coffee intake against neurodegenerative diseases, particularly AD [51]. Additionally, previous studies have highlighted the gender-related aspect of beverages consumption and cognitive impairment [32]. Therefore, further investigation into gender differences in diet and neuroprotection is warranted [36].
AD is a highly complex disease influenced by both genetic and environmental factors, and the exact role of nutrients in its pathogenesis remains unclear. Mechanisms underlying the association between dietary habits and changes in AD risk have not been fully elucidated in previous studies [44]. Epigenetic, gut microbiome, and brain imaging studies could shed light on these mechanisms and warrant further investigation. While no causal association between dietary habits and AD was found in our study, this does not negate the possibility that dietary preferences could hasten cognitive decline in AD patients. Evidence suggests that chronic neuroinflammation plays a crucial role in AD pathogenesis [13], with peripheral inflammatory responses linked to AD pathology [52]. A recent prospective study on the dietary inflammatory index (DII) indicated that increased DII may elevate the risk of AD [HR: 1.391, 95% CI: 1.085–1.784] [13]. Thus, specific dietary interventions may exacerbate AD through the mediation of neuroinflammation rather than dietary habits alone.
This study provides valuable insights into the causal relationship between dietary habits and AD risk, but caution is needed in interpreting the causal evidence due to the need for further validation. Several limitations need to be acknowledged. Firstly, the study was limited to individuals of European ancestry, potentially affecting the generalizability of the findings. Future research should include diverse populations to confirm the results. Secondly, the study focused solely on dietary habits and did not consider principal component (PC) analyses conducted by Cole et al. [53]. Thirdly, due to data limitations, specific dietary patterns’ effects on dementia risk could not be explored. Moreover, using GWAS data from multiple consortia may introduce heterogeneity into the analysis. Efforts to address pleiotropy in the MR study may not have eliminated all instances, potentially biasing the results. Additionally, effect sizes and dose–response relationships could not be accurately estimated in the study.
Conclusions
In summary, our MR analysis did not reveal any causal genetic associations between dietary habits and AD risk. However, due to the intricate interplay and limited research evidence on the pathophysiological mechanisms connecting AD and dietary patterns, further studies are warranted to validate our findings and elucidate potential mechanisms.
Data availability
The original contributions presented in the study are included in the article/Supplementary Material. The dataset generated during and analyzed during the current study are available from the MR Base database (http://www.mrbase.org/).
References
Chen Y, Dang M, Zhang Z. Brain mechanisms underlying neuropsychiatric symptoms in Alzheimer’s disease: a systematic review of symptom-general and -specific lesion patterns. Mol Neurodegener. 2021;16(1):38.
Cummings JL, Goldman DP, Simmons-Stern NR, Ponton E. The costs of developing treatments for Alzheimer’s disease: a retrospective exploration. Alzheimer’s Dement. 2022;18(3):469–77.
Collaborators GBDDF. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7(2):e105-e125.
Alzheimer’s A. 2016 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2016;12(4):459–509.
Jia L, Quan M, Fu Y, et al. Dementia in China: epidemiology, clinical management, and research advances. Lancet Neurol. 2020;19(1):81–92.
McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863–72.
Lee SH, Cho H, Choi JY, et al. Distinct patterns of amyloid-dependent tau accumulation in Lewy body diseases. Mov Disord. 2018;33(2):262–72.
Pope ED, Cordes L, Shi J, Mari Z, Decourt B, Sabbagh MN. Dementia with Lewy bodies: emerging drug targets and therapeutics. Expert Opin Investig Drugs. 2021;30(6):603–9.
Holscher C. Evidence for pathophysiological commonalities between metabolic and neurodegenerative diseases. Int Rev Neurobiol. 2020;155:65–89.
Connors MH, Quinto L, McKeith I, et al. Non-pharmacological interventions for Lewy body dementia: a systematic review. Psychol Med. 2018;48(11):1749–58.
Maraki MI, Yannakoulia M, Xiromerisiou G, et al. Mediterranean diet is associated with a lower probability of prodromal Parkinson’s disease and risk for Parkinson’s disease/dementia with Lewy bodies: a longitudinal study. Eur J Neurol. 2023;30(4):934–42.
