Abstract
Background
Prone position is used in acute respiratory distress syndrome and in coronavirus disease 2019 (Covid-19) acute respiratory distress syndrome (ARDS). However, physiological mechanisms remain unclear. The aim of this study was to determine whether improved oxygenation was related to pulmonary shunt fraction (Q’s/Q’t), alveolar dead space (Vd/Vtalv) and ventilation/perfusion mismatch (V’A/Q’).
Methods
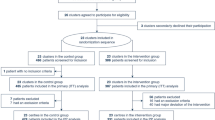
This was an international, prospective, observational, multicenter, cohort study, including six intensive care units in Sweden and Poland and 71 mechanically ventilated adult patients.
Results
Prone position increased PaO2:FiO2 after 30 min, by 78% (83–148 mm Hg). The effect persisted 120 min after return to supine (p < 0.001). The oxygenation index decreased 30 min after prone positioning by 43% (21–12 units). Q’s/Q’t decreased already after 30 min in the prone position by 17% (0.41–0.34). The effect persisted 120 min after return to supine (p < 0.005). Q’s/Q’t and PaO2:FiO2 were correlated both in prone (Beta -137) (p < 0.001) and in the supine position (Beta -270) (p < 0.001). V’A/Q’ was unaffected and did not correlate to PaO2:FiO2 (p = 0.8). Vd/Vtalv increased at 120 min by 11% (0.55–0.61) (p < 0.05) and did not correlate to PaO2:FiO2 (p = 0.3). The ventilatory ratio increased after 30 min in the prone position by 58% (1.9–3.0) (p < 0.001). PaO2:FiO2 at baseline predicted PaO2:FiO2 at 30 min after proning (Beta 1.3) (p < 0.001).
Conclusions
Improved oxygenation by prone positioning in COVID-19 ARDS patients was primarily associated with a decrease in pulmonary shunt fraction. Dead space remained high and the global V’A/Q’ measure could not explain the differences in gas exchange.
Similar content being viewed by others
Introduction
About 20% of hospitalized patients [1, 2] and up to 70% of critically ill patients [2] with coronavirus disease 2019 (COVID-19) develop acute respiratory distress syndrome (ARDS), increasing mortality [3]. The majority of patients with COVID-19 ARDS require intubation and mechanical ventilation [1,2,3]. Prone positioning is considered as one of the most effective treatment strategies for patients with severe ARDS, it may improve oxygenation due to perfusion redistribution and more homogeneous ventilation [4, 5]. Prone positioning is used in up to 76% of mechanically ventilated COVID-19 patients and improves oxygenation in ~ 80%. This response is associated with improved survival independently of the oxygenation response [6,7,8]. However, the underlying physiological mechanisms of improved oxygenation remain unclear [9]. We aimed to investigate the lung physiology of prone positioning in mechanically ventilated patients with COVID-19 ARDS, and to determine whether improved oxygenation was related to three clinically accessible measures of lung function: 1. ventilation/perfusion mismatch (V’A/Q’), that represents the heterogeneity of ventilation and perfusion distribution in the lungs and is defined as the rate of alveolar ventilation to the rate of pulmonary blood flow. 2. The pulmonary shunt fraction (Q’s/Q’t) that is a way to determine how much perfused but poorly or not ventilated lung regions contribute to hypoxemia in arterial blood. 3. The alveolar dead space (Vd/Vtalv) that represents the volume of air present in the respiratory zone of the lungs not taking part in gas exchange. In lung disease, alveolar dead space corresponds to alveoli that are ventilated, but not perfused by the pulmonary circulation. In a brief report, hypoxemia in COVID-19 ARDS correlated to dead space but was dissociated from lung mechanics [10]. A report of 10 intubated Covid-19 patients monitored with Electrical Impedance Tomography suggested that the observed elevated ventilation-perfusion mismatch was more due to lung units with dead space than shunt [11]. A report of 12 patients suggested that prone positioning had little effect on dead space fraction [12]. In previous studies, we reported that proning increased PaO2:FiO2 mainly in patients with PaO2:FiO2 < 120 mmHg [13] and Q’s/Q’t and Vd/Vtalv increased in early mild to moderate COVID-19, but their relative contributions were highly variable (14). We hypothesized that prone positioning would reduce V’A/Q’, Q’s/Q’t and Vd/Vtalv.
