Abstract
Background
Inflammatory myofibroblastic tumor (IMT) is an uncommon cardiac tumor that primarily affects infants, children, and young adults. While complete surgical resection generally leads to a favorable prognosis, accurate diagnostic tests remain limited.
Case presentation
We describe the case of a 26-year-old female who had a dual tumor inside and outside the heart and was misdiagnosed by echocardiography and MRI. We also review 71 cases of cardiac IMTs from the literature regarding their epidemiology, clinical presentation, and outcome.
Conclusion
Early detection of this rare disorder is essential for optimal surgical management.
Similar content being viewed by others
Introduction
Inflammatory myofibroblastic tumor (IMT) is an exceptionally rare benign tumor with an unknown etiology, consisting of plasma cells, lymphocytes, histiocytes, and vascular tissue. Since it was first discovered in 1939 by Brunn [1], very few cases are reported and especially the cardiac origin. Although rhabdomyomas, myxomas, and other mesenchymal tumors are more commonly encountered intracardiac tumors, IMTs represent a distinct subset within cardiac neoplasms. [2, 3] Consequently, determining the epidemiological trends associated with this specific type of IMT has posed a significant challenge. The first cIMT case was described in 1975 [4], describing a tumor located in the left atrium of a pediatric patient. Subsequent to this report, there have been sporadic case accounts and limited literature reviews concerning cardiac IMTs [5,6,7,8,9,10,11,12,13,14,15,16,17,18]. These tumors were previously believed to primarily affect children and young adults. In this report, we present an intriguing case study of a 26-year-old female who experienced chronic chest tightness and had a large IMT occupying a large portion of the atrial septum and invading the aortic sinus. Additionally, a similar tumor was observed within the wall of the ascending aorta.
Case report
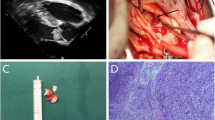
A 26-year-old female was referred to our department with dyspnea and mild chest oppression on exertion for more than 9 months, worsened for 1 month. She had no family history of hypertension, no history of chest trauma or other cardiac health comorbidities. Upon physical examination, a soft blowing murmur was auscultated in the second costal margin of the left sternum. No other significant clinical findings were noted during the examination. The trans-thoracic echocardiogram (Fig. 1A and B) pointed out a marked intra-atrial septal mass with irregular margins. The electrocardiogram demonstrated first-degree atrioventricular block. The cardiac magnetic resonance (CMR) demonstrated invasion of the left atrium and aortic sinus by an abundant solid tissue in the atrial septal with a clear boundary (Fig. 1C and D).
A Echocardiography and color Doppler blood flow image (B) from left atrium angle, white arrow indicates the tumor. C Nuclei magnetic resonance image from long axis, white arrow indicates the tumor. D Nuclei magnetic resonance image from aortic valve plane. White arrow indicates the tumor. LA left atrium, AO aortic, RA right atrium, LV left ventricle, RV right ventricle, DAO descending aorta, LCS left coronary sinus, RCS right coronary sinus, NCS left coronary sinus
Upon admission, the patient underwent open-heart surgery. A regular median sternotomy incision was made, providing access to the surgical area. A 15 × 10 mm solid mass was found at the lateral to the ascending aorta near the root (Fig. 2A). With cannulation of the aorta superior to the mass, and separate cannulas in the superior vena cava and inferior vena cava, cardiopulmonary bypass was established as per standard protocol. Following the opening of the right atrium, the mass was found to locate inside the atrial septal, with body length 45 × 30 mm, exhibited a grayish-yellow color, and had a lobulated structure with a firm and smooth surface, upper limit reached the roof of left atrium and invasive to posterior aortic sinus and left outflow tract (Fig. 2B). Both the masses were completely removed (Fig. 2C) and a bovine pericardial patch was used to rebuild the atrial septum and the posterior aortic sinus, another patch was used to fix the ascending aorta.
Cosmetically the two masses should be of the same origin, but it was not possible to determine the nature of the masses visually, pathologic examination and anaplastic lymphoma kinase (ALK) gene testing were subsequently performed. In both sections of the two tumors, a large amount of proliferative vitreous fibrous tissue infiltrated by lymphoplasmacytic cells and histiocytes and scattered adipocytes were seen under the microscope, plump spindle cells were arranged in a disorganized manner and were seen surrounding myocytes in a focal manner. Notably, a substantial influx of chronic inflammatory cells, including plasma cells (Fig. 3A and B), macrophages (CD68 + , Fig. 3C) are also present, suggesting fibrous histiocytoma-like hyperplasia. Immunohistochemistry (IHC) showed CK(-), CD20(+), CD38(+), SMA(+), DES(+), CD38(+), CD138(+), IgG4(+), CD34(-), S-100(+), P53(-). ALK staining through VENTANA(D5F3) method resulted positive in the positive controlling group but was negative in negative controlling group. To further confirm the absence of ALK-1 translocation, ALK break-apart fluorescence in situ hybridization (FISH) probe analysis was performed, resulting in a negative finding.
