Abstract
Background
The reactivation of herpesviruses (HHV) in COVID-19 patients is evident in the literature. Several reports have been published regarding the reactivation of these viruses (HSV, VZV, EBV, and CMV) among those who got COVID-19 vaccines. In this study, we aimed to review the current evidence to assess whether HHVs reactivation has any association with the prior administration of COVID-19 vaccines.
Methods
A systematic search was conducted on 25 September 2022 in PubMed/MEDLINE, Web of Science, and EMBASE. We included all observational studies, case reports, and case series which reported the reactivation of human herpesviruses following administration of COVID-19 vaccines.
Results
Our systematic search showed 80 articles that meet the eligibility criteria. Among the evaluated COVID-19 vaccines, most of the vaccines were mRNA based. Evidence from observational studies showed the possible relation between COVID-19 vaccine administration and VZV and HSV reactivation. The results of our proportion meta-analysis showed that the rate of VZV reactivation among those who received the COVID-19 vaccine was 14 persons per 1000 vaccinations (95% CI 2.97–32.80). Moreover, our meta-analysis for HSV reactivation showed the rate of 16 persons per 1000 vaccinations (95% CI 1.06–46.4). Furthermore, the evidence from case reports/series showed 149 cases of HHV reactivation. There were several vaccines that caused reactivation including BNT162b2 mRNA or Pfizer–BioNTech (n = 76), Oxford-AstraZeneca (n = 22), mRNA-1273 or Moderna (n = 17), Sinovac (n = 4), BBIBP-CorV or Sinopharm (n = 3), Covaxin (n = 3), Covishield (n = 3), and Johnson and Johnson (n = 1). Reactivated HHVs included varicella-zoster virus (VZV) (n = 114), cytomegalovirus (CMV) (n = 15), herpes simplex virus (HSV) (n = 14), Epstein-Barr virus (EBV) (n = 6), and HHV-6 (n = 2). Most cases reported their disease after the first dose of the vaccine. Many patients reported having comorbidities, of which hypertension, diabetes mellitus, dyslipidemia, chicken pox, and atrial fibrillation were common.
Conclusion
In conclusion, our study showed the possible association between COVID-19 vaccination and herpesvirus reactivation. The evidence for VZV and HSV was supported by observational studies. However, regarding other herpesviruses (EBV and CMV), further research especially from observational studies and clinical trials is required to elucidate the interaction between COVID-19 vaccination and their reactivation.
Similar content being viewed by others
Introduction
Since late 2019, the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), known as coronavirus disease 2019 (COVID-19), has brought up many concerns due to its widespread, which has led to a considerable number of studies evaluating a variety of therapeutic approaches for COVID-19, including chloroquine, ivermectin, remdesivir, nucleoside analogs, hydroxychloroquine, monoclonal antibodies, famotidine, convalescent plasma, herbal medicine, and natural compounds [1,2,3]. To date, utilizing vaccines is one of the most effective ways to control the pandemic. COMIRNATY (the COVID-19 mRNA vaccine BNT162b2 by BioNTech– Pfizer); COVID-19 Vaccine Moderna (mRNA-1273 by Moderna); VAXZEVRIA (ChAdOx1- nCoV19 by AstraZeneca-Oxford University); and COVID-19 Vaccine Janssen (Ad26.COV2.S by Janssen) are among the most popular vaccines used against the COVID-19 [4]. Notwithstanding the different mechanisms of action, all these vaccines that have been administered have some local and systemic side effects after the injection, such as site pain and swallowing, fever, arthralgia, headache, and vomiting [5, 6].
Herpesviridae consists of a DNA virus that falls into a varicella-zoster virus (VZV), cytomegalovirus (CMV), Epstein-Barr virus (EBV), and herpes simplex virus (HSV). Herpesviruses (HHV) are mostly known for their ability to cause latent infection, which can become reactivated by triggers such as stress, lack of sleep, physical fatigue, exposure to sunlight, fever, menstruation, and surgical resection [7]. HHVs are capable of remaining in different types of body cells after the first infection and become reactivated when the host is experiencing an immunocompromised state critically ill patients, sepsis shock, intensive care unit (ICU) administration, usage of anti-inflammatory drugs, and prolonged ventilation are risk factors for the immunocompromised state which can lead to reactivation of these viruses [8,9,10,11]. All these conditions can happen during severe and critical COVID-19. Preliminary work on the incidence of herpesvirus reactivation in COVID-19 patients was undertaken by Simmonet et al., which shows that 85% of critically ill patients with COVID-19 in ICU have developed EBV, CMV, and HHV-6 viremia [9]. It may reasonably be doubted whether the vaccination for COVID-19 can be a reason for the herpes virus’s virus family's reactivation. In this connection, VZV reactivation after vaccine administration was reported in 91 patients who were mostly represented by mild to moderate cutaneous lesions [12].
Reactivation of other HHVs (EBV, CMV, and HSV) following COVID-19 vaccination have been reported in several case reports. Taken together all these reported cases suggest that although vaccines administration rarely results in severe side effect, early diagnosis and prophylaxis would be essential for decreasing the morbidity and side effects. Therefore, the present study was designed to determine the correlation between the COVID-19 vaccine administration and reactivation of herpes virus and review the cases who have experienced this condition, to increase awareness about the clinical manifestation of herpes reactivation following COVID-19 vaccination.
