Abstract
Background
The prevalence of ischaemic heart failure (HF) continues to increase. Diabetes mellitus (DM) concomitant with ischaemic HF increases the risk of major adverse cardiovascular events (MACEs). As a promising predictor for cardiovascular diseases, the predictive value of the monocyte to high-density lipoprotein cholesterol ratio (MHR) for MACE in the ischaemic HF with DM cohort has never been investigated before.
Objective
We aimed to investigate the MHR as a predictor for MACE in ischaemic HF patients with DM who underwent percutaneous coronary intervention (PCI).
Methods
This observational study enrolled 1049 patients with ischaemic HF and DM undergoing PCI from June 2017 to June 2019. The baseline data were collected. MACEs, including all-cause mortality, nonfatal myocardial infarction, and any revascularization, were recorded within the 36-month follow-up. The characteristics and incidence of MACE were analysed in four groups stratified by the quartiles of MHR. The hazard ratio for MACE was analysed with Cox regression models. The incidence of MACE in the four groups was evaluated by Kaplan‒Meier survival analysis. Restricted cubic spline analysis was performed to determine the nonlinear correlation between the MHR and MACE.
Results
After the 36-month follow-up, 407 patients (38.8%) experienced MACEs. The incidence of MACE was significantly higher among patients in the upper MHR quartile than among those in the lower MHR quartiles (23.4% vs. 36.0% vs. 41.4% and 54.6%; P < 0.001, respectively), which was consistent with the Kaplan‒Meier survival analyses (P < 0.0001). A multivariate Cox regression model showed that the MHR was an independent risk factor for MACE after variables were adjusted (adjusted HR: 2.11; 95% CI 1.47–3.03; P < 0.001). Its predictive effects on MACE showed no interaction with hypercholesterolemia (P > 0.05).
Conclusion
The MHR was a significant and independent predictor of MACEs in ischaemic HF patients with DM undergoing PCI.
Similar content being viewed by others
Introduction
Heart failure (HF) has remained a rising global pandemic with a global prevalence of more than 37.7 million individuals currently [1]. HF is usually associated with increased major adverse cardiovascular events (MACEs), which affects the expected lifespan and brings a huge burden to the health care system [2].
Ischaemic HF accounts for nearly 60–70% of HF cases. Coronary artery disease (CAD) remains the most common cause of ischaemic HF [3]. Patients with ischaemic HF often encounter diabetes mellitus (DM). They share similar metabolic risk factors and independently increase the risk for the other. The prevalence of DM in HF cohorts ranges from 10 to 47%, which is markedly higher than the prevalence in the general population [4,5,6,7]. On the other hand, the incidence of HF in individuals with DM was 1.08–6.76 times that in individuals without DM according to many large-scale cohort studies [8,9,10,11]. In addition, HF patients with DM have a higher incidence of MACEs than the nondiabetic HF population [8]. In population-based studies, HF concomitant with DM increases the risk of mortality in both hospitalized and ambulatory patients [12, 13]. Therefore, an effective prognostic factor for MACE in ischaemic HF patients with DM is of great value for clinical management.
Whether percutaneous coronary intervention (PCI) improves the clinical outcomes of ischaemic HF remains ambiguous. Some studies found that PCI in patients with complex CAD and left ventricular systolic dysfunction may reverse LV remodelling and improve clinical outcomes [14, 15]. Patients with ischaemic HF subjected to revascularization showed a lower rate of cardiac mortality by 61% in the first 5 years [15]. However, the REVIVED-BCIS2 study demonstrated that compared to optimal medical therapy, PCI in patients with a left ventricular ejection fraction (LVEF) < 35% and myocardial viability did not reduce the rate of mortality [16, 17]. Therefore, the prognosis of ischaemic HF patients undergoing PCI still needs further investigation.
The monocyte to high-density lipoprotein cholesterol (HDL-C) ratio (MHR) is one of the systemic inflammatory indices. The MHR has been proven to have clinical value in the prognosis of CAD after PCI [18,19,20,21,22,23,24,25,26,27]. However, in the field of ischaemic HF induced by CAD, the investigation of MHR is limited. The two observational retrospective studies showed that the MHR had potential value in the clinical evaluation of patients with HFpEF and chronic HF [28, 29]. Additionally, previous studies have explored cardiovascular disorders combined with DM cohorts, such as non-ST-segment elevation acute coronary syndrome [24], subclinical left cardiac remodelling and dysfunction [30], and arterial stiffness [31]. All of them showed that the MHR is associated with the occurrence and outcome of certain cardiovascular disorders. However, the application value of the MHR in an ischaemic HF combined with DM cohort has never been assessed before.
Therefore, we carried out a retrospective cohort study to identify the prognostic effect of the MHR on MACE in patients with ischaemic HF with concomitant DM undergoing PCI. The predictive value of the MHR may be more pronounced, offering a potential noninvasive approach for prognostic assessment. This research endeavour is expected to provide novel MHR applications in clinical practice and serve as a basis for developing personalized therapeutic strategies.
Methods
Ethics declarations
The Ethics Committee of Beijing Anzhen Hospital, Capital Medical University, and Beijing Institute of Heart, Lung, and Blood Vessel Diseases, Beijing, China approved this study. Informed consent was obtained from all participants in this analysis. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (Registry number: No. 2022235X).