Materials and methods
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the 1964 Helsinki Declaration and its later amendments. The study was approved by the Swedish Ethical Review Authority (2020-02593), the Bioethical Commission, Military Medical Chamber, Poland (KB7/20/178/29), the Bioethical Council, Medical University of Warsaw, Poland (AKBE/219/2020) and the Bioethical Commission, Wrocław Medical University, Poland (KB-764/2020). Patient informed consent was granted as per the approvals. All data were de-identified following collection.
From three centers in Sweden (Södersjukhuset, Danderyd Hospital, Karolinska University Hospital) and three centers in Poland (4-th Military Clinical Hospital in Wroclaw, Medical University of Warsaw, Wroclaw Medical University), we included 71 consecutive adult (> 18 yr) ARDS patients who, while mechanically ventilated, received at least one session of prone positioning, lasting more than or equal to 12 h. All patients had documented COVID-19-positive reverse transcriptase polymerase chain reaction tests from either upper airway swab or bronchoalveolar lavage. All patients fulfilled the Berlin definition of ARDS [15]. The decision to initiate proning and the timing were based on the American–European Consensus Conference criteria for severe ARDS (PaO2:FiO2 ratio of < 150 mm Hg, with a FiO2 of ≥ 0.6) [16] and our experience with COVID-19 ARDS [13]. PaO2:FiO2 ratio was used in the present study to assess changes of pulmonary gas exchange in response to the prone position. This ratio is commonly used in ARDS severity assessment and as a marker of disease progression or to compare patients with different FiO2 (Feiner et al, PMID 27618274 [17]). Patients were included between July 2020 and April 2021. Data were collected before the proning session (baseline), at 30 and 120 min after initiation of proning, just before returning the patient to supine, and 30 and 120 min after returning to supine position. The high temporal resolution of parameters was intended to enable investigation of the pulmonary effects related to the proning intervention. We hypothesized that the response to the first proning session would be the most important indicator of the occurring physiological changes and allow for comparison of the entire patient cohort, based on our earlier experience [13]. Data included respiratory mechanics, ventilatory data, gas exchange and hemodynamic parameters, in addition to demographic and anthropometric variables. Follow-up was conducted at 30 days from the proning session. We intended to include all patients who were mechanically ventilated and proned. However, due to lack of availability of the equipment to measure cardiac output in this intensive phase of the pandemic, we could not include all eligible patients. The selection was random and was not due to patient characteristics.
Physiologic calculations
The Q’s/Q’t was calculated using the equation:
O2Ideal was calculated by:
The fraction of alveolar dead space ventilation (Vd/Vtalv) was calculated using the modified Bohr-Enghoff equation:
Where PaCO2 was a partial pressure of carbon dioxide in arterial blood and PECO2 the tension of the expired carbon dioxide (used as a surrogate of the mixed expired carbon dioxide in the original Bohr-Enghoff formula) and calculated from the equation PECO2 = FECO2 x (Pbar–PH2O). Pbar and PH2O—ambient conditions of the barometric pressure and the partial pressure of water vapor, stated at 760–47 = 713 (mmHg). The fraction of expired carbon dioxide (FECO2) was derived as a function of the carbon dioxide production (VCO2) and minute ventilation of the lungs (VE), from the equation:
The VE was calculated by subtracting the apparatus dead space and the anatomical dead space from the measured tidal volumes (milliliters), multiplied by the respiratory rate (per minute). The anatomical dead space was calculated according to the modified equation proposed by Nunn [20]:
The predicted body weight (PBW) was calculated according to the ARDS-net and the apparatus dead space (DSPapp) was study-site specific [18].