Methods
We conducted a systematic review following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and guideline provided by the Cochrane Handbook for Systematic Reviews of Interventions [13, 14]. The protocol of this study was registered with the following number: IR.ABZUMS.REC.1402.116.
Search strategy
A systematic search was conducted in several international databases including Medline (via PubMed), Embase, and Web of science up to 25 September 2022. No restrictions were applied to the search results we retrieved. Furthermore, studies that were eligible were found by evaluating the references of the papers that might be included. The Boolean operators and the following keywords were combined together to create the right approach for our comprehensive search: COVID-19, SARS-CoV-2, coronavirus, Herpesviridae, HSV, herpes simplex virus, varicella-zoster virus, VZV, Epstein-Barr virus, EBV, cytomegalovirus, CMV. Additional file 1: Table S2 provides a thorough description of the search process for each database, along with exact results and performance times.
Eligibility criteria
Using the PICOT specification, the inclusion criteria were as follows: 1) Population: adults receiving COVID-19 vaccine either first or second dose; 2) Intervention: COVID-19 vaccines; 3) Comparison: If applicable (since most studies did not evaluate a control group), those who were not vaccinated against COVID-19; 4) Outcome: reactivation of Herpesviridae; and 5) Type of Study: Observational studies, case reports, and case series. Conference abstracts were also included. The exclusion criteria included review studies, opinion studies, and letters to the editor devoid of any relevant info.
Screening and data extraction
The papers were initially screened by title and abstract, and then the full texts were screened. Discussions were used to settle disagreements. A spreadsheet in Excel was used to extract the data. The extracted for observational studies were Author, Year, Country, Type of study (Registry/ Duration), Population, Total patients, Vaccine, Reactivated virus, and Main Findings of each cohort. For case reports/series we extracted Author, Year, Country, Total Patients, Age, Vaccine, Clinical manifestations/ Reactivated virus, Detection, Comorbidity, and Treatment from each study.
Quality assessment
For the quality assessment of the included studies, we used the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for case reports [15] and case series [16]. The eight items in the JBI checklist for case reports cover the patient's demographics, medical history, present clinical state, description of diagnostic tests, therapy, post-intervention clinical state, adverse events, and the providing of takeaways. The JBI checklist for case series is a 10-item scale that assesses the inclusion criteria, method of condition measurement, validity of the diagnostic methods, whether participants were consecutively included, the extent to which participants were included, reporting of the demographic characteristics, clinical information, outcomes, presentation of clinic demographic information, and appropriateness of the statistical analysis [17]. We used the Newcastle–Ottawa Scale (NOS) for assessing the quality of observational cohorts [15]. The scale contains 8 signaling question in 3 different domains (Selection, Comparability, and Outcomes).
Data Synthesis
We performed a random effect meta-analysis to estimate the proportions of HHV reactivation among patients vaccinated against COVID-19. Since the incidence of reactivation was rare among the included studies, we decided to present the results as events per 1000 observations. Before pooling the effect estimates, we transformed the raw data using the Logit transformation methods to reduce the variation of the study-specific prevalence. I2 test was evaluated to test the heterogeneity among studies. Sensitivity analysis was performed to found the pooled effects in patients who were clinically diagnosed with herpes zoster. All statistical analyses and graphics were carried out using R (version 4.1.3) [18] and the meta package (version 5.5–0) [19]. Furthermore, we describe the results of individual cohorts and case reports/series in a manner of providing a narrative synthesis.
Results
Search results
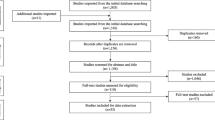
We found a total number of 3542 articles from the mentioned databases. After screening based on the inclusion/exclusion criteria provided, a total number of 80 studies (11 observational cohorts [20,21,22,23,24,25,26,27,28,29,30], 59 case reports [31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88], and 10 case series [89,90,91,92,93,94,95,96,97,98]) were eligible for inclusion. It is important to note that most of the studies included in this review were published in 2022 (Fig. 1).
Qualitative synthesis
Evidence from observational cohorts
All included observational studies regarding the possible effect of COVID-19 administration were published in 2022 and 2023. There were 11 studies with this regard mostly focusing on the reactivation of VZV with herpes zoster presentation, showing the paucity of observational studies for other herpesviruses [20,21,22,23,24,25,26,27,28,29,30] (Table 1). Six studies were retrieved their data through registries [20, 23, 25,26,27,28]. Other studies were center-based observational cohorts [21, 22, 24]. The sample size of the included cohorts varied from 265 to 2190172. Regarding the type of COVID-19 vaccine administered, 8 studies have evaluated BNT162b2 [21,22,23, 25,26,27,28,29], 7 have evaluated mRNA-1273 [23,24,25,26,27,28,29], 3 have evaluated AZD1222 [21, 24, 26], and one has evaluated CoronaVac [22], Sinopharm (Vero Cell), Sinovac COVID‐19 Vaccine (Vero Cell), Sinopharm/WIBP, CanSinoBio, Zhifei Longcom, KCONECAVAC [30], and Ad26.COV2.S [23]. However, it must be mentioned only 5 studies reported the outcome of interest based on the type of each vaccine [22, 24, 25, 27, 28]. Regarding the type of reactivated HHV, most studies have reported the reactivation of VZV. Only two studies have data regarding the reactivation of HSV [21, 22, 27, 29]. Overall, the results of the included cohorts support the possible association between COVID-19 administration and reactivation of VZV. Five studies have found administrating COVID-19 vaccine is accompanied with higher odds of VZV and HSV reactivation [20, 23, 25, 27, 28]. Among these studies, only Birabaharan, M. reported a non-significant different when comparing with a control group using data from TriNetX database registry (risk ratio: 0.91, 95% CI 0.82–1.01) [20]. Another study by Hertel, M. which used the same database the increased rate of reactivation among the COVID-19 vaccinated group (risk ratio: 1.802, 95% CI 1.680–1.932) [23]. It is noteworthy to mention that the length of their cohort was much longer than Birabaharan, M. (2 years compared with 7 months).