Study population
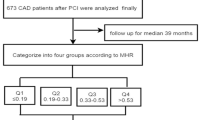
This was a single-centre, observational, retrospective cohort study at Beijing Anzhen Hospital. Our patients from mainland China with the primary diagnosis for this study were ischaemic HF and DM undergoing elective PCI from June 2017 to June 2019 at inclusion. The diagnostic criteria of ischaemic HF were as follows [32]: (1) left ventricular failure, congestive HF, cardiac insufficiency, diastolic HF, or HF, unspecified based on the International Classification of Diseases (ICD) 10th revision (I50.106, I50.001, I50.90, I50.919, I50.905, or I50.911). (2) MVD [left main (LM) disease or coronary artery stenosis > 50% in ≥ 2 vessels]. The diagnosis criteria of DM were according to the 1999 criteria of the World Health Organization: fast blood glucose > 7.0 mmol/L or 2‐hour postprandial glucose > 11.1 mmol/L or already diagnosed with DM by the physicians according to the medical history. A total of 3707 adult patients with HF and concomitant MVD undergoing elective PCI in our hospital were enrolled in this cohort. The exclusion criteria of this study were as follows: (1) patients with no DM; (2) patients lost to follow-up; (3) history of coronary artery bypass grafting; (4) any kind of cancer affecting long-term survival; (5) LVEF ≥ 50%; (6) absolute monocyte count and high-density lipoprotein cholesterol data missing; (7) acute myocardial infarction (MI); (8) chronic, severe diseases of the blood system and immune system or long-term use of glucocorticoids or immunosuppressants; and (9) acute or chronic infectious diseases (such as pneumonia, infective endocarditis, or tuberculosis). Ultimately, 1049 patients were included in the final analysis (Fig. 1).
Data collection
Demographics, vital signs, body mass index (BMI), comorbidities, medical history, New York Heart Association (NYHA) class, echocardiography, angiographic characteristics, procedural results, laboratory examinations, and medication use were collected from the hospital information recording system. Demographics included age, sex. Vital signs included systolic blood pressure (SBP), diastolic blood pressure, and heart rate. Comorbidities included hypertension, hypercholesterolemia, renal insufficiency, and atrial fibrillation. Medical history included prior MI, prior PCI, and prior stroke. Echocardiography data included left ventricular end systolic dimension (LVDs), left ventricular end diastolic dimension (LVDd), and LVEF.
Angiographic characteristics included LM disease, three-vessel disease, chronic total occlusion, diffuse lesion, in-stent restenosis, synergy between PCI with taxus, and cardiac surgery (SYNTAX). The lesion characteristics were defined as follows: (1) LM disease: an angiographically estimated stenosis > 50% or a fractional flow reserve < 0.80 in the LM coronary artery ostium, mid-shaft, or distal bifurcation. (2) Three‑vessel disease: more than two main coronary branches (vessel diameter ≥ 2 mm) with an extent of stenosis ≥ 50%. (3) Chronic total occlusion lesion: lesion with complete obstruction [thrombolysis in myocardial infarction (TIMI) flow grade 0 lasting longer than 3 months, which was judged from the medical history or coronary angiogram results. (4) Diffuse lesion: a single stenotic lesion with a length of ≥ 20 mm. (5) In-stent restenosis: stenosis of ≥ 50% occurring in the segment inside the stent, 5 mm proximal or distal to the stent [33]. The severity of coronary artery lesions was quantified by the synergy between the PCI and SYNTAX score. The SYNTAX score was calculated for each participant using the SYNTAX score algorithm (www.syntaxscore.com) [34]. Procedural results included target vessel territories: LM, left anterior descending artery (LAD), left circumflex artery (LCX), and right coronary artery (RCA), complete revascularization, and the number of stents. PCI strategies were determined and performed by at least two experienced interventional cardiologists and were in accordance with current practice guidelines in China [35].
Laboratory examinations included glucose, glycosylated haemoglobin A1c (HbA1c), glycated albumin, B-natriuretic peptide (BNP), fibrinogen (FBG), D-dimer, International Normalized Ratio (INR), total cholesterol (TC), triglyceride, low-density lipoprotein cholesterol (LDL-C), HDL-C, total bilirubin, direct bilirubin, alanine transaminase(ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), creatinine, blood nitrogen urea, estimated glomerular filtration rate (eGFR), uric acid, sodium, potassium, calcium, magnesium, white blood cell, haemoglobin, platelet, high-sensitivity C-reactive protein (hs-CRP), and MHR. Medications included aspirin, clopidogrel, ticagrelor, statins, ezetimibe, angiotensin-converting enzyme inhibitor (ACEI), angiotensin receptor blocker (ARB), beta-blockers, calcium channel blocker (CCB), loop diuretics, thiazide diuretics, spironolactone, tolvaptan, sacubitril/valsartan, metformin, alpha‑glucosidase inhibitor, sulfonylurea, sodium-dependent glucose transporters 2 (SGLT2) inhibitor, dipeptidyl peptidase inhibitor 4 (DDP4) depressor, insulin, warfarin, factor Xa inhibitors, and factor IIa inhibitors.
