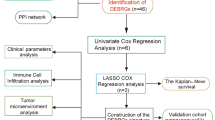
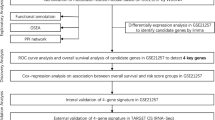
Abstract
Background
Osteosarcoma (OS) has been the most common malignancy of the bone in children and adolescents, and the unsatisfactory prognosis of OS sufferers has long been a hard nut. Here, we delved into the markers with a prognostic value for predicting the prognosis of OS patients.
Methods
The messenger RNA (mRNA) sequencing data and clinical data of OS were retrieved from a Gene Expression Omnibus (GEO) dataset (GSE39058). Next, prognosis-related genes (PRGs) were filtered with the aid of Kaplan–Meier (K-M) curves and Cox regression analysis (CRA). Later, Gene Ontology (GO) biological process analysis was used in verifying the function of different genes. CCK-8 and cell apoptosis assay were performed to evaluate the function of MFNG in U2OS cells.
Results
Among the obtained genes, Manic Fringe (MFNG) had the closest relevance to prognosis and clinical traits, thus becoming the research object herein. In light of the expression level of MFNG, patients fell into high- and low-MFNG groups. Patients with elevated MFNG expression had a worse prognosis, according to the survival analysis. It was unveiled by the univariate and multivariate analyses that MFNG expression was an independent adverse prognostic factor for disease-free survival in OS patients (p = 0.006). Meanwhile, MFNG expression was linked to gender and tumor recurrence, and it was higher in patients with OS recurrence. Moreover, overexpression of MFNG promoted the cell proliferation and inhibited the cell apoptosis of U2OS cells.
Conclusions
The expression level of MFNG negatively correlated with OS progression, and as an independent adverse prognostic factor for disease-free survival in OS patients. Moreover, MFNG regulated the cell proliferation and apoptosis of OS cells.
Similar content being viewed by others
Background
Osteosarcoma (OS), with primitive mesenchymal cells as its origin, is one of the most found primary solid malignancy of the bone, showing an annual prevalence of around 4.8 per million [1,2,3]. OS, frequently arising in the metaphysis of long bone, features high disability rate. By reason that OS inclines to metastasizing to other sites, especially the lung, OS cases have been plagued by unsatisfactory prognosis [4]. In spite of a 60–70% five-year survival rate of localized OS cases following surgical excision, neoadjuvant chemotherapy and postoperative chemotherapy, the rate was under 30% in OS cases experiencing metastasis [5, 6]. As unveiled by a report, advancement in therapies for OS is limited by the complicated and unstable genome [7], which manifest a necessity for advances in early diagnosis, therapy, and prognosis prediction of OS regarding molecular genetics.
Manic Fringe (MFNG) encodes β-1,3-N-acetylglucosaminyltransferase manic fringe that modifies epidermal growth factor repeats in the extracellular domain of Notch [8]. Reports show that MFNG functions as an oncogene and exhibits a high expression in claudin-low breast cancer (CLBC) [9]. A lowered MFNG level limits neovascularization and the migration of renal carcinoma cells [10]. May wa's team reported that an elevation of MFNG was detectable in Ewing's sarcoma cells, and that NIH 3T3 cells with overexpressed MFNG display tumorigenicity in mice suffering severe combined immunodeficiency disease [11]. Ewing's sarcoma is one of the most frequently found malignancies of the bone besides OS, usually arise in childhood and adolescence [12, 13]. Nonetheless, the function of MFNG in OS prognosis is far from understood.
We found that MFNG was an independent prognosis-related gene (PRG) in OS by analyzing the GSE39058. Thus, with the aid of gene expression data in GSE39058, we delved into the interrelation between MFNG expression and OS progression, recurrence, and survival. It was unveiled that high MFNG expression was indicative of adverse outcomes of OS.
Methods
Data acquisition
We retrieved gene expression microarray of OS by simultaneously entering "osteosarcoma" and "survival" in the search box in the gene expression omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo/), and singled out GSE39058 as a training dataset herein. Later, we directly filtered clinical prognostic data from the matrix file of microarray datasets in the GEO database, and the messenger RNA (mRNA) sequencing data were utilized for mRNA profiling.
Screening of PRGs
Kaplan–Meier (K-M) curves and Cox regression analysis (CRA) were adopted for filtering PRGs by use of "survival" package in R with the thresholds of K-M < 0.01 and p < 0.01 (Additional file 1: Table S1).