Cornelis MC, Bennett DA, Weintraub S, Schneider JA, Morris MC. Caffeine consumption and dementia: are Lewy bodies the link? Ann Neurol. 2022;91(6):834–46.
Shi Y, Lin F, Li Y, et al. Association of pro-inflammatory diet with increased risk of all-cause dementia and Alzheimer’s dementia: a prospective study of 166,377 UK Biobank participants. BMC Med. 2023;21(1):266.
Pritam P, Deka R, Bhardwaj A, et al. Antioxidants in Alzheimer’s disease: current therapeutic significance and future prospects. Biology (Basel). 2022;11(2):212.
Du Z, Guo S, Sun Y, et al. Causal relationships between dietary habits and five major mental disorders: a two-sample Mendelian randomization study. J Affect Disord. 2023;340:607–15.
Woolf B, Di Cara N, Moreno-Stokoe C, et al. Investigating the transparency of reporting in two-sample summary data Mendelian randomization studies using the MR-Base platform. Int J Epidemiol. 2022;51(6):1943–56.
Lv W, He J, Shao J, Chen Y, Xia L, Zhang L. Causal relationships between short-chain fatty acids and L-isoleucine biosynthesis and susceptibility and severity of COVID-19: evidence from Mendelian randomization. J Infect. 2023;87(1):e16–8.
Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey SG. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27(8):1133–63.
Boef AG, Dekkers OM, le Cessie S. Mendelian randomization studies: a review of the approaches used and the quality of reporting. Int J Epidemiol. 2015;44(2):496–511.
Chen TT, Chen CY, Fang CP, Cheng YC, Lin YF. Causal influence of dietary habits on the risk of major depressive disorder: a diet-wide Mendelian randomization analysis. J Affect Disord. 2022;319:482–9.
Sudlow C, Gallacher J, Allen N, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3): e1001779.
Kunkle BW, Grenier-Boley B, Sims R, et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Abeta, tau, immunity and lipid processing. Nat Genet. 2019;51(3):414–30.
Li J, Wang J, Yang M, Wang G, Xu P. The relationship between major depression and delirium: a two-sample Mendelian randomization analysis. J Affect Disord. 2023;338:69–73.
Burgess S, Thompson SG, Collaboration CCG. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. 2011;40(3):755–64.
Burgess S, Scott RA, Timpson NJ, Davey Smith G, Thompson SG, Consortium E-I. Using published data in Mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol. 2015;30(7):543–52.
Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–25.
Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304–14.
Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–8.
Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32(5):377–89.
Hemani G, Zheng J, Elsworth B, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7.
Yavorska OO, Burgess S. Mendelian randomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol. 2017;46(6):1734–9.
Jiang X, Cui L, Huang L, Guo Y, Huang G, Guo Q. The relationship between beverages consumption and cognitive impairment in middle-aged and elderly Chinese population. Nutrients. 2023;15(10):2309.
Charisis S, Ntanasi E, Yannakoulia M, et al. Mediterranean diet and risk for dementia and cognitive decline in a Mediterranean population. J Am Geriatr Soc. 2021;69(6):1548–59.
Talebi S, Asoudeh F, Naeini F, Sadeghi E, Travica N, Mohammadi H. Association between animal protein sources and risk of neurodegenerative diseases: a systematic review and dose-response meta-analysis. Nutr Rev. 2023;81(9):1131–43.
Wood H. Pro-inflammatory diet is linked to dementia risk. Nat Rev Neurol. 2022;18(1):1.
Arora S, Santiago JA, Bernstein M, Potashkin JA. Diet and lifestyle impact the development and progression of Alzheimer’s dementia. Front Nutr. 2023;10:1213223.
Bakre AT, Chen R, Khutan R, et al. Association between fish consumption and risk of dementia: a new study from China and a systematic literature review and meta-analysis. Public Health Nutr. 2018;21(10):1921–32.