Grouping and endpoints
The MHR was determined as the absolute monocyte count (× 109/L) divided by HDL-C (mmol/L). All patients were stratified into four groups according to MHR quartiles: Quartile 1 (MHR < 0.33[× 109/mmol]), Quartile 2 (0.33 ≤ MHR < 0.46[× 109/mmol]), Quartile 3 (0.46 ≤ MHR < 0.62[× 109/mmol]), and Quartile 4 (MHR ≥ 0.62 × 109/mmol]). After baseline PCI, the outcome data of patients were collected at 3, 6, 9 and 12, 24, and 36 months after PCI by trained physicians in the outpatient follow-up centre. The collected information was confirmed according to the medical records when necessary. MACE was defined as the combination of all-cause mortality, nonfatal MI, and any revascularization. The definition of MI was determined by the fourth universal definition of MI [36]. Any revascularization was defined as coronary revascularization due to any reason. The primary endpoint was MACE, and the secondary endpoint was any of the components of the defined MACE. In patients who had multiple adverse outcomes during follow-up, we first selected all-cause mortality, followed by nonfatal MI and any revascularization. Only the first incidence was analysed when events occurred more than once. The present study lasted until June 2022.
Statistical analysis
Stata software (StataCorp LLC, TX, USA, ver. 15.0) and R software (R-project®, Vienna, AUS, ver. 4.2.1) were used for analysis. P < 0.05 was considered statistically significant. Normally distributed continuous variables are presented as the mean ± SD. The abnormally distributed continuous variables are presented as the median (25th, 75th). Categorical variables are presented as numbers (percentages). P values were calculated using analysis of variance. Numerical variable comparisons among the groups were performed by ANOVA (for normally distributed continuous data) and the Kruskal‒Wallis test (for abnormally distributed continuous data). Chi-square tests were applied for comparisons of categorical data. Variables for which the P value was < 0.05 in the univariate analysis were assessed by Cox regression analysis to evaluate the independent predictors of 36-month primary and secondary endpoints and the results are shown as hazards ratios (HRs) with 95% confidential intervals (CIs). Model 2 was adjusted for age and gender. Model 3 was built to control for confounders and adjusted for age, sex, heart rate, BMI, LVDd, LM disease, chronic total occlusion, diffuse lesion, target vessel of LM, complete revascularization, number of stents, total bilirubin, direct bilirubin, TC, triglyceride, glucose, platelet, ARB, sacubitril/valsartan, metformin, and SGLT2 inhibitor. The incidence rates of MACE and its components among MHR quartiles were calculated by the Kaplan‒Meier method and verified by the log-rank test. Subgroup analysis was developed to determine the influence of the MHR on MACE and its components in hypercholesterolemia and nonhypercholesterolemia patients. Spearman correlation was used to analysis the association between MHR and conventional risk factor. Nonlinear correlations between MHR and risk of MACE were demonstrated by restricted cubic spline models based on the Cox proportional hazards model with four knots (5th, 35th, 65th, and 95th percentiles of MHR) based on the lowest value of the Akaike information criterion. The adjusted variables in the model were consistent with Model 3.
Results
In total, 1049 patients with a mean age of 61.4 ± 10.7 years (75.5% male) were enrolled in this study. The median follow-up was 36 months (IQR 18–36 months). Any missing data on outcomes, monocyte count, and HDL-C were deleted. Patients were divided into four quartiles based on MHR levels (MHR 0.26 ± 0.05 in Quartile 1, 0.40 ± 0.03 in Quartile 2, 0.54 ± 0.05 in Quartile 3, and 0.84 ± 0.22 in Quartile 4). According to the MHR quartiles, the baseline demographics, vital signs, BMI, comorbidities, medical history, NYHA class, echocardiography, angiographic data, procedural results, laboratory findings, and medication use are shown in Table 1. Apart from monocytes, HDL-C, and MHR, we also found that sex, SBP, BMI, FBG, D-dimer, INR, TC, triglycerides, ALT, AST, GGT, uric acid, potassium, calcium, white blood cells, platelets, hs-CRP, loop diuretics, sulfonylurea, and DDP4 depressor showed differences in MHR quartiles.
The MACE was 62 (23.4%) in MHR Quartile 1, 94 (36.0%) in Quartile 2, 108 (41.4%) in Quartile 3, and 143 (54.6%) in Quartile 4. The incidence of MACE was highest in the MHR Quartile 4 group, followed by the Quartiles 3, 2, and 1 groups (P < 0.001). The all-cause mortality event and incidence were 31 (11.7%) in Quartile 1, 47 (18.0%) in Quartile 2, 54 (20.7%) in Quartile 3, and 63 (24.1%) in Quartile 4. The incidence of all-cause mortality was highest in the MHR Quartile 4 group, followed by the MHR Quartiles 3, 2, and 1 (P = 0.003). The events and incidence of any revascularization showed a similar trend [63 (24.1%), 42 (16.1%), 38 (14.6%), and 26 (9.8%) in Quartiles 4–1, respectively, P < 0.001]. However, among these groups, no significant difference was noted in the incidence of nonfatal MI (1.9%, 3.5%, 4.6%, and 6.5% in Quartiles 1, 2, 3, and 4, respectively, P = 0.056) (Table 2).