Evidence from case reports/series
There are 149 cases included in this review, which were from 30 different countries around the world. USA (n = 21), India (n = 15), Greece (n = 15), Taiwan (n = 9), Saudi Arabia (n = 7), Spain (n = 7), China (n = 6), Switzerland (n = 5), Germany (n = 4), and Kuwait (n = 5) have the most patients. From a 12-year-old adolescent to an elderly patient who was 84 years old, the age range of the patients was varied (Table 2). There were several vaccines that caused reactivation: BNT162b2 mRNA or Pfizer–BioNTech (n = 76), Oxford-AstraZeneca (n = 22), mRNA-1273 or Moderna (n = 17), Sinovac (n = 4), BBIBP-CorV or Sinopharm (n = 3), Covaxin (n = 3), Covishield (n = 3), and Johnson and Johnson (n = 1). In some of the cases, the exact model of the vaccine was not reported in the paper [53, 90, 99, 100]. Reactivated HHVs included varicella-zoster virus (n = 114), cytomegalovirus (n = 15), HSV-1 (n = 14), Epstein-Barr virus (n = 6), and HHV-6 (n = 2). The detection methods varied depended on the symptoms of each specific case, but the most common ones were as follows: history and physical examination, clinical symptoms, slit lamp examination, PCR, serum tests, and laboratory evaluation. There were four papers that did not specify the exact method of diagnosing [32, 38, 47, 95]. As a result of the variety of symptoms caused by the reactivation of virus, treatment varied greatly as well. In addition to antiviral drugs (such as acyclovir, valacyclovir, ganciclovir, and valganciclovir), antibiotics, steroids (such as prednisolone), and glucocorticoids (such as dexamethasone) were the most commonly prescribed medicines. The treatment for the patient was not presented in seven studies [32, 59, 72, 74, 75, 97, 101] Many patients reported having comorbidities, of which hypertension, diabetes mellitus, dyslipidemia, chicken pox, and atrial fibrillation were the common ones. There is a detailed description of the specific method of diagnosing and treatment for each case in Additional file 1: Tables S2 and S3.
Results of meta-analysis
The results of our proportion meta-analysis showed that the rate of VZV reactivation among those who received COVID-19 vaccine was 14 persons per 1000 vaccinations (95% CI 2.97–32.80). Moreover, our meta-analysis for HSV reactivation showed the rate of 16 persons per 1000 vaccinations (95% CI 1.06–46.4) (Fig. 2).
Quality assessment
The results of our quality assessment for observational studies showed 6 and 5 studies with low and high risk of bias, respectively. The most domain which differed among the cohorts was regarding providing a comparable group (e.g., control group) which was only present in three studies.
Quality assessment for case reports was performed by JBI checklist, and five studies [33, 40, 49, 61, 78] received an overall score of 8 out of 8, while one study[74] received the lowest score of 4 out of 8, for an overall mean score of 6.2. In terms of scoring, the highest scoring criteria were reporting the demographic characteristics of patients (100%) and the clinical condition of the patients (96%). A precise diagnosis method (49%) and clear reporting of adverse events (55%) received the lowest scores.
Among the case series, one study[96] received a 10/10 score and the lowest score was received by one study (5/10)[89], with an overall mean score of 7.5. Reporting a complete inclusion criteria, demographic information, and clinical information of participants were the highest scoring criteria (10/10, 100%), while the least reported score was for valid methods used for identification of the condition for all participants (4/10, 40%).
The detailed results of quality assessment for all included studies are available in supplementary material.
Discussion
This study set out with the aim of literature reviewing to examine the potential correlation between the COVID-19 vaccine administration and possible reactivation of the herpesviruses. In our study, 76 reports were included, which comprised patients who had experienced reactivation of different types of herpesviruses after administration of different types of COVID-19 vaccines. The results from observational cohorts showed that the administration of COVID-19 vaccine, especially mRNA-based ones, could be associated with VZV reactivation. It should be noted that most information available was regarding VZV, and not many reports were available for other types of herpesviruses. Few numbers of published records and the nature of observational study would suggest the evidence regarding the association between COVID-19 vaccine and VZV reactivation to be low. Therefore, in addition to the cohorts included for this study, we also reviewed the reported cases of different HHVs reactivation among those who got COVID-19 vaccines. Among different vaccines, BNT162b2 mRNA or Pfizer–BioNTech have been administrated in more than half of the reported cases. Also, among the reactivated HHVs, including VZV, EBV, CMV, HSV-1 and HSV-6, most cases had experienced the reactivation of VZV, which was reported in nearly 70% of case reports, and the less common one was HSV-6 with only 2 cases.