Target gene identification
After screening of PRGs, multivariate CRA was employed to perform independent prognostic analysis (p < 0.01) to obtain genes significantly associated with independent prognosis. Then, we analyzed the correlation between independent PRGs and clinical traits by wilcox.test or kruskal.test. As MFNG had the highest correlation with clinical traits, it was selected as the research object.
All OS patients fell into high- (> median, n = 21) and low- MFNG groups (≤median, n = 21) as per the MFNG expression level. Survival analysis of MFNG in the prognostic model was implemented with the aid of the K-M curve and log-rank test via the "survival" and "survminer" packages in R.
Statistical assessment
R-3.5.0 was utilized for assessment of all data. Hypothesis testing in categorical and continuous variables was implemented by the Fisher exact and Wilcoxon rank-sum tests, respectively. The limma package was employed for the assessment of differentially expressed genes (DEGs). Further, the survival was estimated by use of the K-M method and multivariate CRA, and the log-rank test was carried out for intergroup comparisons. Additionally, enriched Gene Ontology (GO) terms were filtered by use of "Clusterprofiler" package.
Results
Identification of MFNG as a target gene
We got GSE39058 from GEO database (https://www.ncbi.nlm.nih.gov/geo/). As per the probe information from Illumina HumanHT-12 WG-DASL V4.0 R2 Expression BeadChip (GPL14951), the annotation of 20,791 genes in 47 OS samples from GSE39058 was annotated. The results of screening of PRGs in OS with K-M curves (KM < 0.01) and CRA (coxPvalue < 0.01) by “survival” R package are shown in Additional file 1: Table S1. After multivariate CRA was adopted for independent prognostic analysis (p < 0.01), we obtained the genes significantly associated with independent prognosis. Next, wilcox.test or kruskal.test (p < 0.05) displayed the correlation between independent PRGs and clinical traits (age,gender,and recurrence) (Table 1). It was uncovered that MFNG was associated with two clinical traits (gender and recurrence), so we chose MFNG for follow-up study.
Survival outcomes in high-MFNG group and low-MFNG group
All OS patients fell into high- (> median, n = 21) and low- MFNG groups (≤median, n = 21) as per the MFNG expression level. Survival analysis of MFNG in the prognostic model was implemented with the aid of the K-M curve and log-rank test via the "survival" and "survminer" packages in R. As shown in Fig. 1, OS patients in high-MFNG group had worse outcome than those in low-MFNG group.
In univariate and multivariate CRA, MFNG exhibited notable relevance to the prognosis of OS and was proven to be an independent prognostic factor (PF) (Fig. 2A, B; Additional file 2: Table S2).
Differences in clinical data of OS cases between high-MFNG group and low-MFNG group
A report shows that worldwide, 15–19-year-old boys and 10–14-year-old girls displayed the highest OS prevalence, in line with age of puberty [14]. Age groups include < 10-year-old group, 10–20-year-old group, and > 20-year-old group. In the current study, the differences in clinical data of OS cases between high-MFNG group and low-MFNG group were analyzed through "ggpubr" package. There was no significant difference in the age between high-MFNG group and low-MFNG group (Fig. 3A), while there were significant differences in the gender and recurrence (Fig. 3B, C). It can be concluded that male OS patients have a higher tendency of low MFNG expression, and OS patients with no recurrence have a higher tendency of low MFNG expression.
Analysis of DEGs and pathways in high-MFNG group versus low-MFNG group
With the aim for determining MFNG-associated genes, the expression of DEGs was compared between high-MFNG group and low-MFNG group. The results showed 19 up-regulated genes and 3 down-regulated genes (adj.P < 0.05, (FC, log2) > 0.5 or < -0.5, Fig. 4A, B, Additional file 3: Table S3). Later, the enriched GO terms were assessed by use of DEGs. It was uncovered that a majority of DEGs were enriched in GO terms involving the biological process, including Notch signaling pathway (GO:0007219), positive regulation of phosphatidylinositol 3-kinase signaling pathway (GO:0008593), regulation of Notch signaling pathway (GO:0008593), regulation of phosphatidylinositol 3-kinase (PI3K) signaling pathway (GO:0014066) and regulation of mRNA processing (GO:0050684) (Fig. 4C, Additional file 4: Table S4).
