Abstract
Background
Although the number of patients with bladder cancer and lung metastasis is increasing there is no accurate model for predicting survival in these patients.
Methods
Patients enrolled in the Surveillance, Epidemiology, and End Results database between 2010 and 2015 were selected for the study. Univariate and multivariate Cox regression were used to determine independent prognostic factors, followed by development of a nomogram based on the multivariate Cox regression models. The consistency index, receiver operating characteristic curve, and calibration curve were used to validate the prognostic nomogram.
Results
506 eligible bladder cancer patients with lung metastasis were enrolled in the study and then divided randomly into training and validation sets (n = 356 vs. n = 150). Multivariate Cox regression analysis indicated that age at diagnosis, primary site, histological type, surgery of the primary site, chemotherapy, bone metastasis, and liver metastasis were prognostic factors for overall survival (OS) in patients with lung metastasis in the training set. The C-index of the nomogram OS was 0.699 and 0.747 in the training and validation sets, respectively. ROC curve estimation of the nomogram in the training and validation sets showed acceptable accuracy for classifying 1-year survival, with an area under the curve (AUC) of 0.766 and 0.717, respectively. More importantly, the calibration plot showed the nomogram had favorable predictive accuracy in both the training and validation sets.
Conclusions
The prognostic nomogram created in our study provides an individualized diagnosis, remedy, and risk evaluation for survival in patients with bladder cancer and lung metastasis. The nomogram would therefore enable clinicians to make more precise treatment decisions for patients with bladder cancer and lung metastasis.
Similar content being viewed by others
Introduction
Cancer is not only the leading cause of death but is also the most important barrier to increased life expectancy in the world. Bladder cancer (BCa) is the tenth most common form of cancer globally, with an estimated 573,278 new BCa patients occurring in 185 countries during 2020, with 212,536 of these cases dying as a result of the tumor [1]. BCa included several subtypes such as urothelial carcinoma, squamous cell carcinoma, and adenocarcinoma. Among these, urinary bladder urothelial carcinoma was the major common histological subtype. Untreated metastatic bladder carcinoma has a poor prognosis, with the median survival time rarely exceeding 3 to 6 months [2].
Bladder carcinoma metastases include several patterns such as lymph node involvement (25.4%) and distant organ metastasis including bone (24.7%), brain (3.1%), liver (18.1%), and lung (19.4%). However, distant organ metastasis in BCa is considered a unique situation and although occurring rarely has an important significance as it is associated with significantly shortened survival [3,4,5,6,7]. Unfortunately, approximately 10–15% of BCa patients are diagnosed with distant metastases at the time of the initial diagnosis, with up to 30% of patients with high-grade BCa eventually developing metastases that lead to a poor prognosis [8]. BCa with distant metastases can be treated with immunotherapy and systemic chemotherapy, resulting in 5-year survival rates of 36% for regional metastasis and 5% with distant metastasis [9]. The overall survival (OS) rate of BCa patients with metastases remains quite low despite multiple therapeutic modalities. For this reason, it is essential to construct prognostic models for OS of BCa patients with lung metastasis, as identifying patients with evaluated poor survival outcomes may guide enhanced therapeutics and improve prognosis [10].
According to a previous analysis of the Surveillance, Epidemiology, and End Results (SEER) database, up to 38.9% (724/1862) of BCa patients with distant metastasis had lung metastases [11]. Considering the rarity of lung metastases at presentation, there are currently no randomized clinical trial that have investigated the outcomes of this group.
However, we are aware of a few studies on metastatic BCa that have focused on the prognostic significance of lung metastasis from BCa detected at de novo diagnosis. Prognostic nomograms are currently used widely in oncologic medicine as prognostic devices. Because the knowledge of the prognosis of lung metastasis is essential for pretherapeutic assessment the aim of the current study was to describe the frequency of occurrence based on the SEER database. Another aim of the study was to construct a nomogram to predict OS in de novo diagnosed patients with BCa and lung metastasis. The study may also help to choose suitable management strategies by increasing the understanding of prognosis in newly diagnosed patients with BCa and lung metastasis.
