Abstract
Background
Fatal forms of strongyloidiasis, hyperinfection syndrome (HS) and disseminated strongyloidiasis (DS), are caused by exaggerated autoinfection of the intestinal nematode, Strongyloides stercoralis (S. stercoralis). Corticosteroids, frequently administered to patients with severe COVID-19, can transform chronic asymptomatic strongyloidiasis into the above-mentioned fatal diseases. This study aimed to investigate the prevalence of strongyloidiasis in COVID-19 patients receiving corticosteroids in a hypoendemic region.
Methods
The present cross-sectional study enrolled 308 COVID-19 patients admitted to two hospitals in Ahvaz and Abadan in the southwest of Iran between 2020 and 2022. A real-time reverse transcription polymerase chain reaction (RT-PCR) test and chest computed tomography (CT) scan were employed to detect and monitor the disease’s severity in the patients, respectively. All patients were evaluated for IgG/IgM against S. stercoralis using Enzyme-linked immunosorbent assay (ELISA) test. Subsequently, individuals with a positive ELISA test were confirmed using parasitological methods, including direct smear and agar plate culture (APC).
Results
The patients were between 15 and 94 years old, with a mean age of 57.99 ± 17.4 years. Of the 308 patients, 12 (3.9%) had a positive ELISA test, while 296 (96.1%) had negative results. Three of the 12 patients with a positive ELISA result died, and three failed to provide a stool sample. To this end, only six cases were examined parasitologically, in which S. stercoralis larvae were observed in five patients. Significant differences were found between S. stercoralis infection with sex (p = 0.037) and age (p = 0.027). Binary regression analysis revealed that strongyloidiasis was positively associated with sex (odds ratio [OR]: 5.137; 95% confidence interval [CI]: 1.107–23.847), age (OR: 5.647; 95% CI 1.216–26.218), and location (OR: 3.254; 95% CI: 0.864–12.257).
Conclusions
Our findings suggest that screening for latent strongyloidiasis in COVID-19 patients in endemic areas using high-sensitivity diagnostic methods, particularly ELISA, before receiving suppressive drugs should be given more consideration.
Similar content being viewed by others
Background
Strongyloides stercoralis (S. stercoralis), a soil-borne intestinal nematode, has a unique life cycle with the potential of autoinfection. The parasite-induced infection can range from asymptomatic chronic infection to fatal disseminated infection. Evidence suggests that immunosuppression caused by corticosteroid use, one of the main risk factors, can lead to uncontrolled proliferation of the nematode in asymptomatic patients, resulting in severe strongyloidiasis, hyperinfection syndrome/ disseminated syndrome (HS/DS), with mortality rate of up to 100% [1, 2]. On the other hand, chronic strongyloidiasis is frequently misdiagnosed due to the low sensitivity of routine parasite diagnostic tests, such as direct smear examination [3]. Thus, diagnosing the infection is typically challenging before and during immunosuppression [4]. Serial stool examinations, culturing on nutrient agar plate, or searching for specific antibodies in the serum using the enzyme-linked immunosorbent assay (ELISA) technique can increase the likelihood of detecting these cases [5, 6].
Coronavirus disease 2019 (COVID-19) is a respiratory disease induced by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Diagnosis of the disease is mainly based on viral nucleic acid detection using reverse transcription polymerase chain reaction (RT-PCR) and chest computed tomography (CT) [7]. The overproduction of the pro-inflammatory cytokines, also known as cytokine storm syndrome (CSS), is an important manifestation of critical COVID-19 [8]. Patients with moderate or severe infections are often prescribed cytokine inhibitors, such as tocilizumab (TCZ), and the corticosteroid dexamethasone due to the tissue damage caused by this phenomenon, particularly in the respiratory system [8,9,10,11]. Using these medications in patients co-infected with SARS-CoV-2, and S. stercoralis can reactivate latent strongyloidiasis [9]. Several cases of severe strongyloidiasis in natives of or recent arrivals to endemic areas were reported during the COVID-19 pandemic [12,13,14,15].
Considering the background provided above, screening for strongyloidiasis in COVID-19 patients, increasing public health system and healthcare provider awareness, and refining diagnostic approaches for S. stercoralis detection are essential [16].