Due to the unavailability of volumetric capnometry data, VCO2 was computed from the oxygen consumption (VO2) and respiratory quotient (RQ) (arbitrarily set at 0.8). CO—measured cardiac output. CaO2—arterial oxygen content. CcvO2—central venous oxygen content used as a surrogate of the mixed venous oxygen content.
The Vd/Vt and Q’s/Q’t were calculated individually for all patients at protocol-derived measurement points and PaO2 and PaCO2 values were corrected for temperature, according to Bradley’s correction factor [19]. We calculated ventilation/perfusion ratio V’A/Q’ by dividing the estimated alveolar ventilation by the cardiac output, measured with the pulse contour analysis (FloTrac system, Edwards). The Ventilatory ratio was calculated using the equation [20]:
The Oxygenation index was calculated using the Eq. (20):
Statistical analyses
Statistical analyses were done using GraphPad Prism version 9.4.0 (GraphPad Software) and R (v 3.5.1). The primary outcome was the Q’s/Q’t. All continuous data are presented as medians with interquartile range (IQR). All temporal data sets were analyzed with mixed effects model (REML), fixed effects (type III) and Tukey's multiple comparisons test. A GEE model with an autoregressive structure (AR-1) was used for correlation analyses under an assumption of linear function of regressors and gaussian residuals. We inspected residual plots to exclude large deviations from normality, under the assumption that gaussian models are usually stable under minor such deviations. Regression models used to predict PaO2:FiO2 included available baseline variables thought to have possible causal effects of respiration and circulation. α = 0.05 was considered significant. *p < 0.05, **p < 0.01, ***p < 0.005, ****p < 0.001.
Results
The characteristics of the participants at study inclusion are presented in Table 1. All diseases reported were already present at the time of admission. The observed mortality of 63% was higher than the one estimated by the average SAPS III of 64 points, which would predict a mortality rate of 43%.
The ventilatory, metabolic and circulatory data from the first prone positioning session are presented in Table 2.
The percent changes in PaO2:FiO2 were plotted as a function of baseline PaO2:FiO2, at 30 min, 120 min, and at the end of the proning period (just before returning to supine), as well as the percent changes in PaO2:FiO2 from the end of proning (prone baseline) to 30 min and 120 min in supine position. Improvements from baseline PaO2:FiO2 were larger for low baseline PaO2:FiO2, and more pronounced with time. When returning from prone to supine position, patients with a relatively higher PaO2:FiO2 at prone baseline decreased as much as 100 units back to supine. Patients with lower improvement of PaO2:FiO2 did not drop as much in PaO2:FiO2 when returning to supine (Fig. 1A, B).
The pulmonary physiological response to proning is shown in Fig. 2. Proning increased PaO2:FiO2, starting at 30 min from initiation, by a maximum of 78% (83–148 mm Hg). The effect remained throughout the period of study observation, up to 120 min after return to supine (p < 0.001) (Fig. 2A). Oxygenation index decreased at 30 min after prone positioning by a maximum of 43% (21–12 units) and remained decreased throughout the observation period (Fig. 2B). V’A/Q’ remained unchanged (Fig. 2C). Q’s/Q’t decreased during proning by a maximum of 17% (0.41–0.34). The effect was consistent to the end of the observation and was still improved 120 min after return to supine (p < 0.005) (Fig. 2D). Vd/Vtalv remained unchanged in comparison to baseline and differed at 120 min after initiation of proning in comparison to 120 min after returning to supine by 13% (0.71–0.63) (p < 0.01) (Fig. 2E). Ventilatory ratio increased at 30 min after initiation of proning (p < 0.01) and continued to increase before returning to supine (p < 0.001) to a maximum of 58% of baseline (1.9–3.0) (Fig. 2F). Individual responses are shown in Additional file 1: Figure S1.