In Cox regression analyses, independent predictors of MACE (Table 3) were assessed. The MHR Quartile 1 was defined as the reference. No variables were adjusted in Model 1. Age and sex were adjusted for Model 2. The variables with P < 0.05 in stepwise regression were adjusted for Model 3, and these variables included age, sex, heart rate, BMI, LVDd, LM disease, chronic total occlusion, diffuse lesion, SYNTAX score, target vessel of LM, complete revascularization, number of stents, total bilirubin, direct bilirubin, TC, triglyceride glucose, platelet, ARB, sacubitril/valsartan, metformin, and SGLT2 inhibitor. In Models 1 and 2, the MHR was proven to be an independent risk factor for MACE, all-cause mortality, nonfatal MI, and any revascularization. Model 3, after adjusting for other variables, indicated that the MHR was an independent predictor for MACE (adjusted HR: 2.11; 95% CI 1.47–3.03; P < 0.001). The MHR was also an independent risk factor for all the defined components of MACE [for all-cause mortality (adjusted HR: 1.94 95% CI 1.14–3.29; P = 0.015), nonfatal MI (adjusted HR: 4.53 95% CI 1.66–12.39; P = 0.003), and any revascularization (adjusted HR: 1.93 95% CI 1.10–3.39; P = 0.023) (Table 3).
Kaplan‒Meier analysis according to MACE-free survival revealed a higher occurrence of MACE in MHR Quartile 4, followed by Quartiles 3, 2, and 1 (P < 0.0001) (Fig. 2A). Kaplan‒Meier analysis according to all-cause mortality-free survival revealed the highest occurrence of all-cause mortality in the MHR Quartile 4 group, followed by Quartiles 3, 2, and 1 (P = 0.0002) (Fig. 2B). Kaplan‒Meier curves based on the stratified values of MHR also showed that the patients with a higher MHR were found to have a significantly higher nonfatal-MI probability and revascularization probability (P = 0.001 and P < 0.0001) (Fig. 2C, D).
Kaplan‒Meier analysis. Kaplan‒Meier survival curves showing the incidence of major adverse cardiovascular events, all-cause mortality, nonfatal myocardial infarction, and any revascularization among ischaemic heart failure patients with diabetes mellitus. The curves were stratified into 4 quartiles by different levels of monocyte to high-density lipoprotein cholesterol ratio (MHR). The deep blue line is for Quartile 1 (Q1), crimson for Quartile 2 (Q2), green for Quartile 3 (Q3), and orange for Quartile 4 (Q4). Log-rank test P < 0.05 for each
Previous studies have established both hypercholesterolemia and the MHR as risk factors for MACE. To explore whether there are potential interaction effects between hypercholesterolemia and MHR, we conducted interaction relationship analysis based on Cox regression. We found no significant interaction effect between hypercholesterolemia and MHR (P for interaction > 0.05 in Models 1, 2, and 3). The existence of hypercholesterolemia did not significantly affect the association between MHR and MACE among the subjects in Models 1, 2, and 3. The results of the subgroup analysis are shown in Table 4. We also made further investigation into correlations between MHR and known risk factors of MACE. Spearman correlation analysis showed that MHR was positively correlated with BMI (r = 0.094, P = 0.003), HbA1c (r = 0.074, P = 0.018), homocysteine (r = 0.081, P = 0.009), haemoglobin (r = 0.065, P = 0.038), and hs-CRP (r = 0.374, P < 0.001), but negatively correlated with gender female (r = − 0.220, P < 0.001). No correlations were found between MHR levels and age, heart rate, hypertension, renal insufficiency, NYHA class, and LDL-C (Table 5). The heatmap of Spearman correlation coefficients is shown in Fig. 3.
Heatmap of Spearman correlations analysis. Spearman correlations between MHR and conventional risk factors for MACE. MHR, monocyte to high-density lipoprotein cholesterol ratio; MACE, major adverse cardiovascular events; HR, heart rate; BMI, body mass index; HP, hypertension; RI, renal insufficiency; NYHA, New York Heart Association; HBA1c, haemoglobin A1c; LDL-C, low-density lipoprotein cholesterol; Hcy, homocysteine; Hb, Haemoglobin; hs-CRP, high-sensitivity C-reactive protein
The restricted cubic spline curve based on Cox proportional hazards models was used to visualize the nonlinear relation of MHR with MACE. MHR showed an overall positive relationship with the risk of MACE in ischaemic HF patients with DM undergoing PCI. The curve increased rapidly with a steep slope until an MHR of approximately 0.65 was reached. When the MHR was higher than 0.65, the slope was relatively flat. This represented a significant increase in MACE risk when the MHR was lower than 0.65. The risk of MACE increased slowly when the MHR exceeded 0.65. Therefore, the MHR might have higher discrimination for the risk of MACE when the MHR was less than 0.65, while the discrimination was limited when the MHR had a higher value (Fig. 4).