HE staining (A and B) and CD68 staining (C) of the tumorous tissue confirm the diagnosis of inflammatory myofibroblastic tumor, scale bar = 20 μm. The red-stained long strip-shaped indicated cardiomyocytes, blue-stained spindle-shaped indicated myofibroblasts, other blue-stained cells indicated plasma cells or macrophages. Echocardiography 8 months post-operatively (D) shows no hemodynamic abnormality
The postoperative outcomes were satisfactory. Three days following the surgery, echocardiography revealed complete removal of the mass, with normal functioning of the aortic valve. The patient did not experience any significant discomfort and was discharged from the hospital after 2 days. During the 1-month and 6-month follow-up period, no adverse events or unforeseen complications were reported. The echocardiography at 8-month post-operatively showed no hemodynamic abnormality (Fig. 3D), no metastasis and recurrence were found.
Discussion
Cardiac tumors are a rare occurrence in children, with an incidence ranging from 0.01% to 0.32% [19]. Most of these tumors are hamartomas and benign neoplasms, including rhabdomyomas, myxomas, teratomas, and fibromas [3, 20]. However, IMT of the heart is extremely rare, since B. Xu’s review in 2014 [5], and currently, there are only 71 cases documented in the English literature (Table 1). The case we reported here is the first one which characterized as dual IMT appear both inside and outside the heart. It is noteworthy that both tumors were located in the interatrial septum and the vessel wall, a finding not described in any previous reports. This makes us realize that cardiac IMT does not only involve myocardium and valves, it further enhances the diversity of sites involved in IMT and the importance of early diagnosis of cardiac IMT.
Cardiac IMT usually be benign, most of the cases did not show any syndromes, and the presence always to be hidden. Most of the patients did not seek help until the tumor grew and blocked the valve structure, outflow tract, or coronary orifice [21,22,23,24,25].
Histopathology plays a critical role in diagnosing IMT, with a distinctive histological pattern characterized by spindle-shaped myofibroblastic proliferation and a chronic inflammatory infiltrate. The histological section through the tumor shows the spindle-shaped myofibroblasts and the scattering of small lymphocytes in the stroma that are characteristic of this tumor. Although there are some similarities with myosarcomas [26], cIMTs exhibit less pleomorphism, atypia, and fewer mitotic figures.
The predominant differential diagnoses for the observed histologic appearance include cardiac IMT and IgG4-related disease. However, in comparison to IgG4-related disease, which can erode muscle, IMT has not been widely reported to involve cardiac muscle. In the IHC result of this case, we found a sporadic IgG4 positive, but according to the reported IgG4 cardiac tumor cases [27] and review [28], IgG4-related cardiac disease is extremely rare to see, its histological appearance commonly includes features such as storiform fibrosis, obliterative phlebitis, an increased presence of IgG4-positive plasma cells crowded with significant numbers of lymphocyte. Although the reported tumor site already expanded from cardiac muscle to vessel structure [29], the above characteristics are not supportive for matching our case here.
Cardiac IMTs occur predominantly in children and young adults, according to B. Xu’s review and our summary, the mean patient age of presentation is about 16.9 years. Among the documented cases, 26 out of 71 patients (36.6%) were 1 year of age or younger, while 24 patients (33.8%) fell within 1 to 20-year age. The majority of these cases were characterized by polypoid lesions originating from the endocardium, protruding into the cardiac cavity. The cases invasive in the right heart system (RA, RV, PA, PV) versus left heart system (LA, LV, MV, AV) is ratio 37:27, only two cases just seen in IVS, and only one just found in pericardium, no case ever reported tumor inside and outside heart at the same time like our case. 28 of 71 cases found valve affected, implies that tumor sources include myogenic and fibrous origin. In general, cardiac IMTs are considered benign, with a low rate of metastasis. However, their dangerous nature requires special attention. Of the 56 cases with follow-up data available, 12 resulted in death, for a mortality rate of 21.4%. Death was attributed to sudden death due to the tumor or perioperative complications.
In the majority of cases, the tumor is removed without recurrence or other cardiac complications. Pharmacological intervention is not commonly used, and health care professionals should attempt to remove the tumor as completely as possible. Surgical resection is the most common treatment approach for cIMT. Comparing to a recurrence rate 5% of total reported case by year 2012 [30], However, among the 36 patients who underwent complete resection and had subsequent clinical follow-ups, 29 remained asymptomatic with no evidence of disease/recurrence, resulting in a recurrence rate of 19.4%. On the other hand, in the four patients who only received partial resection, a recurrence or progression of the tumor was documented in all cases, leading to a 100% recurrence rate. Additionally, two out the four patients who underwent partial resection eventually died of the disease. It is crucial to highlight the importance of complete surgical resection in achieving more favorable outcomes and reducing the risk of recurrence or disease progression.