Pulmonary physiology for six time points during proning. A: PaO2:FiO2. B: oxygenation index. C V’A/Q’ (ventilation/perfusion ratio). D: Q’s/Q’t (pulmonary shunt fraction). E Vd/Vtalv (ratio of airway dead space to alveolar tidal volume). F ventilatory ratio. Displayed as medians with IQR. Asterisks mark in comparison to baseline, if not specifically marked with lines. *p < 0.05, **p < 0.01, ***p < 0.005, ****p < 0.001
Changes in Q’s/Q’t, Vd/Vtalv, and V’A/Q’ were visualized in scatter plots, showing individual patient values. The higher the distance from the diagonal, the higher the decrease in Q’s/Q’t on proning (white area) or increase in Q’s/Q’t (beige area), while returning to supine position (Fig. 3). The highest level of scatter (variance) was detected in Q’s/Q’t.
The relationship between Q’s/Q’t, Vd/Vtalv, V’A/Q’, and their supine and prone baselines (prone position just before return). Points close to the diagonal indicate no change. Values below the diagonal indicate decrease, and values above the diagonal indicate increase. Baseline PaO2:FiO2 below median marked as dots, PaO2:FiO2 above median marked as circles. P-values which were not significant are specified in exact numbers
The correlation of time dependent variables with PaO2:FiO2 was then investigated for the intervention supine to prone position. In prone position, a strong negative correlation between Q’s/Q’t and PaO2:FiO2 was detected, with an increase in PaO2:FiO2 of 137 units per unit Q’s/Q’t decrease in the linear approximation (Beta = 137, p < 0.001). Vd/Vtalv and V’A/Q’ did not correlate to PaO2:FiO2 (p = 0.3 and p = 0.8, respectively). When returning from prone to supine position, an even stronger correlation between Q’s/Q’t and PaO2:FiO2 was detected (Beta -270, p < 0.001), meaning that for every increase in Q’s/Q’t, PaO2:FiO2 decreased 270 units. Neither Vd/Vtalv (p = 0.6) nor V’A/Q’ (p = 0.8) did correlate to PaO2:FiO2. We identified PaO2:FiO2 at baseline as the only variable predictive of the PaO2:FiO2 at the different time points after proning (Beta 1.3; p < 0.001 and the other tested variables in Table 3).
Discussion
In this study, we show that the oxygenation improvement by prone positioning was primarily associated with a decrease in pulmonary shunt fraction, in mechanically ventilated patients with COVID-19 associated ARDS.
COVID-19 ARDS is a heterogeneous disease. The lung morphology is characterized by coexisting signs of alveolar damage and interstitial injury: ground-glass opacity with or without consolidation and septal thickening are common findings on CT images [22]. Progression of pulmonary injury is characterized by alterations of the pulmonary vasculature tree, with dynamic increase in the size of vessels [23, 24]. Both alveolar and vascular pathology exist in early, less severe COVID-19 and the late, fatal cases [14, 25]. It is possible that the heterogenous nature of the disease causes diverse physiological responses in the lung, before and after the established method of prone positioning.
We investigated the physiological effects of proning as treatment intervention initiated by the caregiver, in a cohort of mechanically ventilated COVID-19 patients with moderate to severe ARDS, according to the Berlin definition of ARDS [15]. Median PaO2:FiO2 at time of intervention was 83 mmHg, a lower starting point than in our previous report [13]. This may reflect that the decision to prone was taken later, or that the cohort was more severely ill. Also, the time in prone position was longer than in our previous report, 19 h, compared to 14.5 h [13].
First, we evaluated the change in PaO2:FiO2 as a function of baseline PaO2:FiO2. Changes were both higher and more pronounced over time for patients with low baseline PaO2:FiO2. Proning improved PaO2:FiO2, starting at 30 min, and the improvement was consistent throughout the observation period. The 82% (47–148 mm Hg) increase (Additional file 1: Table S1) was higher than the 55–60% previously reported [8, 13]. Hence, we detected a heterogeneity in response and the patients with a more severe ARDS responded better to prone positioning, in line with previous investigations [8]. When returning to supine, patients who had a higher improvement in oxygenation in prone position lost up to 100 mmHg in PaO2:FiO2. Most patients with a lower improvement in PaO2:FiO2 did not lose as much oxygenation when turned back to supine. Thus, the reversibility of the response to proning was higher in patients with a more severe ARDS. Proning also caused a decrease in oxygenation index, which describes the severity of hypoxic respiratory failure including the mean airway pressure, an important determinant of oxygenation [21].