Restricted cubic spline analysis. Multivariable adjusted hazards ratio for major adverse cardiovascular events according to the monocyte/high-density lipoprotein cholesterol ratio (MHR) on a continuous scale. The adjusted variables are age, sex, heart rate, body mass index, left ventricular end diastolic diameter, left main disease, chronic total occlusion, diffuse lesion, target vessel of left main artery, complete revascularization, number of stents, total bilirubin, direct bilirubin, total cholesterol, triglyceride glucose, platelet, angiotensin receptor blocker, sacubitril/valsartan, metformin, and sodium-dependent glucose transporters 2 inhibitor. The solid blue line represents the multivariable adjusted hazards ratio, and the light blue area represents the 95% confidence interval. The cut-off point of the MHR is 0.65
Discussion
To the best of our knowledge, this is the first study to implicate the MHR in an ischaemic HF cohort with DM. Furthermore, the MHR was also first proposed to be a predictor of MACEs in ischaemic HF patients with concomitant DM undergoing PCI. A higher MHR value was found to have a higher incidence of MACE. It was also determined that the MHR was an independent predictor of MACE in ischaemic HF patients with DM undergoing PCI and had no interaction with hypercholesterolemia.
The mortality of ischaemic HF has increased in recent decades by 20.6% [37, 38]. CAD is the leading cause of this condition [1]. It is well acknowledged that DM and CAD are homogeneous or equivalent. Additionally, DM and HF independently increase the risk for the other [4]. Meanwhile, most of them have received PCI treatment. Therefore, the population of ischaemic HF combined with DM undergoing PCI is an underestimated large group. Although all-cause chronic HF combined with DM has been widely investigated, the population of specific ischaemic HF combined with DM undergoing PCI has been investigated.
MHR, deprived from routine test items, is monocyte count divided by HDL-C. It has emerged as a marker for systemic oxidative stress and inflammation [23]. Monocytes have chemotaxis effects. Activated monocytes with oxidized LDL and other lipids transform into foam cells, taking an essential part in plaque formation, which leads to the deteriorating development of ACS and MACE [39]. In addition, activated monocytes differentiate into macrophages and trigger the release of various inflammatory cytokines in chronic heart diseases [40, 41]. In contrast, HDL-C has antioxidant and anti-inflammatory effects [42,43,44]. HDL-C was able to inhibit monocyte activation, adhesion, migration, and LDL-C oxidation [45, 46]. Murphy et al. found that HDL-C participated in the formation of apolipoprotein, which inactivated CD1b and acted on its anti-inflammatory effects [42]. In addition, HDL-C is also a well-known protective factor for cardiovascular disease [47]. Therefore, through the ratio of “oxidative and inflammatory promotion” and “oxidative and inflammatory inhibition”, the increasing MHR value represents the severity of inflammation, hence indicating exacerbations of HF, aggravated progression, and a higher incidence of MACE. In addition, the MHR also partially reflected the greater burden of comorbidities, such as DM, hypertension, obesity, and renal insufficiency, in patients with HF [30]. These comorbidities also caused a higher incidence of MACEs.
MHR as a prognostic factor for MACE after PCI in CAD has been investigated before. There are some studies consistent with ours. In patients with ACS undergoing PCI, the prognostic effects of the MHR have been demonstrated [20]. In patients with ST‐segment elevation myocardial infarction (STEMI) undergoing primary PCI who had elevated MHR values, the incidences of in-hospital or long-term MACE and the rates of short or late mortality were significantly higher [48,49,50]. In non-ST‐segment elevation myocardial infarction patients undergoing PCI, the MHR was also an independent risk factor for in-hospital MACE with an OR of 8.36, which exhibited similar predictive performance to STEMI [24]. Additionally, the MHR was associated with increased MACE risk after an adjusted HR of 2.734 in unstable angina patients scheduled for selective PCI [51]. These studies as well as our study indicate that the MHR has a close relationship with the CAD spectrum. MHR is also related to many cardiovascular disorders combined with DM. Previous studies have explored cardiovascular disorders combined with DM cohorts, such as non-ST-segment elevation acute coronary syndrome [24], subclinical left cardiac remodelling and dysfunction [30], and arterial stiffness [31]. All of them showed that the MHR is associated with the occurrence and outcome of certain cardiovascular disorders. However, the application value of the MHR in an ischaemic HF combined with DM cohort has never been investigated before. Therefore, our study filled the research gap and provided a rational background for the clinical management of this population.
Epidemiological research indicates a high prevalence of hypercholesterolemia in patients with concurrent ischaemic HF and DM. Previous studies have established both hypercholesterolemia and MHR as risk factors for adverse prognosis in these patients. Therefore, this study investigated the potential relationship between hypercholesterolemia and MHR. Hypercholesterolemia was identified as a confounding factor in the stepwise regression analysis. However, even after adjusting for hypercholesterolemia, MHR remained an independent risk factor for MACE in Model 3. Furthermore, there was no significant difference in the predictive ability of MHR between the subgroup of patients with hypercholesterolemia and the subgroup without hypercholesterolemia. These findings suggest that MHR possesses distinct underlying mechanisms for predicting MACE compared to hypercholesterolemia. Overall, hypercholesterolemia did not compromise the independent predictive capacity of MHR for MACE, as evidenced by both the subgroup analysis and multivariable regression analysis. These findings further strengthen the robustness of MHR in predicting MACE. However, prospective prespecified subgroup analysis is still necessary to validate the reliability of MHR and assess potential influences from other variables.