Analysis of DEGs and pathways in high-MFNG group versus low-MFNG group. A Heatmap and B volcano plot show DEGs between high-MFNG group versus low-MFNG groups. Cut-off criteria for DEGs significance was adj.P < 0.05 and the absolute value of the log2 fold change > 0.5. C GO result for differential expression genes
Module screening from the protein–protein interaction (PPI) network
We measured the correlation among the top 22 DEGs in high-MFNG group and low-MFNG group (Fig. 5A). Additionally, the PPI network of 22 DEGs was built with the use of the String database, which unveiled interactions among most of the up-regulated genes and all down-regulated genes (Fig. 5B).
MFNG promotes the cell proliferation and inhibits the cell apoptosis of U2OS cells
In Fig. 5B, we can see that hematopoietic cell signal transducer (HCST) is co-expression with MFNG. Reports showed that HCST affects cell proliferation and survival by participating in the natural killer and T cell responses and in the activation of PI3K-dependent signaling pathway [15]. Thus, we want to explore the role of MFNG in OS cells. We overexpressed MFNG in OS cell line U2OS and found that MFNG overexpression (MFNG OE) promoted the cell proliferation and inhibited the cell apoptosis of U2OS cells (Fig. 6A, B).
Discussion
OS is the most found malignancy of the bone arising in childhood and adulthood, showing a high tendency of invasion and metastasis [1, 16, 17]. Since 2000, threefold elevation of the OS incidence rate has been observed in 0–24-year-old cases [2]. Up-to-now therapies for OS patients are neoadjuvant chemotherapy, surgery, radiotherapy and chemotherapy [18, 19]. Nonetheless, the high malignancy of OS results in poor overall survival of OS patients, especially those in the advanced stage [2, 20, 21]. Hence, in-depth deciphering of the molecular pathomechanism by which OS occurs and progresses appears to be a necessity, which benefits filtering of pivotal molecules or biomarkers for early diagnosis, targeted therapy, and prognosis assessment of OS.
Our research demonstrated that lower MFNG expression in OS cases showed relevance to satisfactory prognosis and low recurrence. MFNG has also been reported to be associated with the recurrence of ovarian carcinoma [22]. As unveiled by a study of Zhang et al., a high MFNG expression was detectable in CLBC and served as an oncogene by activating Notch signaling, thus hastening tumor cell migration, tumorsphere formation, and epithelial-to-mesenchymal transition (EMT) [9]. Beyond that, MFNG turns out to be a determinant player in optimal B and T cell development. In detail, it leverages a facilitating role in Th1 cell development and a suppressing role in Th2 cell development [23, 24].
Likewise, enrichment of the GO pathways is mainly detectable in the Notch signaling pathway and regulation of PI3K signaling pathway. Notch signaling pathways are a class of highly conserved signaling pathways in multicellular organisms that mediate the influence and regulation of the external environment on cells by participating in intercellular interactions, and programmatically manipulate cell fate and tissue differentiation in the early development of organisms [25]. Notch signaling pathways modulate cell invasion, adhesion, proliferation, apoptosis, and differentiation by virtue of cell–cell interactions [26, 27]. Beyond that, the growth and progression of human malignant tumors including OS appear to be subjected to influences by the Notch signaling pathway [28, 29]. For example, the Notch signaling pathway probably drives the differentiation of bone marrow mesenchymal stem cells into osteoblasts, thus facilitating ectopic bone formation [30]. DLX5 activates the Notch signaling pathway to benefit OS progression [31]. Suppressing the Notch signaling pathway put a brake on the progression of cell motility, metastasis, and EMT-like phenomena resulting from low-level cisplatin in OS [32]. Overexpression of lncRNA CEBPA-AS1 keeps a rein on OS cell proliferation and migration and elicits their apoptosis via the Notch signaling pathway [33]. On the other hand, activating the Notch signaling pathway can control the differentiation of macrophages into M1 and benefits inflammation and antitumor activity, but impeding the Notch signaling pathway polarizes macrophages to M2, so as to hinder inflammation and drive tumor growth [34].