Next, we evaluated changes in pulmonary physiology during and after proning. Primarily, we investigated three established variables of pulmonary function: Q’s/Q’t, V’A/Q’ and Vd/Vtalv [26]. The rationale for selecting these parameters was that they could be obtained in clinical practice and did not require invasive procedures or transfers, which could jeopardize patient safety, especially in a cohort with high mortality (63% in this cohort). We primarily calculated Q’s/Q’t, which decreased by 7 percentage points (41% before proning to 34% before returning to supine). Interestingly, the effect lasted throughout the observation period, also when returning to baseline. Q’s/Q’t is quantified as fraction of cardiac output distributed to nonventilated units. ARDS of other origins is characterized by severe hypoxemia due to shunt that might exceed 50% [27]. Q’s/Q’t and PaO2:FiO2 were highly correlated for the intervention supine to prone position, and for every unit decrease in Q’s/Q’t, the PaO2:FiO2 increased 137 units (p < 0.001), which further confirmed the pathophysiological mechanism behind the improvement in oxygenation.
V’A/Q’ varied between 1.13 and 1.32, it did not change significantly in prone position, and it did not correlate to PaO2:FiO2 for the intervention supine to prone position or when returning to supine. The Riley three-compartment model is convenient [28], but assumes that the effects of V’A/Q’ mismatch on PaO2 and PaCO2 are entirely due to shunt and physiological dead space ventilation [26] and ignores gas exchange in units with other V’A/Q’ ratios, which may be assessed with multiple inert gas elimination technique [29]. Also, the calculated shunt corresponds to the amount of shunt of mixed venous blood that would result in the observed arterial oxygenation in the absence of low V’A/Q’ regions. But venous admixture may be increased even in the absence of true shunt [26]. In our study cohort, alveolar ventilation and cardiac output remained relatively unchanged. Thus, the global V’A/Q’ measure was not sensitive enough to explain the differences in gas exchange.
Vd/Vtalv varied between 0.69 and 0.75 and did not correlate to PaO2:FiO2 for the intervention supine to prone position, thus confirming previous findings suggesting that prone positioning had little effect on dead space fraction, although dead space decreased at 120 min after returning to supine, in comparison to 120 min after initiation of proning [12]. Despite its well-established role in lung physiology, dead space is not routinely assessed in critically ill patients [30]. In COVID-19 ARDS, increased alveolar dead space may be related to obstruction of small pulmonary arteries due to microthrombosis [25], and is associated with high D-dimer levels and a lower likelihood of being discharged alive [31]. Different methodological approaches have been used for the calculation of the physiological dead space in ICU settings, where reliable VCO2 measurements are challenging to obtain [32, 33]. The Ventilatory ratio is a useful estimate of impaired lung ventilation in terms of CO2 elimination, it is strongly correlated to dead space in ARDS, and it is associated with increased risk of an adverse outcome [20]. The Ventilatory ratio increased 1.1 units during prone positioning (from 1.9 at baseline to 3 before return to supine). Earlier studies reported a lower increase (of 0.03 units) on proning [8].
Q’s/Q’t, Vd/Vtalv, and V’A/Q’ were then visualized in scatter plots, showing changes in the individual patients. The highest level of variance was detected in Q’s/Q’t, confirming that Q’s/Q’t was the physiologic parameters mostly affected by proning. Possible predictors of the oxygenation response were then evaluated. PaO2:FiO2 at baseline predicted PaO2:FiO2 at 30 min and no further variables proved to correlate to changes in PaO2:FiO2, confirming that baseline oxygenation may predict patient response.