Close to 100% of the adult population is at least once in a lifetime infected by one of the herpes viridea family viruses [102]. This family is known for its ability to indicate latent infection after the primary infection, which can reactivate by external or internal triggers. The latent phase of infection is defined as a situation in which the virus is quiescent, meaning the virus is not replicating which prevents the lytic infection and release of new progeny virus particles; in this mode of infection, external or internal stimuli can reactivate the virus, which defined as switching the latent phase to lytic [103]. Expression of a variety of virus genes during lytic infection leads to make progeny virions. Based on the time of their expression concerning the initial onset of reactivation, they fall into three groups, including IE genes, early (E) genes, and late (L) genes, which encode the proteins whose role in the gene transcription, viral replication, and structural proteins, which result in virion formation and reactivation [103]. There are different sites in which the viruses become latent; VZV mostly stays latent in neurons of dorsal root ganglia, cranial nerve ganglia, and autonomic ganglia, and EBV displays a latent phase in B lymphocytes and epithelial cells. CMV becomes latent in cells of the myeloid and HSV-1 and HSV-2 reactivate from trigeminal ganglia and sacral ganglia, respectively [12, 104,105,106]. Based on the reactivation of which type of herpes virus family, different kinds of triggers are capable of reactivating the virus. However, on balance, the most typical stimuli are fever, microbial co-infection, tissue injury, stress, immunocompromised situations, hyperthermia, hormonal imbalance, UV light, allogenic stimulation, and cytokines [107].
Vaccine administration can provide some of these triggers, such as hyperthermia and tissue injury as other side effects and also immunodeficiency state; in other words, it may theoretically result in the reactivation of herpes viruses. DNA repair and the immune system are known as the two essential systems for defending against threats; loss of function of DNA repair may lead to disability of production of B and T cells resulting in immunodeficiency [108]. A recent study by Liu et al. involved the pathophysiological alterations after the COVID-19 vaccine in which CD8+ T cells reduction, increase in classic monocyte contents, increased NF-κB signaling, and reduced type I interferon responses were reported; they have admitted that in the first 28 days after a vaccine injection, the immune system is in the vulnerable state [109]. Type I IFN receptor signaling in CD8+ T cells has an essential role in regulating memory cell response to viral infection and blockage of reactivation [109, 110]. These examples suffice to show that after COVID-19 vaccine administration, reactivation of the herpes virus family may occur. One of the more significant findings to emerge from this study is that, although vaccines are critical for controlling the COVID-19 pandemic, vaccine administration could lead to the reactivation of the herpes virus family. It is true that only few complicated cases have been reported. However, the fact remains that it can influence a large number of people all around the world. Clinical awareness about ways to the early onset diagnosis, preparing the best treatment for patients, and recognizing the patients who are at risk of reactivation are essential.
The results from our study are in line with recent systematic reviews which also reported an association between COVID-19 vaccine and VZV reactivation [111,112,113,114]. All previous systematic reviews only included case reports/series regarding the reactivation of VZV. In addition to case reports/series, our systematic review evaluated the available observational evidence regarding VZV reactivation following COVID-19 vaccination, including 6 cohorts. Moreover, our study focused not only on VZV but also on reporting the reported cases available in literature for HSV, EBV, CMV, and HHV-6. Recent systematic review by Martinez-Reviejo eta al. [112] showed most reported cases of VZV reactivation have their symptoms following the first dose of mRNA vaccination and most of the patients were presented with uncomplicated course, with few having serious disease. These results were in line with our findings for HSV, EBV, and CMV.
A number of limitations need to be considered. First, the number of cases that have been reported is inadequate for certainly assessing the correlation between vaccines and HHVs reactivation. Second, these findings are limited by not using the clinical trial design and lack of comparison between vaccinated and non-vaccinated participants. Considerably more work will need to be done to determine the effect of vaccination on HHVs reactivation. On the other hand, our study is the first to review the possible correlation between COVID-19 and HHVs reactivation. The present study provides a comprehensive overview of the published literature and highlights the available data with rigorous quality assessment.
In conclusion, although vaccination has played an essential role in controlling the COVID-19 pandemic, many different side effects should be considered before administration. However, more research on this topic needs to be undertaken before the association between vaccination and reactivation of the herpes virus family is more clearly understood. To date, the reported cases have shown that clinical physicians should be prepared and aware, so they are capable of recognizing their patients who present with the symptoms of herpes virus reactivation after vaccination and providing them with the best prophylaxis and treatment.
Availability of data and materials
All relevant data are within the manuscript and its Additional file 1.
References
Han F, et al. Current treatment strategies for COVID-19 (Review). Mol Med Rep. 2021. https://doi.org/10.3892/mmr.2021.12498.
Shafiee A, et al. Ivermectin under scrutiny: a systematic review and meta-analysis of efficacy and possible sources of controversies in COVID-19 patients. Virol J. 2022;19(1):102.
Cheema HA, et al. No evidence of clinical efficacy of famotidine for the treatment of COVID-19: a systematic review and meta-analysis. J Infect. 2022. https://doi.org/10.1016/j.jinf.2022.11.022.
Loubet P, et al. A french cohort for assessing COVID-19 vaccine responses in specific populations. Nat Med. 2021;27(8):1319–21.
Pormohammad A, et al. Efficacy and safety of COVID-19 vaccines: a systematic review and meta-analysis of randomized clinical trials. Vaccines (Basel). 2021. https://doi.org/10.3390/vaccines9050467.