Iran is a hypoendemic area for strongyloidiasis, with a prevalence rate of 4.8% (2.5–7.2%) [17]. In immunocompetent and immunocompromised Iranian populations, the prevalence of strongyloidiasis has been estimated to be 2% and 4%, respectively [18]. Due to the region’s humid and tropical climate, the prevalence of strongyloidiasis in Khuzestan province is 8.7% using serological methods and 2.7% using parasitological methods [3]. In addition, Khuzestan was severely impacted by the COVID-19 pandemic and was highly prevalent among patients [19]. As a result, the current study aimed to determine the prevalence of S. stercoralis infection in COVID-19 patients admitted to the province's two regional hospitals.
Methods
Study location
With a population of over 4,700,000 and an area spanning 63,238 Km2 [19], Khuzestan province is one of the most important agricultural and industrial regions in southwestern Iran [20]. Due to the region’s hot and humid climate, most neglected tropical diseases (NTDs) may be endemic in the area [3]. To this end, the current study was conducted in two of Iran’s most populous counties, Ahvaz and Abadan.
Study design and sampling
During multiple peaks of the coronavirus pandemic from 2020 to 2022, this cross-sectional study was conducted on patients hospitalized with moderate to severe COVID-19 in Ahvaz and Abadan counties, southwestern Iran, where strongyloidiasis is endemic. The inclusion criteria were hospitalized patients with COVID-19 who had a fever, headache, sore throat, cough, chills, rhinorrhea, and respiratory distress and whose infection with SARS-CoV-2 was confirmed using an RT-PCR test on nasopharyngeal swabs. A chest CT scan was utilized to assess the severity of the disease. According to their electronic medical records, all patients had received dexamethasone and tocilizumab. Patients who received antiparasitic medications within the previous six months were excluded from the study.
The required sample size was determined using single population proportion formula: (Zα/2)2 × p × (1 − p)/d2, where n is the sample size, z is the standard normal score set at 1.96, d is the desired degree of accuracy and p is the estimated proportion of the target population. By taking a prevalence rate of 8.7% [3], Zα/2 = 1.96 and 95% confidence interval, and d = 0.033 the computed sample size was 305. Using simple random sampling, finally 308 patients, including 157 from Ahvaz and 151 from Abadan, were selected and included in the study. Twenty cases were randomly selected each week, and five patients were enrolled in the study using a lottery system based on the inclusion criteria.
Data collection
A questionnaire was used to collect demographic data such as age, sex, and place of residence, but clinical and laboratory findings, as well as information about medications used, were obtained from the hospital’s electronic database.
Enzyme-linked immunosorbent assay (ELISA)
To determine the prevalence of S. stercoralis/SARS-CoV-2 co-infections, all COVID-19 patients were screened for S. stercoralis infection using ELISA test. Each patient had 2 mL of venous blood drawn and centrifuged at 2000×g for 5 min; the isolated sera were stored at − 20 °C until ELISA testing. The positive and equivocal ELISA test patients were subsequently examined using parasitological techniques, such as direct smear and agar plate culture (APC), for additional confirmation.
The test was conducted using an IgG/IgM ELISA kit (NovaTec Immundiagnostica GmbH, Dietzenbach, Germany) according to the manufacturer’s instructions. The plate was coated with recombinant immunodiagnostic antigens (NIE) that had a diagnostic sensitivity and specificity of 89.47% (95% CI: 75.2–97.06%) and 94.12% (95% CI: 83.76–98.77%) respectively. A DS2® ELISA reader (Dynex, VA, USA) measured optical density (OD) at 450 nm. The cut-off point was set at 11 NovaTec Units (NTU). A 9–11 NTU range was considered equivocal, while less than 9 NTU was considered negative.
Direct smear examination and agar plate culture (APC)
All patients who tested positive for strongyloidiasis or were suspected of having the disease based on ELISA results were asked to provide stool samples. Each patient provided two stool samples on separate days, which were analyzed by direct smear and APC to confirm the ELISA results. Four slides were prepared from each sample for direct smear examinations and examined at low magnification to increase the sensitivity of larva detection. APC was determined by placing 3–4 g of fresh stool sample on the center of a nutrient agar plate. The plates’ lids were then sealed to prevent the larvae from escaping. The plates were then placed in a plastic box and incubated for 1 week at 25–30 °C.