Materials and methods
Database and patient selection
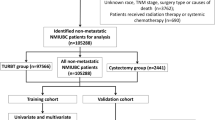
All patient data in the study were selected from the National Cancer Institute-funded SEER database (http://seer.cancer.gov/seerstat), which includes approximately 28% of the population of the USA. The database includes clinicopathologic and demographic information and survival outcomes, such as age, sex, race, year of diagnosis, marital status at diagnosis, primary site, histological type, tumor grade, tumor-node metastasis (TNM) stage based on the American Joint Commission on Cancer (AJCC) 7th edition, treatment methods of the primary site, cause of death, and survival time [12]. Site‐specific metastasis data in the SEER database only includes the lung, brain, bone, and liver at diagnosis since 2010. The variable (C67.0–C67.9, positive histology, one primary, or 1st of 2 or more primaries) was used in the SEER*Stat software (version 8.3.9) to identify 76,686 BCa patients enrolled in the database between January 1, 2010 to December 31, 2015.
As shown in Fig. 1, the exclusion criteria for the study were as follows: (I) without or unsure of lung metastasis (n = 75,725); (II) patients aged < 40 years (n = 14); (III) T stage unknown or unclear (n = 156), N stage unknown or unclear (n = 74), grade stage unknown (n = 99); (IV) lymph nodes surgery definitive (n = 6); (V) survival time < 1 month (n = 60); (VI) marital status unknown (n = 22); and (VII) bone metastases unknown (n = 11), brain metastases unknown (n = 5), liver metastases unknown (n = 4); and (VIII) radiation unsure (n = 4).
Due to BCa occurring late in life, with a mean age at diagnosis of approximately 67 years, patients younger than 40 years were excluded for the number of reasons. Because the study was a retrospective analysis, potential selection bias and confounding bias were inevitable. Finally, 506 eligible BCa patients with lung metastasis were enrolled in the study. The study was performed in compliance with the Declaration of Helsinki.
Study variables
The variables included the participants’ sex, year of diagnosis, ethnicity, marital status at diagnosis, primary site, histology type, grade stage, TNM stage, surgery of the primary site, surgery of lymph nodes, surgery of other sites, radiotherapy, chemotherapy, distant metastatic site, survival months, and vital status. The primary end point was OS according to the database. OS was defined as the time from diagnosis to death from any cause.
For convenient analysis we processed some variables in the SEER database using continuous variables for radiation, chemotherapy, bone metastasis, liver metastasis, brain metastasis, and surgery of another site, classified as either yes or no. Age was transformed into categorical variables: < 50, 50–59, 60–69, 70–79, or ≥ 80 years. Ethnicity included black, white, and others which included American, Native, Asian, Alaska, and Pacific Island people. We defined marital status as unmarried, married, divorced, separated, or widowed. The histology of the tumors in the study patients included transitional cell carcinoma, squamous cell carcinoma, adenocarcinoma, and other types. Precise information on the TMN system was recorded based on the seven editions of the AJCC stages.
Statistical analysis
All cases were included in the study set, which was then divided into training and validation sets at a ratio of 7:3. Categorical data were expressed as numbers and percentage in the three datasets, with the chi-square test used for intergroup comparisons. Continuous variables were expressed as mean ± standard deviation (SD), with Student’s t-test used to compare the baseline characteristics of the patients in the two groups. Categorical variables were expressed as frequencies and proportions and comparisons carried out using the Chi-square test.
A nomogram incorporating selected variables was constructed from the multiple Cox model, using a critical P-value of 0.05. To ascertain the risk factors associated with the OS of BCa patients with lung metastasis at diagnosis, we determined the hazard rations (HR) and 95%CI for the training set using univariate and multivariable Cox proportional analyses. The survival curves were plotted using the Kaplan–Meier curve and then compared using the log-rank test.
Subsequently, based on the results of the multivariate Cox regression model, we used the rms-R package to construct a prognostic nomogram to predict OS probability in BCa patients with lung metastasis. In addition, the consistency index (C-index), receiver operating characteristic (ROC) curve, and calibration curve were used to estimate the predictive performance of the nomogram, and also to calibrate the prediction capacity of the nomogram for 1- and 3-year OS. The C-index and an area under the curve (AUC) of > 0.65 in the receiver operating characteristic graph were considered to indicate acceptable classification. The calibration plot was used to assess the predictive accuracy by comparison with the actual survival rate of the nomogram in the training set. Similarly, the C-index, ROC curve, and calibration curve were used to analyze the validation set. The statistical analyses were carried out using the SPSS software program (version 25.0) and R software (www.rproject.org). P values < 0.05 indicated statistical significance.