Tavoosian A, Arabzadeh Bahri R, Zahmatkesh P, Khoshchehreh M, Aghsaee Fard Z, Abedi Yarandi V. Safe Medication with Remdesivir for COVID-19 in Patients with Infertility. Translational Research in Urology. 2023 May 1;5(2):89-94.
Suzich JB, Cliffe AR. Strength in diversity: understanding the pathways to herpes simplex virus reactivation. Virology. 2018;522:81–91.
Ong DSY, et al. Epidemiology of multiple herpes viremia in previously immunocompetent patients with septic shock. Clin Infect Dis. 2017;64(9):1204–10.
Simonnet A, et al. High incidence of Epstein-Barr virus, cytomegalovirus, and human-herpes virus-6 reactivations in critically ill patients with COVID-19. Infect Dis Now. 2021;51(3):296–9.
Shafiee A, et al. Epstein-Barr virus and COVID-19. J Med Virol. 2022;94(9):4040–2.
Shafiee A, Teymouri Athar MM, Amini MJ, Hajishah H, Siahvoshi S, Jalali M, Jahanbakhshi B, Mozhgani SH. Reactivation of herpesviruses during COVID-19: A systematic review and meta-analysis. Rev Med Virol. 2023 May;33(3):e2437. https://doi.org/10.1002/rmv.2437. Epub 2023 Mar 7. PMID: 36880642.
Katsikas Triantafyllidis K, et al. Varicella zoster virus reactivation following COVID-19 vaccination: a systematic review of case reports. Vaccines (Basel). 2021. https://doi.org/10.3390/vaccines9091013.
Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89.
Cumpston M, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane database syst reviews. 2019. https://doi.org/10.1002/14651858.ED000142.
JB Institute. The Joanna Briggs Institute critical appraisal tools for use in JBI systematic review: checklists for case reports. The Joanna Briggs Institute; 2019.
JB Institute. The Joanna Briggs Institute critical appraisal tools for use in JBI systematic reviews. checklist for case series; 2017.
Fazlollahi A, et al. Cardiac complications following mRNA COVID-19 vaccines: a systematic review of case reports and case series. Rev Med Virol. 2022;32(4): e2318.
Balduzzi S, Rücker G, Schwarzer G How to perform a meta-analysis with R: a practical tutorial BMJ Ment Health 2019;22:153-160.
Wells, G.A., et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Oxford; 2000.
Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153–60.
Birabaharan M, Kaelber DC, Karris MY. Risk of herpes zoster reactivation after messenger RNA COVID-19 vaccination: a cohort study. J Am Acad Dermatol. 2022;87(3):649–51.
Català A, et al. Cutaneous reactions after SARS-CoV-2 vaccination: a cross-sectional Spanish nationwide study of 405 cases. Br J Dermatol. 2022;186(1):142–52.
Cebeci Kahraman F, et al. Cutaneous reactions after COVID-19 vaccination in Turkey: a multicenter study. J Cosmet Dermatol. 2022;21(9):3692–703.
Hertel M, et al. Real-world evidence from over one million COVID-19 vaccinations is consistent with reactivation of the varicella-zoster virus. J Eur Acad Dermatol Venereol: JEADV. 2022;36(8):1342–8.
Lee TJ, Lu CH, Hsieh SC. Herpes zoster reactivation after mRNA-1273 vaccination in patients with rheumatic diseases. Ann Rheum Dis. 2022;81(4):595–7.
Préta LH, et al. Association study between herpes zoster reporting and mRNA COVID-19 vaccines (BNT162b2 and mRNA-1273). Br J Clin Pharmacol. 2022;88(7):3529–34.
Machado PM, et al. Safety of vaccination against SARS-CoV-2 in people with rheumatic and musculoskeletal diseases: results from the EULAR Coronavirus Vaccine (COVAX) physician-reported registry. Ann Rheum Dis. 2022;81(5):695–709.
Gringeri M, et al. Herpes zoster and simplex reactivation following COVID-19 vaccination: new insights from a vaccine adverse event reporting system (VAERS) database analysis. Expert Rev Vaccines. 2022;21(5):675–84.
Florea A, et al. Risk of herpes zoster following mRNA COVID-19 vaccine administration. Expert Rev Vaccines. 2023;22(1):643–9.
Fathy RA, et al. Varicella-zoster and herpes simplex virus reactivation post-COVID-19 vaccination: a review of 40 cases in an international dermatology registry. J Eur Acad Dermatol Venereol. 2022;36(1):e6–9.
Chen J, et al. Varicella zoster virus reactivation following COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a cross-sectional Chinese study of 318 cases. J Med Virol. 2023;95(1): e28307.
Zheng F, Willis A, Kunjukunju N. Acute retinal necrosis from reactivation of varicella zoster virus following BNT162b2 mRNA COVID-19 vaccination. Ocul Immunol Inflamm. 2022;30(5):1133–5.
Zhang LW, et al. Disseminated herpes zoster following inactivated SARS-CoV-2 vaccine in a healthy old man. Eur J dermatol: EJD. 2022;32(3):415–6.
You IC, Ahn M, Cho NC. A case report of herpes zoster ophthalmicus and meningitis after COVID-19 vaccination. J Korean Med Sci. 2022;37(20): e165.
Wang CS, Chen HH, Liu SH. Pityriasis Rosea-like eruptions following COVID-19 mRNA-1273 vaccination: a case report and literature review. J Formos Med Assoc. 2022;121(5):1003–7.
van Dam CS, et al. Herpes zoster after COVID vaccination. Int J Infect Dis. 2021;111:169–71.