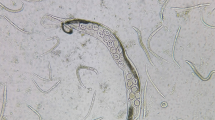
The plates were examined macroscopically and microscopically from the third day for the presence of the characteristic tracks and juvenile and adult stages of the parasite (Fig. 1). The surfaces of the plates were then washed with a formalin solution containing 10% formaldehyde, and the resulting solution was centrifuged at 1500 rpm for 5 min. The obtained sediments were examined using a light microscope for the presence of larvae or adult worms. The second-stage larvae (rhabditiform larvae) could be distinguished by their short buccal cavity, bulbous esophagus, and genital primordium. The third-stage larvae (filariform larvae) could be identified by their closed buccal cavity, long esophagus, and notched tail (Fig. 2) [21].
Statistical analysis
All data were analyzed using the SPSS version 26.0 software package (IBM, Chicago, IL, USA). Chi-square (χ2) test or Fisher’s exact test was used for comparisons. In addition, Logistic regression models was used to determine Odds ratios (ORs) with 95% confidence intervals (CIs). A two-tailed p < 0.05 was considered statistically significant.
Results
Of the 308 patients who participated in this study, 157 were admitted to a referral hospital for COVID-19 in Ahvaz, and the remaining patients were hospitalized in a hospital dedicated to COVID-19 in Abadan.
Patients enrolled in the study were aged 15–94, where the mean age of the participants was 57.99 ± 17.4 years. In addition, there were 156 (50.6%) male patients and 152 (49.4%) female patients. Furthermore, 12 patients (3.9%) had a positive ELISA test, 4 cases (1.3%) were equivocal, and 292 (94.8%) had negative ELISA tests. The ELISA test was repeated 2 to 4 weeks later in the four cases with equivocal results. However, because no rising antibody titer was found, it was deemed negative according to the kit protocol; nevertheless, parasitological tests were also performed, and all were negative (Table 1). Of the 12 ELISA-positive cases, three patients died prior to parasitological testing, three failed to provide stool samples, and only six underwent stool examinations. Two patients were found to be infected with S. stercoralis using both the direct smear examination and the APC method. However, only APC confirmed the presence of S. stercoralis infection in three cases (Table 2).
In this study, there were statistically significant differences between S. stercoralis infection and sex (p = 0.037) and age (p = 0.027). However, there was no meaningful relationship between strongyloidiasis and the residence location of the patients (p = 0.081). Of the 12 cases with positive ELISA results, 9 (75.0%) resided in Abadan. The analysis confirmed the significant association between strongyloidiasis and sex (OR: 5.137; 95% CI 1.107–23.847), age (OR: 5.647; 95% CI 1.216–26.218), and location (OR: 3.254; 95% CI 0.864–12.257) (Table 1).
The five patients diagnosed with strongyloidiasis were administered 200 μg/kg/day ivermectin orally for 2 days, followed by another dose 2 weeks later [22]. In addition, they were followed up with APC and direct smear examination after one month, and all of their test results were negative.
Discussion
Despite the strong emphasis on screening immunocompromised patients for asymptomatic strongyloidiasis due to the transformation of chronic strongyloidiasis to HS, clinicians in the clinic do not give this subject enough consideration, particularly in S. stercoralis endemic areas [1, 2].
During the coronavirus pandemic, the risk of HS increased, particularly in endemic regions, due to the increased use of tocilizumab and immunosuppressive drugs such as dexamethasone in COVID-19 patients [9, 14, 23]. Some evidence indicated that a dexamethasone dose of 6 mg/day for ten days (≈ 40 mg of prednisone) could induce HS in patients with chronic strongyloidiasis [24]. In addition, the accessibility and low cost of corticosteroids such as dexamethasone in most S. stercoralis endemic regions may increase the likelihood of this risk [24].