The assumed physiological mechanisms of the improved oxygenation may be explained in part by previously published experimental studies. Using positron emission tomography imaging of nitrogen in sheep, prone position was shown to improve gas exchange by restoring aeration while preserving perfusion in dorsal lung regions, and by making the distribution of ventilation more uniform [34]. While we could not assess the specific contribution of different mechanisms, we detected a decrease in shunt, and it is possible that proning also improved the distribution of ventilation, although the global V’A/Q’ index was not sufficiently sensitive to detect it. Prone positioning was previously shown to increase transpulmonary pressures while improving oxygenation and hemodynamics in patients with moderate to severe ARDS [35], which may be a mechanism of improvement also in COVID-19 ARDS.
There are some limitations to be discussed. First, there was no prespecified sample size for the cohort enrolled since, at the study time, no knowledge was available regarding gas exchange response or physiological variable changes to be expected in Covid-19 ARDS patients during prone positioning. Second, a potential inclusion bias may exist since the initiation of prone positioning may ultimately reflect the practice of the treating clinical team. Differences in patient selection among centers may potentially reflect different resource availability at the time of the pandemic. The issues of staffing, burden of patients, and ICU occupancy may have affected the timeliness of delivery of proning and the use of the non-invasive ventilatory support prior to intubation. Third, this study was not powered to determine survival, so the reported mortality data are not a predefined outcome variable of interest in our study. Fourth, the assumptions and populational data-based gas exchange modelling are clinically applicable but may require further investigations of validity. However, it should be noted that the investigation was performed during a severe phase of the pandemic, and the patients in need of prone positioning were severely ill. Therefore, further investigations which would require a lung physiology laboratory were not possible, and we believe that the results reflect important insights of changes occurring in the lungs as a result of proning. Fifth, although PaO2:FiO2 is a surrogate of venous admixture and thus correlates with improvements in pulmonary shunt fraction, differences in FiO2 and cardiac output may substantially influence venous admixture and thus the calculated pulmonary shunt fraction [36]. At a given venous admixture, the PaO2:FiO2 ratio may differ, depending on oxygen consumption and cardiac output. Conversely, for the same PaO2:FiO2, venous admixture may vary with FiO2, while cardiac output did not differ depending on proning in our cohort (p = 0.56). Selecting PEEP according to PaO2:FiO2 ratio may also be misleading if hemodynamics are not taken into account. PEEP was not changed throughout the study neither in relation to PaO2:FiO2 nor to prone positioning (p = 0.90). Sixth, tidal volumes were higher (mean 7.1–8.2) than the recommended < 6 mL/kg PBW for lung protective ventilation [37]. While a confounder of the PaO2:FiO2 by increased tidal volumes cannot be excluded, tidal volumes were not affected by proning (p = 0.8). Seventh, the choice of the ventilator mode and use of neuromuscular relaxants were decisions of the attending clinician. Therefore, the ventilation strategy differed among patients but was largely kept unchanged within patients throughout the study period (Additional file 1: Table S3). For this reason, no conclusion can be drawn regarding the effect of mode of ventilation or neuromuscular blockers on the study results.
Conclusions
Improved oxygenation by prone positioning in COVID-19 ARDS patients was primarily associated with a decrease in pulmonary shunt fraction. Dead space remained high and the global V’A/Q’ measure could not explain the differences in gas exchange.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. Jama. 2020. https://doi.org/10.1001/jama.2020.1585.
Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–81.
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62.
Guérin C, Reignier J, Richard J, Beuret P, Gacouin A, Boulain T, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159.
Gattinoni L, Busana M, Giosa L, Macri MM, Quintel M. Prone positioning in acute respiratory distress syndrome. Semin Respir Crit Care Med. 2019;40(1):94–100.
Ziehr D, Alladina J, Petri C, Maley J, Moskowitz A, Medoff B, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care Med. 2020. https://doi.org/10.1164/rccm.202004-1163LE.