In Model 3, some adjusted variables were related to the comorbidities of obesity, abnormal liver function, hyperlipidaemia, and DM, such as body mass index, total bilirubin, direct bilirubin, TC, triglyceride, fast blood glucose, metformin, and SGLT2 inhibitor. They partly contribute to the incidence of MACE. Notably, other adjusted variables related to the severity of coronary artery lesions and the results of PCI procedures, such as left main artery disease, chronic total occlusion, diffuse lesion, target vessel of left main artery, complete revascularization, and number of stents, suggested that severe lesions had negative impacts on ischaemic HF and its prognosis, especially for lesions significantly reducing the myocardial blood supply, such as left main artery disease, chronic total occlusion, and diffuse lesion. In contrast, complete revascularization ameliorated these impacts. These findings are consistent with the aetiology and pathophysiology of ischaemic HF, as well as a previous study showing that the values of MHR were positively related to myocardial infarct sizes [19].
In our study, we found that the MHR may be a stronger predictor of MACE at MHR values lower than 0.65 in the restricted cubic spline model. One possible explanation is that the restricted cubic spline in this study was based on Model 3 and adjusted for several confounders, including well-established factors known to influence MACE, such as age, sex, TC, BMI, SGLT2 inhibitor, and sacubitril/valsartan. Adjusting for these factors diminished the predictive capacity of MHR for MACE, with this reduction being more pronounced at higher MHR values. This observation suggests that MHR is more susceptible to the influence of confounding factors at higher values. Therefore, caution should be exercised when applying MHR in practice, especially when it exhibits an exceptionally high value.
Additionally, we conducted comparisons of the discrimination ability of the MHR for incidence of MACE between cohorts of ischaemic HF with and without DM. Following the same exclusion criteria as illustrated in Fig. 1, we identified 2584 eligible cases for analysis in the ischaemic HF cohort. Kaplan–Meier curves were generated to illustrate the incidence rates of MACE in different MHR quartiles within the ischaemic HF cohort. The MHR exhibited superior discrimination in predicting observed outcomes in the ischaemic HF cohort with DM compared to the ischaemic HF cohort alone (see Fig. 2 and Additional file 1: Fig. S1). These findings suggest that MHR performs well in patients with ischaemic HF and DM, indicating that this particular cohort may derive greater benefit from the predictive ability of MHR.
There are some limitations in this study. Given that this study is a single-centre study, the generalizability of the conclusions derived from this research to other centres or regions may be limited. Further data from additional centres are necessary in the future to enhance the robustness and applicability of our findings. Due to the characteristics of retrospective studies, the results might have been affected by recall deviation, especially medication use. Besides, we were unable to further compare the predictive role of MHR for MACE in ischaemic HF cohort and ischaemic HF with DM cohort because of the incomprehensive clinical baseline data in ischaemic HF cohort.
Conclusion
In conclusion, a high MHR is an independent predictor of MACE, all-cause mortality, nonfatal MI, and any revascularization at 36 months in ischaemic HF combined with DM patients who have undergone PCI, suggesting that the MHR might be a promising predictor for identifying this population with a higher risk of poor clinical outcomes after PCI treatment.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ACEI:
-
Angiotensin-converting enzyme inhibitor
- ACS:
-
Acute coronary syndrome
- ALT:
-
Aspartate transaminase
- ARB:
-
Angiotensin receptor blocker
- AST:
-
Alkaline phosphatase
- BMI:
-
Body mass index
- BNP:
-
B-natriuretic peptide
- CAD:
-
Coronary artery disease
- CI:
-
Confidential interval
- DDP4:
-
Dipeptidyl peptidases inhibitor 4
- DM:
-
Diabetes mellitus
- eGFR:
-
Estimated glomerular filtration rate
- FBG:
-
Fibrinogen
- GGT:
-
Gamma-glutamyl transferase
- HDL-C:
-
High-density lipoprotein cholesterol
- HF:
-
Heart failure
- hs-CRP:
-
High-sensitivity C-reactive protein
- HR:
-
Hazards ratio
- INR:
-
International normalized ratio
- LAD:
-
Left anterior descending artery
- LCX:
-
Left circumflex artery
- LDL-C:
-
Low-density lipoprotein cholesterol
- LM:
-
Left main artery
- LVDd:
-
Left ventricular end diastolic dimension
- LVDs:
-
Left ventricular end systolic dimension
- LVEF:
-
Left ventricular ejection fraction
- MACE:
-
Major adverse cardiovascular events
- MHR:
-
Monocyte/HDL-C ratio
- MI:
-
Myocardial infarction
- NHR:
-
Neutrophil to high-density lipoprotein ratio
- NLR:
-
Neutrophil to lymphocyte ratio
- NYHA:
-
New York Heart Association
- PCI:
-
Percutaneous coronary intervention
- RCA:
-
Right coronary artery
- SGLT2:
-
Sodium-dependent glucose transporters 2
- STEMI:
-
ST‐segment elevation myocardial infarction
- SYNTAX:
-
Synergy between PCI with taxus and cardiac surgery
- TC:
-
Total cholesterol
References
Truby LK, Rogers JG. Advanced heart failure: epidemiology, diagnosis, and therapeutic approaches. JACC Heart Fail. 2020;8(7):523–36.