In the present study, a seroprevalence of 3.9% for strongyloidiasis underscores the significance and necessity of screening and treatment for co-infection with strongyloidiasis and COVID-19. Several cases of hyperinfection or disseminated infection in COVID-19-positive patients have been reported over the past 3 years. These severe conditions were observed during hospitalization or shortly after patients’ discharge [9, 25,26,27].
Strongyloidiasis is typically asymptomatic and challenging to diagnose using routine parasitological methods such as the direct smear method. Diagnostic methods with high sensitivity, particularly APC, are important when diagnosing patients; however, these methods are time-consuming and laborious and require fresh stool samples [21, 28]. In our study, the severity of gastrointestinal symptoms, specifically diarrhea, was observed in two patients due to an increasing parasite load and abundant larval excretion, which was easily detectable using the direct smear technique. However, in the remaining positive cases, only APC confirmed the presence of S. stercoralis infection.
The higher sensitivity and negative predictive value (NPV) of the ELISA test in detecting the mild form of strongyloidiasis, as well as the easier use of blood sampling for critically ill patients as opposed to stool sampling, have rendered the ELISA test a suitable method for screening for strongyloidiasis in COVID-19 patients [29].
The co-infection of nematode and virus is debatable in two additional ways: decreased eosinophil levels in the peripheral blood of COVID-19 patients and overlap of pulmonary and gastrointestinal symptoms during the acute phases of both infections [9, 30, 31].
In addition to its protective role in strongyloidiasis, eosinophilia is regarded as a screening factor with a high positive predictive value for asymptomatic patients. On the other hand, researchers recognize eosinopenia as a sign of the progression to severe cases of strongyloidiasis [28, 32,33,34]. Previous research indicates that eosinopenia is a common laboratory finding in 50–70% of hospitalized COVID-19 patients with mild to moderate symptoms [35, 36]. It is believed that the migration of eosinophils from the peripheral blood to the interstitial space and pulmonary alveoli, the decrease in the production of these cells, and their suppression by endogenous glucocorticoids are the leading causes of this decrease [37,38,39]. Some seropositive patients in the present study had a history of eosinophilia. Still, the rate of peripheral blood eosinophils in most of them had decreased significantly during hospitalization due to SARS-CoV-2 infection.
Corticosteroid therapy decreased the eosinophil count in a COVID-19 patient with a history of chronic hypereosinophilia due to strongyloidiasis, as reported by Stylemans et al. [40]. In addition, Feria et al. (2022) and Lier et al. (2020) found no increase in the number of peripheral blood eosinophils in patients co-infected with Strongyloides and COVID-19 [13, 14, 25].
Another attribute of strongyloidiasis-COVID-19 co-infection is the overlap of pulmonary and gastrointestinal manifestations caused by COVID-19 and those induced by HS, such as coughing, dyspnea, diarrhea, and fever [23]. In these instances, the physician will likely disregard the reactivation of Strongyloides. As a result, the patient is not referred to the parasitology laboratories and continues to take corticosteroids, which can exacerbate the infection [41].
Intriguingly, ten of the twelve positive ELISA cases involved patients older than 60. These findings are consistent with previous research indicating that a significant proportion of strongyloidiasis patients co-infected with COVID-19 are older adults [26, 27, 40, 42]. The higher prevalence of strongyloidiasis in elderly populations [43] can overlap with the higher COVID-19 mortality in these patients [44].
Similar to previous studies [45, 46] S. stercoralis infection was correlated with sex. The higher prevalence of infection in males may be attributable to prolonged contact with soil in occupations such as agriculture [47].
Strengths and limitations
This study was one of the few studies conducted to screen strongyloidiasis in COVID-19 patients in an endemic region with serological and parasitological methods. The study had several limitations. First, the patient's medical records information could not be accessed. Second, the study was limited by the inability to conduct parasitological tests on some seropositive cases due to patient non-cooperation or death. Third, we were unable to evaluate the potential relationship between steroid dose and treatment of COVID-19 patients infected with S. stercoralis; therefore, future research is required.
Conclusion
In conclusion, our findings demonstrated that screening for latent strongyloidiasis using high-sensitivity diagnostic methods, particularly ELISA, in COVID-19 patients before receiving suppressive drugs in endemic areas should be given greater consideration.