Vallianou NG, et al. Herpes zoster following COVID-19 vaccination in an immunocompetent and vaccinated for herpes zoster adult: a two-vaccine related event? Metabol Open. 2022;13: 100171.
Tripathy DM, et al. Postherpetic granulomatous dermatitis and herpes zoster necroticans triggered by Covid-19 vaccination. Dermatol Ther. 2022;35(10): e15707.
Thonginnetra S, Limtanyakul P, Tawinprai K. Herpes zoster after COVID-19 vaccination in an adolescent. Dermatol online J. 2022. https://doi.org/10.5070/D328458533.
Thimmanagari K, et al. Ipsilateral zoster ophthalmicus post COVID-19 vaccine in healthy young adults. Cureus. 2021;13(7): e16725.
Tanizaki R, Miyamatsu Y. Zoster sine herpete following BNT162b2 mRNA COVID-19 vaccination in an immunocompetent patient. IDCases. 2022;29: e01563.
Tang WR, et al. A case report of posttransplant lymphoproliferative disorder after astrazeneca coronavirus disease 2019 vaccine in a heart transplant recipient. Transplant Proc. 2022;54(6):1575–8.
Tang LV, Hu Y. Hemophagocytic lymphohistiocytosis after COVID-19 vaccination. J Hematol Oncol. 2021;14(1):87.
Singh J, et al. Herpes simplex virus retinitis following ChAdOx1 nCoV- 19 (Covishield) vaccination for SARS CoV 2: a case report. Ocul Immunol Inflamm. 2022;30(5):1282–5.
Santovito LS, Pinna G. A case of reactivation of varicella-zoster virus after BNT162b2 vaccine second dose? Inflamm Res. 2021;70(9):935–7.
Sangoram R, et al. Herpes simplex virus 1 anterior uveitis following coronavirus disease 2019 (COVID-19) vaccination in an asian indian female. Ocul Immunol Inflamm. 2022;30(5):1260–4.
Said JT, et al. Disseminated varicella-zoster virus infections following messenger RNA-based COVID-19 vaccination. JAAD Case Rep. 2021;17:126–9.
Ryu KJ, Kim DH. Recurrence of varicella-zoster virus keratitis after SARS-CoV-2 vaccination. Cornea. 2022;41(5):649–50.
Poudel S, et al. Bell’s palsy as a possible complication of mRNA-1273 (Moderna) vaccine against COVID-19. Ann Med Surg (Lond). 2022;78: 103897.
Plüß M, et al. Case report: cytomegalovirus reactivation and pericarditis following ChAdOx1 nCoV-19 vaccination against SARS-CoV-2. Front Immunol. 2021;12: 784145.
Pedrazini MC, da Silva MH. Pityriasis rosea-like cutaneous eruption as a possible dermatological manifestation after Oxford-AstraZeneca vaccine: case report and brief literature review. Dermatol Ther. 2021;34(6): e15129.
Papasavvas I, de Courten C, Herbort CP Jr. Varicella-zoster virus reactivation causing herpes zoster ophthalmicus (HZO) after SARS-CoV-2 vaccination - report of three cases. J Ophthalmic Inflamm Infect. 2021;11(1):28.
Palanivel JA. Herpes zoster after COVID-19 vaccination-Can the vaccine reactivate latent zoster virus? J Cosmet Dermatol. 2021;20(11):3376–7.
Özdemir AK, Kayhan S, Çakmak SK. Herpes zoster after inactivated SARS-CoV-2 vaccine in two healthy young adults. J Eur Acad Dermatol Venereol. 2021;35(12):e846–7.
Ota M. SARS-CoV-2 mRNA vaccination and subsequent herpes zoster: possible immune reconstitution by mRNA vaccination. JAAD Case Rep. 2022. https://doi.org/10.1016/j.jdcr.2022.02.035.
Nishimoto M, Sogabe N, Hino M. Visceral disseminated varicella zoster virus infection following COVID-19 vaccination in an allogeneic stem cell transplant recipient. Transpl Infect Dis. 2022;24(2): e13810.
Nastro F, et al. Small vessel vasculitis related to varicella-zoster virus after Pfizer-BioNTech COVID-19 vaccine. J Eur Acad Dermatol Venereol. 2021;35(11):e745–7.
Munasinghe BM, et al. Reactivation of varicella-zoster virus following mRNA COVID-19 vaccination in a patient with moderately differentiated adenocarcinoma of rectum: a case report. SAGE Open Med Case Rep. 2022. https://doi.org/10.1177/2050313X221077737.
Muhie OA, et al. Herpes zoster following covaxin receipt. Int Med Case Rep J. 2021;14:819–21.
Mishra SB, et al. Reactivation of varicella zoster infection presenting as acute retinal necrosis post COVID 19 vaccination in an asian indian male. Eur J Ophthalmol. 2021. https://doi.org/10.1177/11206721211046485.
Medhat R, et al. Varicella-Zoster virus (VZV) meningitis in an immunocompetent adult after BNT162b2 mRNA COVID-19 vaccination: a case report. Int J Infect Dis. 2022;119:184–6.
Maruki T, et al. A case of varicella zoster virus meningitis following BNT162b2 mRNA COVID-19 vaccination in an immunocompetent patient. Int J Infect Dis. 2021;113:55–7.