Results
Demographic and clinicopathologic characteristics
A total of 506 (training cohort, 356 patients; validation cohort, 150 patients) BCa patients with lung metastasis enrolled in the database between January 1, 2010 and December 31, 2015, were eligible for selection in the study cohort. The clinicopathological features and demographic characteristics of these 506 patients are described in Table 1. The differences in clinical data between the training and validation set were not statistically significant (P > 0.05). The majority of patients in both cohorts were aged 60–80 years, married, and white. There were 154 (30.4%) females and 352 (69.6%) males in the full study cohort, with the most common tumor locations being bladder, NOS (209, 41.3%), followed by overlapping lesions of the bladder (113, 22.3%) and lateral wall of the bladder (72, 14.2%). The most common tumors were transitional cell carcinoma (448, 88.5%), T2 stage (246, 48.6%), and N0 stage (314, 62.1%). There were 123 (24.3%) bone metastasis, 15 (3.0%) brain metastasis, and 96 (19.0%) liver metastasis in the full study cohort. The median survival time was 6 months (range, 1–98 months).
Identification of prognostic factors of OS
Univariate and multivariate Cox regression models were performed on all factors in the training set, with the exception of the year of diagnosis. Univariate Cox regression analysis showed that age at diagnosis, sex, marital status, histological type, surgery of the primary site, surgery of lymph nodes, chemotherapy, bone metastasis, and liver metastasis were factors related to OS in BCa patients with lung metastasis. Multivariate Cox regression model results also showed that age at diagnosis, primary site, histological type, surgery of the primary site, chemotherapy, bone metastasis, and liver metastasis were prognostic factors for OS in these patients (Table 2). For example, higher age (70–79 years: HR = 1.936, 95%CI 1.074–3.490, P < 0.05; ≥ 80 years: HR = 2.361, 95%CI 1.244–4.481, P < 0.05), tumor located in the dome of bladder (HR = 2.352, 95%CI 1.028–5.381, P < 0.05), squamous cell carcinoma (HR = 6.458, 95%CI 3.121–13.364, P < 0.001), and combined with bone (HR = 1.437, 95%CI 1.096–1.885, P < 0.05) or liver (HR = 1.624, 95%CI 1.187–2.223, P < 0.05) metastasis were associated with a worse OS. In contrast, surgery of the primary site [transurethral resection of the bladder (TURB): HR = 0.548, 95%CI 0.337–0.890, P < 0.05; other type: HR = 0.423, 95%CI 0.238–0.752, P < 0.05], or chemotherapy (HR = 0.465, 95%CI 0.359–0.604, P < 0.001) were associated with a favorable OS. Figures 2, 3 and 4 show the Kaplan–Meier curves for these relevant variables.
Development of a prognostic nomogram
The variables with a P value < 0.05 in the multivariate Cox regression models were included in the prognostic nomogram. We constructed the nomogram using the prognostic factors identified in the multivariate Cox regression model of the training set. The TNM stage was an important prognostic factor for tumor patients, although it did not show statistically significant power to predict outcomes in BCa patients with lung metastasis. The results of the multivariate Cox regression models showed that T stage (P > 0.05) and N stage (P > 0.05) were not prognostic factors for OS in BCa patients with lung metastasis and therefore we did not integrate TNM stage into the nomogram. The nomogram based on the prognostic factors is shown in Fig. 5. Using this nomogram individual survival at 1- and 3-year could be predicted by the available clinical information.
Each subgroup variable could get a corresponding score in the nomogram. The scores ranged from 0 to 100 for each variable depending on its contribution, and produced total scores of the subscales that were then transformed to predict the related OS (Table 3). Using the nomogram, a vertical line is drawn up to the top row of the points and points assigned for each variable. The sum of the scores is located on the total points axis, and a downward line drawn on the survival axis to determine the likelihood of survival for 1- or 3-years.
Validation and calibration of the nomograms
The C-index and ROC curves were compared to determine, whether the survival months predicted by the nomograms were in accordance with the actual survival times. The C-index of the nomogram OS was 0.699 and 0.747 in the training and validation sets, respectively. The ROC curve estimation of the nomogram in both the training and validation sets also showed acceptable accuracy, with a 1-year AUC of 0.766 and 0.717, respectively (Fig. 6a, c). In addition, the 3-year AUCs were 0.699 and 0.696, respectively (Fig. 6b, d). These results indicated that the model we constructed was relatively accurate.