Availability of data and materials
All data analyzed during this study are included in this manuscript.
Abbreviations
- S. stercoralis :
-
Strongyloides stercoralis
- APC:
-
Agar plate culture
- CSS:
-
Cytokine storm syndrome
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- TCZ:
-
Tocilizumab
- ARDS:
-
Acute Respiratory Distress Syndrome
- ELISA:
-
Enzyme-linked immunosorbent assay
- NIE:
-
Recombinant immunodiagnostic antigens
- OD:
-
Optical density
- NTU:
-
NovaTec units
- NPV:
-
Negative predictive value
References
Nutman TB. Human infection with Strongyloides stercoralis and other related Strongyloides species. Parasitology. 2017;144(3):263–73.
Mejia R, Nutman TB. Screening, prevention, and treatment for hyperinfection syndrome and disseminated infections caused by Strongyloides stercoralis. Curr Opin Infect Dis. 2012;25(4):458–63.
Ashiri A, Rafiei A, Beiromvand M, Khanzadeh A, Alghasi A. Screening of Strongyloides stercoralis infection in high-risk patients in Khuzestan Province, Southwestern Iran. Parasit Vectors. 2021;14(1):37.
Keiser PB, Nutman TB. Strongyloides stercoralis in the immunocompromised population. Clin Microbiol Rev. 2004;17(1):208–17.
Khieu V, Schär F, Marti H, Sayasone S, Duong S, Muth S, et al. Diagnosis, treatment and risk factors of Strongyloides stercoralis in schoolchildren in Cambodia. PLoS Negl Trop Dis. 2013;7(2): e2035.
Buonfrate D, Formenti F, Perandin F, Bisoffi Z. Novel approaches to the diagnosis of Strongyloides stercoralis infection. Clin Microbiol Infect. 2015;21(6):543–52.
Umakanthan S, Sahu P, Ranade AV, Bukelo MM, Rao JS, Abrahao-Machado LF, et al. Origin, transmission, diagnosis and management of coronavirus disease 2019 (COVID-19). Postgrad Med J. 2020;96(1142):753–8.
Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20(5):269–70.
Marchese V, Crosato V, Gulletta M, Castelnuovo F, Cristini G, Matteelli A, et al. Strongyloides infection manifested during immunosuppressive therapy for SARS-CoV-2 pneumonia. Infection. 2021;49(3):539–42.
Fara A, Mitrev Z, Rosalia RA, Assas BM. Cytokine storm and COVID-19: a chronicle of pro-inflammatory cytokines. Open Biol. 2020;10(9):200160.
Radbel J, Narayanan N, Bhatt PJ. Use of Tocilizumab for COVID-19-induced cytokine release syndrome: a cautionary case report. Chest. 2020;158(1):e15–9.
Patel A, Bender W, Gonzalez E, Williamson M. A case of disseminated strongyloidiasis during treatment for COVID-19. Chest. 2021;160(4):A278.
Gautam D, Gupta A, Meher A, Siddiqui F, Singhai A. Corticosteroids in Covid-19 pandemic have the potential to unearth hidden burden of strongyloidiasis. IDCases. 2021;25: e01192.
Lier AJ, Tuan JJ, Davis MW, Paulson N, McManus D, Campbell S, et al. Case report: disseminated strongyloidiasis in a patient with COVID-19. Am J Trop Med Hyg. 2020;103(4):1590–2.
Salam R, Sharaan A, Jackson SM, Solis RA, Zuberi J. Strongyloides hyperinfection syndrome: a curious case of asthma worsened by systemic corticosteroids. Am J Case Rep. 2020;21:e925221.
Tilli M, Olliaro P, Gobbi F, Bisoffi Z, Bartoloni A, Zammarchi L. Neglected tropical diseases in non-endemic countries in the era of COVID-19 pandemic: the great forgotten. J Travel Med. 2021;28(1)
Buonfrate D, Bisanzio D, Giorli G, Odermatt P, Fürst T, Greenaway C, et al. The global prevalence of Strongyloides stercoralis infection. Pathogens. 2020;9(6):468.