Ferrando C, Suarez-Sipmann F, Mellado-Artigas R, Hernández M, Gea A, Arruti E, et al. Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS. Intensive Care Med. 2020. https://doi.org/10.1007/s00134-020-06251-8.
Camporota L, Sanderson B, Chiumello D, Terzi N, Argaud L, Rimmelé T, et al. Prone position in COVID-19 and -COVID-19 acute respiratory distress syndrome: an international multicenter observational comparative study. Crit Care Med. 2022;50(4):633–43.
Berg RMG, Hartmann JP, Iepsen UW, Christensen RH, Ronit A, Andreasen AS, et al. Therapeutic benefits of proning to improve pulmonary gas exchange in severe respiratory failure: focus on fundamentals of physiology. Exp Physiol. 2022;107(7):759–70.
Vasques F, Sanderson B, Formenti F, Shankar-Hari M, Camporota L. Physiological dead space ventilation, disease severity and outcome in ventilated patients with hypoxaemic respiratory failure due to coronavirus disease 2019. Intensive Care Med. 2020;46:2092–3.
Mauri T, Spinelli E, Scotti E, Colussi G, Basile MC, Crotti S, et al. Potential for lung recruitment and ventilation-perfusion mismatch in patients with the acute respiratory distress syndrome from coronavirus disease 2019. Crit Care Med. 2020. https://doi.org/10.1097/CCM.0000000000004386.
Sharp T, Al-Faham Z, Brown M, Martin-Lazaro J, Cevallos MJ. Prone position in covid-19: can we tackle rising dead space? J Intensive Care Soc. 2022;23(2):240–3.
Gleissman H, Forsgren A, Andersson E, Lindqvist E, Lipka Falck A, Cronhjort M, et al. Prone positioning in mechanically ventilated patients with severe acute respiratory distress syndrome and coronavirus disease 2019. Acta Anaesthesiol Scand. 2021;65(3):360–3.
Harbut P, Prisk GK, Lindwall R, Hamzei S, Palmgren J, Farrow CE, et al. Intrapulmonary shunt and alveolar dead space in a cohort of patients with acute COVID-19 pneumonitis and early recovery. Eur Respir J. 2022. https://doi.org/10.1183/13993003.01117-2022.
Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–33.
Bernard G, Artigas A, Brigham K, Carlet J, Falke K, Hudson L, et al. The American-European Consensus Conference on ARDS definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818.
Feiner JR, et al. Evaluating pulmonary function: an assessment of PaO2/FIO2. Crit Care Med. 2071;45(1):e40–e48. https://doi.org/10.1097/CCM.0000000000002017. PMID 27618274.
Linares-Perdomo O, East TD, Brower R, Morris AH. Standardizing predicted body weight equations for mechanical ventilation tidal volume settings. Chest. 2015;148(1):73–8.
Bradley AF, Stupfel M, Severinghaus JW. Effect of temperature on Pco2 and Po2 of blood in vitro. J Appl Physiol. 1956;9(2):201–4.
Sinha P, Calfee CS, Beitler JR, Soni N, Ho K, Matthay MA, et al. Physiologic analysis and clinical performance of the ventilatory ratio in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2019;199(3):333–41.
Thomas NJ, Shaffer ML, Willson DF, Shih MC, Curley MA. Defining acute lung disease in children with the oxygenation saturation index. Pediatr Crit Care Med. 2010;11(1):12–7.
Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–34.
Albarello F, Pianura E, Di Stefano F, Cristofaro M, Petrone A, Marchioni L, et al. 2019-novel coronavirus severe adult respiratory distress syndrome in two cases in Italy: an uncommon radiological presentation. Int J Infect Dis. 2020;93:192–7.
Li Y, Xia L. Coronavirus disease 2019 (COVID-19): role of chest CT in diagnosis and management. AJR Am J Roentgenol. 2020. https://doi.org/10.2214/AJR.20.22954.
Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N Engl J Med. 2020;383(2):120–8.