PI3K, a family of lipid kinases, modulates a cascade of physiological cell processes such as metabolism, motility, exocytosis differentiation, proliferation, apoptosis, exocytosis and autophagy [35]. Subsequent to activation at the cell surface, the PI3K signaling pathway integrates signals from cytokines, growth factors and environmental signals and delivers them to effector molecules controlling protein synthesis, growth, survival and proliferation via protein kinase B (AKT) and mammalian target of rapamycin (mTOR) [36, 37]. AKT is the vital mediator of the PI3K signaling pathway, and its abnormal activation is interrelated to a multifold of malignant tumors [38, 39], including OS. For example, LINC00968 activates the PI3K/AKT/mTOR signaling pathway to exert an oncogene action [40]. PI3K inhibitors impair tumor progression and enhance sensitivity to anlotinib in anlotinib-resistant OS [41]. MDA19 inhibits OS via suppressing the PI3K/Akt/mTOR signaling pathway [42].
The PPI network also revealed that hematopoietic cell signal transducer (HCST) was co-expressed with MFNG. Reports show that HCST participates in the activation of PI3K-dependent signaling pathway and in the natural killer and T cell responses, which affect cell survival and proliferation [15]. Besides, HCST is interrelated to the poor prognosis of clear cell renal cell carcinoma [43]. We also performed CCK-8 and cell apoptosis assay in U2OS cells which were transfected with vector or MFNG OE. We found that MFNG OE promoted the cell proliferation and inhibited the cell apoptosis of U2OS cells.
However, there still some limitations in our study. Firstly, the current research is only based on public databases, we will further collect clinical specimens to verify our conclusion. Secondly, the specific physiopathologic mechanism developed by MFNG to function in OS cells has not yet been fully understood. In the future, we will conduct more detailed molecular mechanism research and gain a deeper understanding of MFNG at the OS genome level.
Conclusions
In closing, the low MFNG expression is a good PF in OS cases, while the high MFNG expression is associated with recurrence of OS. Additionally, enriched GO terms and PPI networks pertinent to OS shed light on the delving into the pathogenesis of high and low MFNG expressions.
Availability of data and materials
Not applicable.
Abbreviations
- OS:
-
Osteosarcoma
- GEO:
-
Gene expression omnibus
- K-M:
-
Kaplan–Meier curve
- PRG:
-
Prognosis-related gene
- CRA:
-
Cox regression analysis
- GO:
-
Gene ontology
- MFNG:
-
Manic fringe
References
Vundavilli H, Datta A, Sima C, Hua J, Lopes R, Bittner M, Miller T, Wilson-Robles HM. Anti-tumor effects of cryptotanshinone (C19H20O3) in human osteosarcoma cell lines. Biomed Pharmacother. 2022;150: 112993.
Cole S, Gianferante DM, Zhu B, Mirabello L. Osteosarcoma: a surveillance, epidemiology, and end results program-based analysis from 1975 to 2017. Cancer. 2022;128(11):2107–18.
Folkert IW, Devalaraja S, Linette GP, Weber K, Haldar M. Primary bone tumors: challenges and opportunities for CAR-T therapies. J Bone Miner Res. 2019;34(10):1780–8.
Sun M, Wang Z, Sun W, Chen M, Ma X, Shen J, Fu Z, Zuo D, Wang G, Wang H, Wang C, Yin F, Wang Z, et al. Correlation between patient-derived xenograft modeling and prognosis in osteosarcoma. Orthop Surg. 2022;14(6):1161–6.
Smeland S, Bielack SS, Whelan J, Bernstein M, Hogendoorn P, Krailo MD, Gorlick R, Janeway KA, Ingleby FC, Anninga J, Antal I, Arndt C, Brown KLB, et al. Survival and prognosis with osteosarcoma: outcomes in more than 2000 patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort. Eur J Cancer. 2019;109:36–50.
Koster R, Panagiotou OA, Wheeler WA, Karlins E, Gastier-Foster JM, Caminada de Toledo SR, Petrilli AS, Flanagan AM, Tirabosco R, Andrulis IL, Wunder JS, Gokgoz N, Patino-Garcia A, et al. Genome-wide association study identifies the GLDC/IL33 locus associated with survival of osteosarcoma patients. Int J Cancer. 2018; 142(8):1594–1601.
Wu CC, Livingston JA. Genomics and the immune landscape of osteosarcoma. Adv Exp Med Biol. 2020;1258:21–36.
Pennarubia F, Nairn AV, Takeuchi M, Moremen KW, Haltiwanger RS. Modulation of the NOTCH1 pathway by LUNATIC FRINGE is dominant over that of MANIC or RADICAL FRINGE. Molecules. 2021;26(19):5942.