Eslahi AV, Olfatifar M, Houshmand E, Johkool MG, Zibaei M, Foroutan M, et al. Prevalence of Strongyloides stercoralis in the immunocompetent and immunocompromised individuals in Iran: a systematic review and meta-analysis. Trans R Soc Trop Med Hyg. 2022;116(2):87–99.
Zarei J, Dastoorpoor M, Jamshidnezhad A, Cheraghi M, Sheikhtaheri A. Regional COVID-19 registry in Khuzestan, Iran: a study protocol and lessons learned from a pilot implementation. Inform Med Unlocked. 2021;23:100520.
Moradi-Majd N, Fallah-Ghalhari G, Chatrenor M. Estimation of greenhouse gas emission flux from agricultural lands of Khuzestan province in Iran. Environ Monit Assess. 2022;194(11):811.
Inês Ede J, Souza JN, Santos RC, Souza ES, Santos FL, Silva ML, et al. Efficacy of parasitological methods for the diagnosis of Strongyloides stercoralis and hookworm in faecal specimens. Acta Trop. 2011;120(3):206–10.
Covid O, Table SA. Ivermectin treatment for Strongyloides infection in patients with COVID-19. Can Commun Dis Rep. 2021;47(7–8):316–21.
Luvira V, Siripoon T, Phiboonbanakit D, Somsri K, Watthanakulpanich D, Dekumyoy P. Strongyloides stercoralis: a neglected but fatal parasite. Trop Med Infect Dis. 2022;7(10):310.
Stauffer WM, Alpern JD, Walker PF. COVID-19 and dexamethasone: a potential strategy to avoid steroid-related Strongyloides hyperinfection. JAMA. 2020;324(7):623–4.
Feria L, Torrado M, Anton-Vazquez V. Reactivation of Strongyloides stercoralis in patients with SARS-CoV-2 pneumonia receiving dexamethasone. Med Clin (Engl Ed). 2022;158(5):242–3.
Kim JM, Sivasubramanian G. Strongyloides hyperinfection syndrome among COVID-19 patients treated with corticosteroids. Emerg Infect Dis. 2022;28(7):1531–3.
Babazadeh S, Shokri-Shirvani J, Ranaee M. Strongyloides hyperinfection syndrome following corticosteroid therapy in a patient with COVID-19 infection: a case report. Iran J Med Microbiol. 2022;16(3):267–70.
Requena-Méndez A, Chiodini P, Bisoffi Z, Buonfrate D, Gotuzzo E, Muñoz J. The laboratory diagnosis and follow up of strongyloidiasis: a systematic review. PLOS Negl Trop Dis. 2013;7(1): e2002.
Buonfrate D, Formenti F, Perandin F, Bisoffi Z. Novel approaches to the diagnosis of Strongyloides stercoralis infection. Clin Microbiol Inf. 2015;21(6):543–52.
Soni M. Evaluation of eosinopenia as a diagnostic and prognostic indicator in COVID-19 infection. Int J Lab Hematol. 2021;43(Suppl 1):137–41.
Shirley DA, Moonah S. COVID-19 and corticosteroids: Unfamiliar but potentially fatal infections that can arise following short-course steroid treatment. Am J Trop Med Hyg. 2021;104(3):790–3.
Marcos LA, Terashima A, Canales M, Gotuzzo E. Update on strongyloidiasis in the immunocompromised host. Curr Infect Dis Rep. 2011;13(1):35–46.
Farthing M, Albonico M, Bisoffi Z, Bundy D, Buonfrate D, Chiodini P, et al. World Gastroenterology Organisation Global Guidelines: management of strongyloidiasis February 2018—Compact Version. J Clin Gastroentoerol. 2020;54(9):747–57.
Jenks NP, Driscoll B, Locke T. Strongyloidiasis hyperinfection syndrome in COVID-19 positive migrants treated with corticosteroids. J Immigr Minor Health. 2022;24(6):1431–4.
Xie G, Ding F, Han L, Yin D, Lu H, Zhang M. The role of peripheral blood eosinophil counts in COVID-19 patients. Allergy. 2021;76(2):471–82.
Lindsley AW, Schwartz JT, Rothenberg ME. Eosinophil responses during COVID-19 infections and coronavirus vaccination. J Allergy Clin Immunol. 2020;146(1):1–7.