Petersson J, Glenny RW. Gas exchange and ventilation-perfusion relationships in the lung. Eur Respir J. 2014;44:1023–41.
Dakin J, Jones AT, Hansell DM, Hoffman EA, Evans TW. Changes in lung composition and regional perfusion and tissue distribution in patients with ARDS. Respirology. 2011;16(8):1265–72.
Riley RL, Cournand A. Ideal alveolar air and the analysis of ventilation-perfusion relationships in the lungs. J Appl Physiol. 1949;1(12):825–47.
Wagner PD. The multiple inert gas elimination technique (MIGET). Intensive Care Med. 2008;34(6):994.
Ferluga M, Lucangelo U, Blanch L. Dead space in acute respiratory distress syndrome. Ann Transl Med. 2018;6(19):388.
Graf J, Pérez R, López R. Increased respiratory dead space could associate with coagulation activation and poor outcomes in COVID-19 ARDS. J Crit Care. 2022;71:154095.
Nuckton TJ, Alonso JA, Kallet RH, Daniel BM, Pittet JF, Eisner MD, et al. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med. 2002;346(17):1281–6.
Kallet RH, Daniel BM, Garcia O, Matthay MA. Accuracy of physiologic dead space measurements in patients with acute respiratory distress syndrome using volumetric capnography: comparison with the metabolic monitor method. Respir Care. 2005;50(4):462–7.
Richter T, Bellani G, Scott Harris R, Vidal Melo MF, Winkler T, Venegas JG, et al. Effect of prone position on regional shunt, aeration, and perfusion in experimental acute lung injury. Am J Respir Crit Care Med. 2005;172(4):480–7.
Boesing C, Graf PT, Schmitt F, Thiel M, Pelosi P, Rocco PRM, et al. Effects of different positive end-expiratory pressure titration strategies during prone positioning in patients with acute respiratory distress syndrome: a prospective interventional study. Crit Care. 2022;26(1):82.
Gattinoni L, Vassalli F, Romitti F. Benefits and risks of the P/F approach. Intensive Care Med. 2018;44:2245–7.
Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–8.
Acknowledgements
Not applicable.
Funding
Open access funding provided by Karolinska Institute. Departmental funding only. The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Contributions
PH, FCJ, MG contributed substantially to the conception and design of the study and analyzation of data. MD analyzed and interpreted the patient data. All authors have contributed to the acquisition of data, drafted, or provided critical revision of the article, and provided final approval of the version submitted for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Swedish Ethical Review Authority (2020-02593), the Bioethical Commission, Military Medical Chamber, Poland (KB7/20/178/29), the Bioethical Council, Medical University of Warsaw, Poland (AKBE/219/2020) and the Bioethical Commission, Wrocław Medical University, Poland (KB-764/2020). Patient informed consent was granted as per the approvals. All data were de-identified following collection.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Individual temporal responses to proning. A. PFI, B. VdVt, C. Qs/Qt, D. VA/Q. Blue area is standard deviation. Table S1. Pulmonary physiology values for six timepoints during the first proning session. Median (IQR). Qs/Qt (pulmonary shunt). B. Vd/Vt (ratio of airway dead space to alveolar tidal volume). C. VA/Q (Ventilation-perfusion ratio), D. PFI. Table S2. Determination of responders to prone positioning based on PaO2:FiO2 increase greater than or equal to 20mmHg, dynamic compliance (Cdyn) improvement, Ventilatory Ratio and CO2 decrease. Δ% = relative percent change. @30min= timepoint 30minutes after proning. @16h = timepoint 16 hours after proning. Table S3. Ventilation modes used during the study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Harbut, P., Campoccia Jalde, F., Dahlberg, M. et al. Improved oxygenation in prone positioning of mechanically ventilated patients with COVID-19 acute respiratory distress syndrome is associated with decreased pulmonary shunt fraction: a prospective multicenter study. Eur J Med Res 28, 597 (2023). https://doi.org/10.1186/s40001-023-01559-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-023-01559-9