Zhang S, Chung WC, Wu G, Egan SE, Miele L, Xu K. Manic fringe promotes a claudin-low breast cancer phenotype through notch-mediated PIK3CG induction. Cancer Res. 2015;75(10):1936–43.
Cheng WK, Oon CE, Kaur G, Sainson RCA, Li JL. Downregulation of Manic fringe impedes angiogenesis and cell migration of renal carcinoma. Microvasc Res. 2022;142: 104341.
May WA, Arvand A, Thompson AD, Braun BS, Wright M, Denny CT. EWS/FLI1-induced manic fringe renders NIH 3T3 cells tumorigenic. Nat Genet. 1997;17(4):495–7.
Zhan H, Mo F, Zhu M, Xu X, Zhang B, Liu H, Dai M. A SEER-based nomogram accurately predicts prognosis in Ewing’s sarcoma. Sci Rep. 2021;11(1):22723.
Zhang J, Huang J, Liu W, Ding L, Cheng D, Xiao H. Identification of common oncogenic genes and pathways both in osteosarcoma and ewing’s sarcoma using bioinformatics analysis. J Immunol Res. 2022;2022:3655908.
Whelan JS, Davis LE. Osteosarcoma, chondrosarcoma, and chordoma. J Clin Oncol. 2018;36(2):188–93.
Wang W, Li S, Lin J, Guo X, Xie Y, Li W, Hao Y, Jiang X. The roles and potential mechanisms of HCST in the prognosis and immunity of KIRC via comprehensive analysis. Am J Transl Res. 2022;14(2):752–71.
Liu H, Shu W, Liu T, Li Q, Gong M. Analysis of the function and mechanism of DIRAS1 in osteosarcoma. Tissue Cell. 2022;76: 101794.
Zhang G, Zhu Y, Jin C, Shi Q, An X, Song L, Gao F, Li S. CircRNA_0078767 promotes osteosarcoma progression by increasing CDK14 expression through sponging microRNA-330-3p. Chem Biol Interact. 2022;360: 109903.
Kokkali S, Kotsantis I, Magou E, Sophia T, Kormas T, Diakoumis G, Spathas N, Psyrri A, Ardavanis A. The addition of the immunomodulator mifamurtide to adjuvant chemotherapy for early osteosarcoma: a retrospective analysis. Invest New Drugs. 2022;40(3):668–75.
Zhu J, Simayi N, Wan R, Huang W. CAR T targets and microenvironmental barriers of osteosarcoma. Cytotherapy. 2022;24(6):567–76.
Zhou C, Sun Y, Gong Z, Li J, Zhao X, Yang Q, Yu H, Ye J, Liang J, Jiang L, Zhang D, Shen Z, Zheng S. FAT1 and MSH2 are predictive prognostic markers for chinese osteosarcoma patients following chemotherapeutic treatment. J Bone Miner Res. 2022;37(5):885–95.
Harris MA, Hawkins CJ. Recent and ongoing research into metastatic osteosarcoma treatments. Int J Mol Sci. 2022;23(7):3817.
Chen F, Liu N. A 10-gene expression signature of Notch pathway predicts recurrence in ovarian carcinoma. Oncol Lett. 2015;10(3):1704–8.
Gu W, Xu W, Ding T, Guo X. Fringe controls naive CD4(+)T cells differentiation through modulating notch signaling in asthmatic rat models. PLoS ONE. 2012;7(10): e47288.
Song Y, Kumar V, Wei HX, Qiu J, Stanley P. Lunatic, manic, and radical fringe each promote T and B cell development. J Immunol. 2016;196(1):232–43.
Shim YS, Lee HS, Hwang JS. Aberrant Notch signaling pathway as a potential mechanism of central precocious puberty. Int J Mol Sci. 2022;23(6):3332.
Guo S, Quan S, Zou S. Roles of the Notch signaling pathway in ovarian functioning. Reprod Sci. 2021;28(10):2770–8.
Yang L, Wang X, Sun J, Liu C, Li G, Zhu J, Huang J. Neuritin promotes angiogenesis through inhibition of DLL4/Notch signaling pathway. Acta Biochim Biophys Sin (Shanghai). 2021;53(6):663–72.
Gao Y, Bai L, Shang G. Notch-1 promotes the malignant progression of osteosarcoma through the activation of cell division cycle 20. Aging (Albany NY). 2020;13(2):2668–80.
Zieba JT, Chen YT, Lee BH, Bae Y. Notch signaling in skeletal development, homeostasis and pathogenesis. Biomolecules. 2020;10(2):332.