More importantly, we calibrated the 1- and 3-year OS nomogram in both the training and validation sets. The calibration plots showed that the nomogram had a favorable predictive accuracy in both the training set (Fig. 6e, f) and validation set (Fig. 6g, h). This result indicated good agreement between the nomogram predictions and the observed results in the training and validation sets.
Discussion
To our knowledge, a considerable number of BCa patients have metastases at distant organs at diagnosis, leading to a shorter OS. Of the metastatic BCa organs, bone is the most common, followed by the lung. Bianchi et al. [4] also showed that the bone metastasis rate was higher than the lung metastasis rate in patients with M1 stage bladder cancer. A previous study studied the independent prognostic factors of outcome in BCa patients with bone metastasis [13]. However, the independent predictive factors of outcome in lung metastasis BCa still remain unknown. The aim of the present research was therefore to determine the independent predictive factors in BCa patients with lung metastasis and develop a predictive nomogram to help predict outcome risks.
Univariate and multivariate analyses were carried out in the current study on a large number of BCa patients with lung metastasis. These analyses showed that age at diagnosis, primary site of the tumor, histology, surgery of the primary site, additional chemotherapy, bone metastasis, and liver metastasis were independent prognostic factors for OS in BCa patients with lung metastasis.
The nomogram we constructed enables more personalized risk prediction and is a well-studied intuitive statistical model based on the results of a multivariate analysis [14, 15]. The integration of multiple independent prognostic factors in the model could further improve its accuracy to appraise the survival probability of an individual patient [16]. To date, several nomograms have been developed for different tumor types and have shown powerful predictive ability that is more accurate than the traditional TNM systems [17]. More importantly, clinicians are able to intuitively evaluate the physical condition of patients and offer individual predictions using nomograms. However to our knowledge before the current study there was no prognostic nomogram model for BCa patients with lung metastasis. Therefore, it is of great prognostic significance for these patients to establish a reliable and efficacious prognostic nomogram and to offer individualized therapies.
The present study investigated and validated a new prognostic tool based on the results of multivariate Cox regression models that included the age at diagnosis, primary site of the tumor, histology, bone metastasis, liver metastasis, chemotherapy, and surgery of the primary site. This tool enhanced prediction of OS in BCa patients with lung metastases. The results of the study demonstrated that the prognostic tool could be used to divide the patients into two groups (low-risk and high-risk) with wide variations in OS. Using a median cut-off value, the patients were divided into either high-risk or low-risk groups. We also assessed the accuracy of the 1- and 3-year OS in the prognostic nomograms, with the results indicating good consistency and reliability. In terms of its content, the prediction model is simple and easy to understand. First, in the nomogram, a vertical line is drawn from each clinicopathological parameter to the ‘points’ line, followed by addition of the score to determine the ‘total points’. A vertical line is then drawn from ‘total points’ to ‘1- or 3-years survival’. Based on the above analysis we are able to calculate the 1- or 3-year survival rates in lung metastatic BCa patients. For example, a 65-year-old male patient was diagnosed with a transitional cell carcinoma located in the lateral wall of bladder, without liver or bone metastases, and had undergone a TURB and chemotherapy. According to the nomogram, the ‘total points’ was 95 and the 1- and 3-years survival rates were approximately 45% and 20%, respectively.
In general, the prognostic factors identified in our study were associated strongly with the choice of therapeutic approach, as well as the metastatic site of the BCa patients. Previous research in BCa patients has demonstrated that a single metastatic site was capable of independently predicting better OS compared with multisite organ metastasis [17]. The results of our research were consistent with this previous study in that it showed better survival with lung metastases compared to a coalesced tumor or simultaneous bone metastasis or liver metastasis.
Treatment of BCa patients with lung metastases is not uniform. In the current study, we included five variables in the treatment of lung metastasis BCa, including surgery of the primary site, surgery of lymph nodes, surgery of other sites, radiation, and chemotherapy.