Maranini B, et al. Herpes zoster infection following mRNA COVID-19 vaccine in a patient with ankylosing spondylitis. Reumatismo. 2021. https://doi.org/10.4081/reumatismo.2021.1445.
Maldonado MD, Romero-Aibar J. The Pfizer-BNT162b2 mRNA-based vaccine against SARS-CoV-2 may be responsible for awakening the latency of herpes varicella-zoster virus. Brain Behav Immun Health. 2021;18: 100381.
Lv Y, Chang Y. Cytomegalovirus proctitis developed after COVID-19 vaccine: a case report and literature review. Vaccines (Basel). 2022. https://doi.org/10.3390/vaccines10091417.
Lo T, et al. Varicella zoster reactivation causing acute retinal necrosis following mrna covid-19 vaccination in a young immunocompetent man. Ocul Immunol Inflamm. 2022. https://doi.org/10.1080/09273948.2022.2033795.
Lin TY, et al. Hemophagocytic lymphohistiocytosis following BNT162b2 mRNA COVID-19 vaccination. Vaccines (Basel). 2022. https://doi.org/10.3390/vaccines10040573.
Lim ZV, Kanagalingam J, Heng YK. Response to “Varicella-zoster virus reactivation after SARS- CoV-2 BNT162b2 mRNA vaccination: report of 5 cases.” JAAD Case Rep. 2022. https://doi.org/10.1016/j.jdcr.2021.09.044.
Li SQ, et al. Herpetic keratitis preceded by COVID-19 vaccination. Vaccines. 2021. https://doi.org/10.3390/vaccines9121394.
Koh S, et al. Varicella zoster virus reactivation in central and peripheral nervous systems following COVID-19 vaccination in an immunocompetent patient. J Clin Neurol. 2022;18(1):99–101.
Kluger N, Klimenko T, Bosonnet S. Herpes simplex, herpes zoster and periorbital erythema flares after SARS-CoV-2 vaccination: 4 cases. Ann Dermatol Venereol. 2022;149(1):58–60.
Kerr C, et al. Zoster meningitis in an immunocompetent young patient post first dose of BNT162b2 mRNA COVID-19 vaccine, a case report. IDCases. 2022;27: e01452.
Jiang ZH, et al. Disseminated and localised herpes zoster following Oxford-AstraZeneca COVID-19 vaccination. Indian J Dermatol Venereol Leprol. 2022;88(3):445.
Jeong J. Vestibular neuritis after COVID-19 vaccination. Hum Vaccin Immunother. 2021;17(12):5126–8.
Herzum A, et al. Epstein-Barr virus reactivation after COVID-19 vaccination in a young immunocompetent man: a case report. Clin Exp Vaccine Res. 2022;11(2):222–5.
Girardin FR, et al. Multifocal lymphadenopathies with polyclonal reactions primed after EBV infection in a mRNA-1273 vaccine recipient. Swiss Med Wkly. 2022;152: w30188.
Fukuoka H, et al. Oral herpes zoster infection following COVID-19 vaccination: a report of five cases. Cureus. 2021;13(11): e19433.
Eid E, et al. Herpes zoster emergence following mRNA COVID-19 vaccine. J Med Virol. 2021;93(9):5231–2.
Dermawan A, et al. Acute herpes zoster radiculopathy mimicking cervical radiculopathy after ChAdOx1 nCoV-19/AZD1222 vaccination. BMJ Case Rep. 2022. https://doi.org/10.1136/bcr-2022-248943.
Chiu HH, et al. Herpes zoster following COVID-19 vaccine: a report of three cases. QJM. 2021;114(7):531–2.
Buranasakda M, et al. Varicella zoster meningitis following COVID-19 vaccination: a report of two cases. Int J Infect Dis. 2022. https://doi.org/10.1016/j.ijid.2022.03.055.
Atiyat R, et al. Varicella-zoster virus reactivation in aids patient after pfizer-bioNTech COVID-19 vaccine. Cureus. 2021;13(12): e20145.
Arora P, et al. Herpes zoster after inactivated COVID-19 vaccine: A cutaneous adverse effect of the vaccine. J Cosmet Dermatol. 2021;20(11):3389–90.
Ardalan M, et al. Herpes-like skin lesion after AstraZeneca vaccination for COVID-19: a case report. Clin Case Rep. 2021;9(10): e04883.
Alkhalifah MI, et al. Herpes simplex virus keratitis reactivation after SARS-CoV-2 BNT162b2 mRNA vaccination: a report of two cases. Ocul Immunol Inflamm. 2021;29(6):1238–40.
Algaadi SA. Herpes zoster after COVID-19 vaccine: A case report. Pak J Med Health Sci. 2021;15(3):1165–6.
Al-Dwairi RA, et al. Reactivation of herpes simplex keratitis on a corneal graft following SARS-CoV-2 mRNA vaccination. Med Arch. 2022;76(2):146–8.
Aksu SB, Öztürk GZ. A rare case of shingles after COVID-19 vaccine: is it a possible adverse effect? Clin Exp Vaccine Res. 2021;10(2):198–201.
Abu-Rumeileh S, et al. Varicella zoster virus-induced neurological disease after COVID-19 vaccination: a retrospective monocentric study. J Neurol. 2022;269(4):1751–7.
Alkwikbi H, et al. Herpetic keratitis and corneal endothelitis following COVID-19 vaccination: a case series. Cureus. 2022;14(1): e20967.