Roger VL. Epidemiology of heart failure: a contemporary perspective. Circ Res. 2021;128(10):1421–34.
Adams KF Jr, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2005;149(2):209–16.
Chia YMF, et al. Clinical correlates and pharmacological management of Asian patients with concomitant diabetes mellitus and heart failure. Heart Fail Rev. 2018;23(3):461–8.
Dei Cas A, et al. Concomitant diabetes mellitus and heart failure. Curr Probl Cardiol. 2015;40(1):7–43.
From AM, et al. Diabetes in heart failure: prevalence and impact on outcome in the population. Am J Med. 2006;119(7):591–9.
Cleland JG, et al. The EuroHeart Failure survey programme—a survey on the quality of care among patients with heart failure in Europe. Part 1: patient characteristics and diagnosis. Eur Heart J. 2003;24(5):442–63.
McAllister DA, et al. Incidence of hospitalization for heart failure and case-fatality among 3.25 million people with and without diabetes mellitus. Circulation. 2018;138(24):2774–86.
Kannel WB, McGee DL. Diabetes and cardiovascular disease The Framingham study. JAMA. 1979;241(19):2035–8.
Bahrami H, et al. Novel metabolic risk factors for incident heart failure and their relationship with obesity: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;51(18):1775–83.
Gottdiener JS, et al. Predictors of congestive heart failure in the elderly: the Cardiovasc Health Study. J Am Coll Cardiol. 2000;35(6):1628–37.
Targher G, et al. In-hospital and 1-year mortality associated with diabetes in patients with acute heart failure: results from the ESC-HFA Heart Failure Long-Term Registry. Eur J Heart Fail. 2017;19(1):54–65.
Johansson I, et al. Prognostic implications of type 2 diabetes mellitus in ischemic and nonischemic heart failure. J Am Coll Cardiol. 2016;68(13):1404–16.
Daubert MA, et al. High-risk percutaneous coronary intervention is associated with reverse left ventricular remodeling and improved outcomes in patients with coronary artery disease and reduced ejection fraction. Am Heart J. 2015;170(3):550–8.
Sawada SG, et al. Effect of revascularization on long-term survival in patients with ischemic left ventricular dysfunction and a wide range of viability. Am J Cardiol. 2010;106(2):187–92.
Perera D, et al. Percutaneous revascularization for ischemic ventricular dysfunction: rationale and design of the REVIVED-BCIS2 trial: percutaneous coronary intervention for ischemic cardiomyopathy. JACC Heart Fail. 2018;6(6):517–26.
Perera D, et al. Percutaneous revascularization for ischemic left ventricular dysfunction. N Engl J Med. 2022;387(15):1351–60.
Ganjali S, et al. Monocyte-to-HDL-cholesterol ratio as a prognostic marker in cardiovascular diseases. J Cell Physiol. 2018;233(12):9237–46.
Eyyupkoca F, et al. Admission monocyte/HDL ratio predicts adverse cardiac remodeling after St-elevation myocardial infarction. Rev Invest Clin. 2022;74(2):104–12.
Yu R, et al. Correlation between monocyte to high-density lipoprotein ratio and major adverse cardiovascular events in patients with acute coronary syndrome after percutaneous coronary intervention. Pak J Med Sci. 2021;37(3):885–9.
Zhang DP, et al. An elevated monocyte-to-high-density lipoprotein-cholesterol ratio is associated with mortality in patients with coronary artery disease who have undergone PCI. Biosci Rep. 2020;40(8):BSR20201108.
Sercelik A, et al. Increased monocyte to high-density lipoprotein cholesterol ratio is associated with TIMI risk score in patients with ST-segment elevation myocardial infarction. Rev Port Cardiol (Engl Ed). 2018;37(3):217–23.
Oylumlu M, et al. Monocyte to high-density lipoprotein cholesterol and lymphocyte to monocyte ratios are predictors of in-hospital and long-term mortality in patients with acute coronary syndrome. Int J Clin Pract. 2021;75(5):e13973.
Li C, et al. The monocyte to high-density lipoprotein cholesterol ratio and outcomes in type 2 diabetes mellitus patients with non-ST-segment elevation acute coronary syndrome. Ann Transl Med. 2021;9(21):1627.
Villanueva DLE, et al. Monocyte to high-density lipoprotein ratio (MHR) as a predictor of mortality and major adverse cardiovascular events (MACE) among ST elevation myocardial infarction (STEMI) patients undergoing primary percutaneous coronary intervention: a meta-analysis. Lipids Health Dis. 2020;19(1):55.
Liu HT, et al. Monocyte to high-density lipoprotein ratio predict long-term clinical outcomes in patients with coronary heart disease: a meta-analysis of 9 studies. Medicine (Baltimore). 2022;101(33):e30109.
Sun M, et al. Prognostic utility of monocyte to high-density lipoprotein ratio in patients with acute coronary syndrome: a meta-analysis. Am J Med Sci. 2020;359(5):281–6.