Guo X, Jiang H, Zong X, Du L, Zhao J, Zhang D, Song G, Jin X. The implication of the notch signaling pathway in biphasic calcium phosphate ceramic-induced ectopic bone formation: a preliminary experiment. J Biomed Mater Res A. 2020;108(5):1035–44.
Zhang X, Bian H, Wei W, Wang Q, Chen J, Hei R, Chen C, Wu X, Yuan H, Gu J, Lu Y, Cai C, Zheng Q. DLX5 promotes osteosarcoma progression via activation of the NOTCH signaling pathway. Am J Cancer Res. 2021;11(6):3354–74.
Dai G, Liu G, Zheng D, Song Q. Inhibition of the Notch signaling pathway attenuates progression of cell motility, metastasis, and epithelial-to-mesenchymal transition-like phenomena induced by low concentrations of cisplatin in osteosarcoma. Eur J Pharmacol. 2021;899: 174058.
Xia P, Gu R, Zhang W, Sun YF. lncRNA CEBPA-AS1 overexpression inhibits proliferation and migration and stimulates apoptosis of OS cells via notch signaling. Mol Ther Nucleic Acids. 2020;19:1470–81.
Chen W, Liu Y, Chen J, Ma Y, Song Y, Cen Y, You M, Yang G. The Notch signaling pathway regulates macrophage polarization in liver diseases. Int Immunopharmacol. 2021;99: 107938.
Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7(8):606–19.
Jean S, Kiger AA. Classes of phosphoinositide 3-kinases at a glance. J Cell Sci. 2014;127(Pt 5):923–8.
Evangelisti C, Evangelisti C, Bressanin D, Buontempo F, Chiarini F, Lonetti A, Soncin M, Sparta A, McCubrey JA, Martelli AM. Targeting phosphatidylinositol 3-kinase signaling in acute myelogenous leukemia. Expert Opin Ther Targets. 2013;17(8):921–36.
Amirani E, Hallajzadeh J, Asemi Z, Mansournia MA, Yousefi B. Effects of chitosan and oligochitosans on the phosphatidylinositol 3-kinase-AKT pathway in cancer therapy. Int J Biol Macromol. 2020;164:456–67.
Li D, Wang D, Liu H, Jiang X. LEM domain containing 1 (LEMD1) transcriptionally activated by SRY-related high-mobility-group box 4 (SOX4) accelerates the progression of colon cancer by upregulating phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) signaling pathway. Bioengineered. 2022;13(4):8087–100.
Liu G, Yuan D, Sun P, Liu W, Wu PF, Liu H, Yu GY. LINC00968 functions as an oncogene in osteosarcoma by activating the PI3K/AKT/mTOR signaling. J Cell Physiol. 2018;233(11):8639–47.
Chen C, Guo Y, Huang Q, Wang B, Wang W, Niu J, Lou J, Xu J, Ren T, Huang Y, Guo W. PI3K inhibitor impairs tumor progression and enhances sensitivity to anlotinib in anlotinib-resistant osteosarcoma. Cancer Lett. 2022;536: 215660.
Liu B, Xu L, Dai EN, Tian JX, Li JM. Anti-tumoral potential of MDA19 in human osteosarcoma via suppressing PI3K/Akt/mTOR signaling pathway. 2018. Biosci Rep. https://doi.org/10.1042/BSR20181501.
Zhou Y, Wang X, Zhang W, Liu H, Liu D, Chen P, Xu D, Liu J, Li Y, Zeng G, Li M, Wu Z, Zhang Y, et al. The immune-related gene HCST as a novel biomarker for the diagnosis and prognosis of clear cell renal cell carcinoma. Front Oncol. 2021;11: 630706.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
YG and QZ wrote the main manuscript text; YG, LL, and YQ prepared figures and Tables. All authors reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. Table S1.
The prognosis-related gene in OS.
Additional file 2. Table S2.
The univariate and multivariate Cox regression analysis.
Additional file 3. Table S3.
The differentially expressed genes in high-MFNG group versus low-MFNG group.
Additional file 4. Table S4.
GO result for differential expression genes.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gao, Y., Luo, L., Qu, Y. et al. MFNG is an independent prognostic marker for osteosarcoma. Eur J Med Res 28, 256 (2023). https://doi.org/10.1186/s40001-023-01139-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-023-01139-x