A previous study revealed that the primary site of surgery might contribute to long-term OS survival in lung metastatic BCa patients. Wang et al. [18] reported that the survival rates of metastatic BCa patients with adenocarcinoma and transitional cell (non)-papillary carcinoma could be improved by surgery of the primary site alone, while the survival rates of metastatic BCa patients with squamous cell carcinoma could be increased only by surgery combined with adjuvant chemotherapy. They also demonstrated that surgery of the primary site alone was an independent predictive factor of outcome, and that surgery could affect the OS of BCa patients with lung metastasis. We found that BCa patients with lung metastasis could also benefit from primary site surgery. Regarding surgery of lung metastasis, the conclusions remain limited due to a lack of consistent reports. However, there is evidence that a pulmonary metastasectomy, combined with chemotherapy may improve OS [19]. Although the efficacy of therapeutic approaches is susceptible to the underlying emergence of drug resistance, as shown in our nomogram, chemotherapy still has the greatest survival benefit for BCa patients with lung metastasis. This finding is consistent with the first-line treatment regimen in the guidelines of the European Urological Association [20]. Besides, in cancer, fibroblast growth factor receptors (FGFRs) have emerged as a novel therapeutic target [21]. As an oncogenic driver in bladder cancer, FGFR3 genomic alterations represent predictive biomarkers that predict the response to FGFR inhibitors. According to research, 50% of bladder cancers have somatic mutations of the FGFR3 gene [22, 23]. Bladder cancer is typically associated with FGFR3 gene rearrangements [24, 25]. While platinum-based chemotherapies have always been the main therapeutic approach for urothelial bladder carcinoma, the advent of antiFGFR-target therapy represents significant advances for bladder cancer patients with FGFR altered.
To our knowledge this is the first cohort study to investigate the risk factors for the prognosis of BCa with lung metastasis. Furthermore, the study is the largest cohort study to examine the prognostic significance of lung metastasis in BCa patients and to determine the effect of numerous treatment modalities on the prognosis of these patients. Nevertheless, we acknowledge that our study has several limitations. First, the retrospective research design of the study limited its conclusions and it was not possible to completely rule out confounding factors, such as smoking history etc. In addition, information on cancer recurrence was lacking, and patients who might have developed distant metastases later in their disease process were not taken into consideration.
Conclusions
Age at diagnosis, primary site of the tumor, histology, surgery of the primary site, additional chemotherapy, bone metastasis, and liver metastasis are independent predictive factors of outcome in BCa patients with lung metastasis. Based on these prognostic factors, we constructed a prognostic nomogram, which could provide the best assessment of OS and indicate appropriate therapy in BCa patients with lung metastasis.
Availability of data and materials
The data analyzed in this study is available at https://seer.Cancer.gov/.
Abbreviations
- OS:
-
Overall survival
- AUC:
-
Area under the curve
- BCa:
-
Bladder cancer
- SEER:
-
Surveillance, Epidemiology, and End Results
- TNM:
-
Tumor-node metastasis
- AJCC:
-
American Joint Commission on Cancer
- HR:
-
Hazard rations
- C-index:
-
Consistency index
- ROC:
-
Receiver operating characteristic
- TURB:
-
Transurethral resection of the bladder
- FGFR:
-
Fibroblast growth factor receptors
References
Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. https://doi.org/10.3322/caac.21660.
Shou J, Zhang Q, Zhang D. The prognostic effect of metastasis patterns on overall survival in patients with distant metastatic bladder cancer: a SEER population-based analysis. World J Urol. 2021. https://doi.org/10.1007/s00345-021-03721-6.
Shinagare AB, Ramaiya NH, Jagannathan JP, et al. Metastatic pattern of bladder cancer: correlation with the characteristics of the primary tumor. AJR Am J Roentgenol. 2011;196(1):117–22. https://doi.org/10.2214/AJR.10.5036.
Bianchi M, Roghmann F, Becker A, et al. Age-stratified distribution of metastatic sites in bladder cancer: a population-based analysis. Can Urol Assoc J. 2014;8(3–4):E148-158. https://doi.org/10.5489/cuaj.787.
Bellmunt J, Choueiri TK, Fougeray R, et al. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol. 2010;28(11):1850–5. https://doi.org/10.1200/JCO.2009.25.4599.
Lin CC, Hsu CH, Huang CY, et al. Prognostic factors for metastatic urothelial carcinoma treated with cisplatin and 5-fluorouracil-based regimens. Urology. 2007;69(3):479–84. https://doi.org/10.1016/j.urology.2006.12.010.
von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23(21):4602–8. https://doi.org/10.1200/JCO.2005.07.757.
Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19(3):666–75. https://doi.org/10.1200/JCO.2001.19.3.666.
Lenis AT, Lec PM, Chamie K, et al. Bladder cancer: a review. JAMA. 2020;324(19):1980–91. https://doi.org/10.1001/jama.2020.17598.