Wang R, et al. Application value of systemic inflammatory indexes in the clinical evaluation of patients with heart failure with preserved ejection fraction (HFpEF). Medicina (Kaunas). 2022;58(10):1473.
Liu Z, et al. Associations of systemic inflammatory markers with the risks of chronic heart failure: a case-control study. Clinics (Sao Paulo). 2022;77:100056.
Zhong J, et al. Monocyte to high-density lipoprotein cholesterol ratio is associated with subclinical left cardiac remodeling and dysfunction in type 2 diabetes mellitus. Int Heart J. 2022;63(3):524–30.
Mayasari DS, et al. Association of monocyte-to-high density lipoprotein ratio with arterial stiffness in patients with diabetes. BMC Cardiovasc Disord. 2021;21(1):362.
Völz S, et al. Long-term mortality in patients with ischaemic heart failure revascularized with coronary artery bypass grafting or percutaneous coronary intervention: insights from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR). Eur Heart J. 2021;42(27):2657–64.
Alfonso F, et al. Current treatment of in-stent restenosis. J Am Coll Cardiol. 2014;63(24):2659–73.
Sianos G, et al. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1(2):219–27.
Chinese Society of Cardiology, Chinese Medical Association, Editorial Board of Chinese Journal of Cardiology. Chinese guideline for percutaneous coronary intervention in patients with left main bifurcation disease. Zhonghua Xin Xue Guan Bing Za Zhi. 2022;50(4):349–60.
Thygesen K, et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018;72(18):2231–64.
Zhou M, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394(10204):1145–58.
Fihn SD, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2014;130(19):1749–67.
Kratofil RM, et al. Monocyte conversion during inflammation and injury. Arterioscler Thromb Vasc Biol. 2017;37(1):35–42.
Tabas I, et al. Macrophage phenotype and function in different stages of atherosclerosis. Circ Res. 2016;118(4):653–67.
Tang J, et al. Inhibiting macrophage proliferation suppresses atherosclerotic plaque inflammation. Sci Adv. 2015;1(3):e1400223.
Murphy AJ, et al. High-density lipoprotein: a potent inhibitor of inflammation. Clin Exp Pharmacol Physiol. 2010;37(7):710–8.
Barter PJ, et al. Antiinflammatory properties of HDL. Circ Res. 2004;95(8):764–72.
Xepapadaki E, et al. Τhe antioxidant function of HDL in atherosclerosis. Angiology. 2020;71(2):112–21.
Xiang Y, et al. Lower HDL-C levels are associated with higher expressions of CD16 on monocyte subsets in coronary atherosclerosis. Int J Med Sci. 2020;17(14):2171–9.
Nazir S, et al. Interaction between high-density lipoproteins and inflammation: Function matters more than concentration! Adv Drug Deliv Rev. 2020;159:94–119.
Rader DJ, et al. HDL and cardiovascular disease. Lancet. 2014;384(9943):618–25.
Çiçek G, et al. The relationship between admission monocyte HDL-C ratio with short-term and long-term mortality among STEMI patients treated with successful primary PCI. Coron Artery Dis. 2016;27(3):176–84.
Karataş MB, et al. Monocyte to high-density lipoprotein ratio as a new prognostic marker in patients with STEMI undergoing primary percutaneous coronary intervention. Am J Emerg Med. 2016;34(2):240–4.
Açıkgöz SK, et al. Monocyte to high-density lipoprotein cholesterol ratio is predictive of in-hospital and five-year mortality in ST-segment elevation myocardial infarction. Cardiol J. 2016;23(5):505–12.
Zhang Y, et al. Is monocyte to HDL ratio superior to monocyte count in predicting the cardiovascular outcomes: evidence from a large cohort of Chinese patients undergoing coronary angiography. Ann Med. 2016;48(5):305–12.
Acknowledgements
The authors are grateful to all the participants in this study.
Funding
This research was funded by the National Natural Science Foundation of China (Nos. 81971302 and 81771494, both to Qian Fan).
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study conception and design. QF, DHZ, JHL, and XLL performed the PCI procedures. QYL, XLL, FQL, XGM, XWB, and SYC collected the demographic characteristics and angiographic characteristics data. QYL performed data analysis. QYL wrote the first draft of the manuscript. QF and JHL revised it. All the authors commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Ethics Committee of Beijing Anzhen Hospital, Beijing, China, approved this study. Informed consent was obtained from all participants in this analysis. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (No. 2022235X).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information

Additional file 1
: Fig. S1. Kaplan–Meier analysis. Kaplan–Meier survival curves showing the incidence of major adverse cardiovascular events, all-cause mortality, nonfatal myocardial infarction, and any revascularization among the ischaemic heart failure patients. The curves were stratified by 4 quartiles by different levels of monocyte to high-density lipoprotein cholesterol ratio (MHR). Deep blue line is for Quartile 1, crimson for Quartile 2, green for Quartile 3, and orange for Quartile 4.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, Q., Lin, X., Bo, X. et al. Monocyte to high-density lipoprotein cholesterol ratio predicts poor outcomes in ischaemic heart failure patients combined with diabetes: a retrospective study. Eur J Med Res 28, 493 (2023). https://doi.org/10.1186/s40001-023-01451-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-023-01451-6