Outh R, Boutin C, Gueudet P, Suzuki M, Saada M, Aumaître H. Eosinopenia <100/μL as a marker of active COVID-19: An observational prospective study. J Microbiol Immunol Infect. 2021;54(1):61–8.
Roca E, Ventura L, Zattra CM, Lombardi C. Eosinopenia: an early, effective and relevant COVID-19 biomarker? QJM. 2021;114(1):68–9.
Eijmael M, Janssens N, le Cessie S, van Dooren Y, Koster T, Karim F. Coronavirus disease 2019 and peripheral blood eosinophil counts: a retrospective study. Infection. 2021;49(6):1325–9.
Stylemans D, Van Cauwelaert S, D’Haenens A, Slabbynck H. COVID-19-associated eosinopenia in a patient with chronic eosinophilia due to chronic strongyloidiasis. Infect Dis Clin Pract (Baltim Md). 2021;29(5):e305–6.
De Wilton A, Nabarro LE, Godbole GS, Chiodini PL, Boyd A, Woods K. Risk of Strongyloides hyperinfection syndrome when prescribing dexamethasone in severe COVID-19. Travel Med Infect Dis. 2021;40: 101981.
Pereira CVM, Mastandrea GRA, Medeiros ACCS, Gryschek RCB, Paula FM, Corral MA. COVID-19 and strongyloidiasis: what to expect from this coinfection? Clinics (Sao Paulo Brazil). 2021;76:e3528.
Paula FM, Costa-Cruz JM. Epidemiological aspects of strongyloidiasis in Brazil. Parasitology. 2011;138(11):1331–40.
Gutman JR, Lucchi NW, Cantey PT, Steinhardt LC, Samuels AM, Kamb ML, et al. Malaria and parasitic neglected tropical diseases: potential syndemics with COVID-19? Am J Trop Med Hyg. 2020;103(2):572–7.
Laoraksawong P, Sanpool O, Rodpai R, Thanchomnang T, Kanarkard W, Maleewong W, et al. Current high prevalences of Strongyloides stercoralis and Opisthorchis viverrini infections in rural communities in northeast Thailand and associated risk factors. BMC Public Health. 2018;18(1):940.
Knopp S, Mohammed KA, Stothard JR, Khamis IS, Rollinson D, Marti H, et al. Patterns and risk factors of helminthiasis and anemia in a rural and a peri-urban community in Zanzibar, in the context of helminth control programs. PLoS Negl Trop Dis. 2010;4(5): e681.
Eamudomkarn C, Ruantip S, Sithithaworn J, Techasen A, Kopoolrat KY, Worasith C, et al. Epidemiology of strongyloidiasis determined by parasite-specific IgG detections by enzyme-linked immunosorbent assay on urine samples using Strongyloides stercoralis, S. ratti and recombinant protein (NIE) as antigens in Northeast Thailand. PLoS ONE. 2023;18(4):e0284305.
Acknowledgements
We would like to thank the patients who participated in this study and the staff of Razi Hospital, Ahvaz County and Hefdah-e-Shahrivar hospital, Abadan County. This research was part of PhD. project of Alireza Ashiri.
Funding
This study was supported by the Student Research Committee, Ahvaz Jundishapur University of Medical Sciences under Grant No. 00s91 to Alireza Ashiri.
Author information
Authors and Affiliations
Contributions
AA: Investigation, Methodology, Formal Analysis, and Writing—Original Draft. MB: Conceptualization, Project administration, Formal Analysis, Writing—Original Draft—view, and editing. AR: Conceptualization and Writing—Original Draft –review, and editing. RH and AT: Collecting samples.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present study protocol was approved by the Ethics Committee of the Ahvaz Jundishapur University of Medical Sciences (IR.AJUMS.REC.1400.635). Moreover, all the patients or their families gave informed consent for participation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ashiri, A., Beiromvand, M., Rafiei, A. et al. Prevalence of asymptomatic strongyloidiasis co-infection in COVID-19 patients residing in endemic areas. Eur J Med Res 28, 281 (2023). https://doi.org/10.1186/s40001-023-01262-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-023-01262-9