Currently, there are no biochemical markers indicative of the prognosis of cardiac IMTs. Immunohistochemistry analysis often shows positive staining for vimentin, smooth muscle actin (SMA), and muscle-specific actin (MSA) in the majority of cases, while ALK staining varies in positivity [5, 31]. Historically, the association between ALK gene and the tumor has been considered. ALK reactivity has been reported to be linked to the local recurrence, but not the distant metastasis [32]. But compared to 40–60% of positive rate in the extra cIMTs, only 10% of cIMTs showed ALK-1 overexpression within IHC [5, 31]. More cases are still needed to judge the diagnostic value of ALK-1 in cIMT, with this reason, in contrast to B. Xu’s review, we did not list the histological and immunohistochemistry features in Table 1. An alternative approach to studying cIMTs involves genetic testing to identify specific genetic abnormalities. This includes examining the 2p23 locus for clonal ALK gene rearrangements and detecting ALK gene fusion with proto-oncogenes, as well as ALK overexpression and other potential genetic alterations like EML4-ALK inversion [33]. This genetic testing can provide valuable information about the molecular characteristics of cardiac IMTs and aid in their diagnosis and management [34].
Availability of data and materials
Data are available from the corresponding author upon reasonable request.
References
Brunn H. two interesting benign lung tumors of contradictory histopathology; remarks on necessity for maintaining chest tumor registry. J Thorac Surg. 1939;9:119–31.
Lam KY, Dickens P, Chan AC. Tumors of the heart. A 20-year experience with a review of 12,485 consecutive autopsies. Arch Pathol Lab Med. 1993;117(10):1027–31.
Uzun O, Wilson DG, Vujanic GM, Parsons JM, De Giovanni JV. Cardiac tumours in children. Orphanet J Rare Dis. 2007;2:11.
Gonzalez-Crussi F, Vanderbilt BL, Miller JK. Unusual intracardiac tumor in a child. Inflammatory pseudotumor or “granulomatous” variant of myxoma? Cancer. 1975;36(6):2214–26.
Xu B, Fraser RS, Renaud C, Youssef S, Gottesman RD, Bernard C. Inflammatory myofibroblastic tumor of the aortic valves causing sudden cardiac death: a case report and review of the literature. Pediatr Dev Pathol. 2014;17(3):231–9.
Bao M, Zheng C, Zhang H, Ruan Y, Cao A, Wu T, Luo YI. Inflammatory myofibroblastic tumor of the left atrium in infant. Int J Cardiol. 2016;222:965–7.
Luo W, Teng P, Ni Y. A rare cardiac inflammatory myofibroblastic tumor involving aortic valve. J Cardiothorac Surg. 2017;12(1):13.
Zorinas A, Austys D, Janusauskas V, Trakymas M, Tamulionyte M, Seinin D, Karalius R, Aidietis A, Stukas R, Rucinskas K. Small intestinal inflammatory myofibroblastic metastasis in the left ventricle. Ann Thorac Surg. 2017;103(1):e31–3.
Deng MD, Han JY, Lin K, Tang H. Cardiac inflammatory myofibroblastic tumor in interventricular septum: a rare case report. Medicine. 2018;97(48): e13219.
Syed AU, Al Mutairi MB, Al Muhaya M, Morsey M, Al Hujailey A, Al Najjar A. Inflammatory myofibroblastic tumor of heart in a 4-month-old infant. Asian Cardiovasc Thorac Ann. 2018;26(1):47–9.
D’Angelo T, Mazziotti S, Inserra MC, De Luca F, Agati S, Magliolo E, Pathan F, Blandino A, Romeo P. Cardiac inflammatory myofibroblastic tumor. Circ Cardiovasc Imaging. 2019;12(9): e009443.
Monzon Diaz D, Cuerpo Caballero G, Irabien Ortiz A, Gonzalez Pinto A. Inflammatory myofibroblastic tumour in the right ventricle of a 66-year-old man. Interact Cardiovasc Thorac Surg. 2019;29(6):967–8.
Donmez YN, Aykan HH, Orhan D, Peker RO, Yilmaz M, Karagoz T. Intrapericardial inflammatory myofibroblastic tumour in a 3-month-old infant associated with Coronavirus OC43 presenting with pericardial tamponade. Paediatr Int Child Health. 2020;40(4):261–7.
Suzuki S, Ohtani M, Matsuo Y, Yoshida M, Goto A, Fukuda M, Mimasaka S. A forensic autopsy case: Sudden unexpected death due to cardiac inflammatory myofibroblastic tumor. Leg Med. 2021;53:101931.