Roberts RO, Roberts LA, Geda YE, et al. Relative intake of macronutrients impacts risk of mild cognitive impairment or dementia. J Alzheimer’s Dis. 2012;32(2):329–39.
Akbaraly TN, Singh-Manoux A, Dugravot A, Brunner EJ, Kivimaki M, Sabia S. Association of midlife diet with subsequent risk for dementia. JAMA. 2019;321(10):957–68.
Flores AC, Jensen GL, Mitchell DC, et al. Prospective study of diet quality and the risk of dementia in the oldest old. Nutrients. 2023;15(5):1282.
Petersson SD, Philippou E. Mediterranean diet, cognitive function, and dementia: a systematic review of the evidence. Adv Nutr. 2016;7(5):889–904.
Ellouze I, Sheffler J, Nagpal R, Arjmandi B. Dietary patterns and Alzheimer’s disease: an updated review linking nutrition to neuroscience. Nutrients. 2023;15(14):3204.
Zhang J, Cao X, Li X, et al. Associations of midlife dietary patterns with incident dementia and brain structure: findings from the UK biobank study. Am J Clin Nutr. 2023;118(1):218–27.
Takeuchi H, Kawashima R. Diet and dementia: a prospective study. Nutrients. 2021;13(12):4500.
Yang W, Yang Y, He L, et al. Dietary factors and risk for asthma: a Mendelian randomization analysis. Front Immunol. 2023;14:1126457.
Boyko EJ. Observational research–opportunities and limitations. J Diabetes Complications. 2013;27(6):642–8.
Liu Z, Wang H, Yang Z, Lu Y, Zou C. Causal associations between type 1 diabetes mellitus and cardiovascular diseases: a Mendelian randomization study. Cardiovasc Diabetol. 2023;22(1):236.
Larsson SC, Wolk A. The role of lifestyle factors and sleep duration for late-onset dementia: a cohort study. J Alzheimer’s Dis. 2018;66(2):579–86.
Larsson SC, Woolf B, Gill D. Plasma caffeine levels and risk of Alzheimer’s disease and Parkinson’s disease: Mendelian randomization study. Nutrients. 2022;14(9):1697.
Kwok MK, Leung GM, Schooling CM. Habitual coffee consumption and risk of type 2 diabetes, ischemic heart disease, depression and Alzheimer’s disease: a Mendelian randomization study. Sci Rep. 2016;6:36500.
Tira R, Viola G, Barracchia CG, et al. Espresso coffee mitigates the aggregation and condensation of Alzheimer’s associated tau protein. J Agric Food Chem. 2023;71(30):11429–41.
Lai KSP, Liu CS, Rau A, et al. Peripheral inflammatory markers in Alzheimer’s disease: a systematic review and meta-analysis of 175 studies. J Neurol Neurosurg Psychiatry. 2017;88(10):876–82.
Cole JB, Florez JC, Hirschhorn JN. Comprehensive genomic analysis of dietary habits in UK Biobank identifies hundreds of genetic associations. Nat Commun. 2020;11(1):1467.
Acknowledgements
We are grateful to the many participants and researchers for collecting, contributing to the GWAS dataset, and making their GWAS summary statistics publicly available.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
M-Q: Study concept, design, software, and paper writing. C–C and Z-MF: Data curation and software. H-Y: Dissertation Revision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Since all analyses were based on publicly available summary statistics, no patients were involved in the design of the study, and no ethical approval from an institutional review board was required.
Competing interests
The authors declare no interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Summary of 20 dietary habits questionnaire. Table S2. Results for Mendelian randomization analyses (IVW). Figure S1. Scatterplot analysis for dietary habits and AD. Figure s2. MR leave-one-out analysis for dietary habits and AD. Figure S3. Funnel plots of the association between dietary habits and AD.
Additional file 2.
SNPS for dietary habits.
Additional file 3.
The results of replication validation.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Meng, Q., Chen, C., Zhu, M. et al. Dietary factors and Alzheimer’s disease risk: a Mendelian randomization study. Eur J Med Res 29, 261 (2024). https://doi.org/10.1186/s40001-024-01821-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-024-01821-8