Yang Z, Bai Y, Liu M, et al. Development and validation of a prognostic nomogram for predicting cancer-specific survival after radical cystectomy in patients with bladder cancer: a population-based study. Cancer Med. 2020;9(24):9303–14. https://doi.org/10.1002/cam4.3535.
Dong F, Shen Y, Gao F, et al. Prognostic value of site-specific metastases and therapeutic roles of surgery for patients with metastatic bladder cancer: a population-based study. Cancer Manag Res. 2017;9:611–26. https://doi.org/10.2147/CMAR.S148856.
Mao W, Zhang Z, Huang X, et al. Marital status and survival in patients with penile cancer. J Cancer. 2019;10(12):2661–9. https://doi.org/10.7150/jca.32037.
Fan Z, Huang Z, Hu C, et al. Risk factors and nomogram for newly diagnosis of bone metastasis in bladder cancer: a SEER-based study. Medicine (Baltimore). 2020;99(42):e22675. https://doi.org/10.1097/MD.0000000000022675.
Iasonos A, Schrag D, Raj GV, et al. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26(8):1364–70. https://doi.org/10.1200/JCO.2007.12.9791.
Eastham JA, Kattan MW, Scardino PT. Nomograms as predictive models. Semin Urol Oncol. 2002;20(2):108–15. https://doi.org/10.1053/suro.2002.32936.
Hou G, Zheng Y, Wei D, et al. Development and validation of a SEER-based prognostic nomogram for patients with bone metastatic prostate cancer. Medicine (Baltimore). 2019;98(39):e17197. https://doi.org/10.1097/MD.0000000000017197.
Wu S, Chen JN, Zhang QW, et al. A new metastatic lymph node classification-based survival predicting model in patients with small bowel adenocarcinoma: a derivation and validation study. EBioMedicine. 2018;32:134–41. https://doi.org/10.1016/j.ebiom.2018.05.022.
Wang P, Zang S, Li G, et al. The role of surgery on the primary tumor site in bladder cancer with distant metastasis: significance of histology type and metastatic pattern. Cancer Med. 2020;9(24):9293–302. https://doi.org/10.1002/cam4.3560.
Nakagawa T, Taguchi S, Kanatani A, et al. Oncologic outcome of metastasectomy for urothelial carcinoma: who is the best candidate? Ann Surg Oncol. 2017;24(9):2794–800. https://doi.org/10.1245/s10434-017-5970-8.
Alfred Witjes J, Lebret T, Compérat EM, et al. Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol. 2017;71(3):462–75. https://doi.org/10.1016/j.eururo.2016.06.020.
Ascione CM, Napolitano F, Esposito D, et al. Role of FGFR3 in bladder cancer: treatment landscape and future challenges. Cancer Treat Rev. 2023;115:102530. https://doi.org/10.1016/j.ctrv.2023.102530.
Cappellen D, De Oliveira C, Ricol D, et al. Frequent activating mutations of FGFR3 in human bladder and cervix carcinomas. Nat Genet. 1999;23(1):18–20. https://doi.org/10.1038/12615.
Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10(2):116–29. https://doi.org/10.1038/nrc2780.
Nelson KN, Meyer AN, Siari A, et al. Oncogenic gene fusion FGFR3-TACC3 is regulated by tyrosine phosphorylation. Mol Cancer Res. 2016;14(5):458–69. https://doi.org/10.1158/1541-7786.MCR-15-0497.
Parker BC, Engels M, Annala M, et al. Emergence of FGFR family gene fusions as therapeutic targets in a wide spectrum of solid tumours. J Pathol. 2014;232(1):4–15. https://doi.org/10.1002/path.4297.
Acknowledgements
The authors would like to express their gratitude to EditSprings (https://www.editsprings.cn) for the expert linguistic services provided. We acknowledge the efforts of the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries for creating the SEER database.
Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
LL: Conceptualization, methodology, software, writing—original draft. FS: Methodology, software, writing—original draft. PZ: Data curation, visualization, methodology. YX: Data curation, visualization, methodology. HN: Validation, writing—review & editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The data of this study were obtained from the SEER database. The patients’ data were public and anonymous, so this study does not require ethical approval and informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, L., Sun, Fz., Zhang, Py. et al. Development and validation a model for predicting overall survival of bladder cancer with lung metastasis: a population-based study. Eur J Med Res 28, 279 (2023). https://doi.org/10.1186/s40001-023-01261-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-023-01261-w