Vijayakumar N, Thattaliyath B, Dundar B, Karimi M, Badheka A, Chegondi M. A Rare Inflammatory Myofibroblastic Tumor of the Mitral Valve With Systemic Embolism in a Child. World J Pediatr Congenit Heart Surg. 2021;12(6):783–4.
Zhu W, Pan W, Li Z. Inflammatory myofibroblastic tumour of the mitral valve. J Paediatr Child Health. 2022;58(11):2104–5.
McKinney LM, Escalera RB, Downs EA. Out of the blue: inflammatory myofibroblastic tumour identified during repair of tetralogy of Fallot with absent pulmonary valve. Cardiol Young. 2023. https://doi.org/10.1017/S1047951123003104.
Soares BM, Soares AM, Aiello VD. Superior caval vein syndrome and cardiac inflammatory myofibroblastic tumour in an infant. Cardiol Young. 2023;33(7):1226–8.
Tzani A, Doulamis IP, Mylonas KS, Avgerinos DV, Nasioudis D. Cardiac tumors in pediatric patients: a systematic review. World J Pediatr Congenit Heart Surg. 2017;8(5):624–32.
Burke A, Virmani R. Pediatric heart tumors. Cardiovasc Pathol. 2008;17(4):193–8.
Hoffmeier A, Sindermann JR, Scheld HH, Martens S. Cardiac tumors–diagnosis and surgical treatment. Dtsch Arztebl Int. 2014;111(12):205–11.
Poterucha TJ, Kochav J, O’Connor DS, Rosner GF. Cardiac tumors: clinical presentation, diagnosis, and management. Curr Treat Options Oncol. 2019;20(8):66.
Burke A, Tavora F. The 2015 WHO classification of tumors of the heart and pericardium. J Thorac Oncol. 2016;11(4):441–52.
Butany J, Nair V, Naseemuddin A, Nair GM, Catton C, Yau T. Cardiac tumours: diagnosis and management. Lancet Oncol. 2005;6(4):219–28.
Neragi-Miandoab S, Kim J, Vlahakes GJ. Malignant tumours of the heart: a review of tumour type, diagnosis and therapy. Clin Oncol (R Coll Radiol). 2007;19(10):748–56.
Fournier E, Séguéla PE, Sauvestre F, Jalal Z, Thomas J, Iriart X, Bonello B, Thambo JB. Imaging aspects of pediatric cardiac tumors. JACC Cardiovasc Imaging. 2020;13(10):2245–53.
Carbajal H, Waters L, Popovich J, Boniuk M, Chevez-Barrios P, Marcus DM, Sessoms S. IgG4 related cardiac disease. Methodist Debakey Cardiovasc J. 2013;9(4):230–2.
Vasaitis L. IgG4-related disease: a relatively new concept for clinicians. Eur J Intern Med. 2016;27:1–9.
Deshpande V, Zen Y, Chan JK, Yi EE, Sato Y, Yoshino T, Kloppel G, Heathcote JG, Khosroshahi A, Ferry JA, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012;25(9):1181–92.
Andersen ND, DiBernardo LR, Linardic CM, Camitta MG, Lodge AJ. Recurrent inflammatory myofibroblastic tumor of the heart. Circulation. 2012;125(19):2379–81.
Sebire NJ, Ramsay A, Sheppard M, Malone M, Harding B, Risdon RA. Intravascular inflammatory myofibroblastic tumors in infancy. Pediatr Dev Pathol. 2002;5(4):400–4.
Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol. 2007;31(4):509–20.
Antonescu CR, Suurmeijer AJ, Zhang L, Sung YS, Jungbluth AA, Travis WD, Al-Ahmadie H, Fletcher CD, Alaggio R. Molecular characterization of inflammatory myofibroblastic tumors with frequent ALK and ROS1 gene fusions and rare novel RET rearrangement. Am J Surg Pathol. 2015;39(7):957–67.
Coffin CM, Patel A, Perkins S, Elenitoba-Johnson KS, Perlman E, Griffin CA. ALK1 and p80 expression and chromosomal rearrangements involving 2p23 in inflammatory myofibroblastic tumor. Mod Pathol. 2001;14(6):569–76.
Acknowledgements
None.
Funding
Funded by Fundamental Research Funds for the Central Universities of Central South University, No. 56021702.
Author information
Authors and Affiliations
Contributions
JL and JY drafted the manuscript. XY and JY designed the study. JL, XY, and JY revised the manuscript. JL and JL were responsible for the collection of data or analysis. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics and consent to participate
The study protocol was approved by the Ethics Committee of the Second Xiangya Hospital of Central South University, Changsha, China.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, J., Liu, J., Yao, X. et al. A rare inflammatory myofibroblastic tumor appearing both inside and outside the heart. Eur J Med Res 29, 132 (2024). https://doi.org/10.1186/s40001-024-01710-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-024-01710-0