Almutairi N, et al. Herpes zoster in the era of COVID 19: A prospective observational study to probe the association of herpes zoster with COVID 19 infection and vaccination. Dermatol Ther. 2022;35(7): e15521.
Chakravorty S, et al. CMV infection following mRNA SARS-CoV-2 vaccination in solid organ transplant recipients. Transplantation direct. 2022;8(7): e1344.
Chew MC, et al. Incidence of COVID-19 vaccination-related uveitis and effects of booster dose in a tertiary uveitis referral center. Front Med. 2022;9: 925683.
Furer V, et al. Herpes zoster following BNT162b2 mRNA COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology (Oxford). 2021. https://doi.org/10.1093/rheumatology/keab345.
Job AM, et al. Herpes zoster following vaccination with ChAdOx1 nCoV-19 Coronavirus vaccine (recombinant). Indian J Public Health. 2022;66(1):83–5.
Monastirli A, et al. Herpes zoster after mRNA COVID-19 vaccination: a case series. Skinmed. 2022;20(4):284–8.
Psichogiou M, et al. Reactivation of varicella zoster virus after vaccination for SARS-CoV-2. Vaccines (Basel). 2021. https://doi.org/10.3390/vaccines9060572.
Rodríguez-Jiménez P, et al. Varicella-zoster virus reactivation after SARS-CoV-2 BNT162b2 mRNA vaccination: report of 5 cases. JAAD Case Rep. 2021;12:58–9.
Saraiva AL, et al. Varicella zoster virus reactivation following COVID-19 vaccination: a report of 3 cases. Fam Pract. 2022. https://doi.org/10.1093/fampra/cmac014.
Zhang Z, et al. Cytomegalovirus reactivation in immunocompetent mechanical ventilation patients: a prospective observational study. BMC Infect Dis. 2021;21(1):1026.
Tang WR, et al. A case report of posttransplant lymphoproliferative disorder after astrazeneca coronavirus disease 2019 vaccine in a heart transplant recipient. Transplant Proc. 2021. https://doi.org/10.1016/j.transproceed.2021.09.006.
Daouk SK, et al. Zoster meningitis in an immunocompetent child after COVID-19 Vaccination, California, USA. Emerg Infect Dis. 2022;28(7):1523–4.
Grinde B. Herpesviruses: latency and reactivation - viral strategies and host response. J Oral Microbiol. 2013. https://doi.org/10.3402/jom.v5i0.22766.
Traylen CM, et al. Virus reactivation: a panoramic view in human infections. Future Virol. 2011;6(4):451–63.
Amon W, Farrell PJ. Reactivation of Epstein-Barr virus from latency. Rev Med Virol. 2005;15(3):149–56.
Mendelson M, et al. Detection of endogenous human cytomegalovirus in CD34+ bone marrow progenitors. J Gen Virol. 1996;77(Pt 12):3099–102.
Yoshikawa T, et al. The characteristic site-specific reactivation phenotypes of HSV-1 and HSV-2 depend upon the latency-associated transcript region. J Exp Med. 1996;184(2):659–64.
Stoeger T, Adler H. “Novel” triggers of herpesvirus reactivation and their potential health relevance. Front Microbiol. 2018;9:3207.
Seneff S, et al. Innate immune suppression by SARS-CoV-2 mRNA vaccinations: the role of G-quadruplexes, exosomes, and MicroRNAs. Food Chem Toxicol. 2022;164: 113008.
Liu J, et al. Comprehensive investigations revealed consistent pathophysiological alterations after vaccination with COVID-19 vaccines. Cell Discov. 2021;7(1):99.
Kolumam GA, et al. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202(5):637–50.
Desai HD, et al. Can SARS-CoV-2 vaccine increase the risk of reactivation of Varicella zoster? A systematic review J Cosmet Dermatol. 2021;20(11):3350–61.
Martinez-Reviejo R, et al. Varicella-Zoster virus reactivation following severe acute respiratory syndrome coronavirus 2 vaccination or infection: new insights. Eur J Intern Med. 2022;104:73–9.
Musialik J, Kolonko A, Więcek A. Increased EBV DNAemia after Anti-SARS-CoV-2 vaccination in solid organ transplants. Vaccines. 2022. https://doi.org/10.3390/vaccines10070992.
Acknowledgements
Not applicable.
Funding
The authors would like to acknowledge Alborz Univerisity of Medical Sciences for their financial grant (Grant no.:5746).
Author information
Authors and Affiliations
Contributions
AS contributed to project administration, conceptualization, investigation, project administration, writing––original draft, and writing––review and editing. MJA and RAB were involved in conceptualization, investigation, and writing––original draft. KJ, HH, SAS, and SHM performed investigation, writing––original draft, and writing––review and editing.
Corresponding author
Ethics declarations
Ethics approval consent to participate
Not applicable.
Competing interests
No conflict of interest is declared.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file1: Table S1
. PRISMA 2020 checklist. Table S2. Databases searched and search strategies employed. Table S3. Detailed results of included case reports. Table S4. Detailed results of included case series. Table S5. The results of quality assessment for observational studies. Table S6. The results of quality assessment for case reports. Table S7. The results of quality assessment for case series.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shafiee, A., Amini, M.J., Arabzadeh Bahri, R. et al. Herpesviruses reactivation following COVID-19 vaccination: a systematic review and meta-analysis. Eur J Med Res 28, 278 (2023). https://doi.org/10.1186/s40001-023-01238-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-023-01238-9