Abstract
Background
There is a great association between the prevalence of obstructive sleep apnea (OSA) and asthma. Nonetheless, whether OSA impacts lung function, symptoms, and control in asthma and whether asthma increases the respiratory events in OSA are unknown. This meta-analysis aimed to examine the relationship between obstructive sleep apnea and asthma severity and vice versa.
Methods
We carried out a systematic search of PubMed, EMBASE, and Scopus from inception to September 2022. Primary outcomes were lung function, parameters of polysomnography, the risk of OSA in more severe or difficult-to-control asthmatic patients, and the risk of asthma in patients with more severe OSA. Heterogeneity was examined with the Q test and I2 statistics. We also performed subgroup analysis, Meta-regression, and Egger’s test for bias analysis.
Results
34 studies with 27,912 subjects were totally included. The results showed that the comorbidity of OSA aggravated lung function in asthmatic patients with a consequent decreased forced expiratory volume in one second %predicted (%FEV1) and the effect was particularly evident in children. %FEV1 tended to decrease in adult asthma patients complicated with OSA, but did not reach statistical significance. Interestingly, the risk of asthma seemed to be slightly lower in patients with more severe OSA (OR = 0.87, 95%CI 0.763–0.998). Asthma had no significant effect on polysomnography, but increased daytime sleepiness assessed by the Epworth Sleepiness Scale in OSA patients (WMD = 0.60, 95%CI 0.16–1.04). More severe asthma or difficult-to-control asthma was independently associated with OSA (odds ratio (OR) = 4.36, 95%CI 2.49–7.64).
Conclusion
OSA was associated with more severe or difficult-to-control asthma with decreased %FEV1 in children. The effect of OSA on lung function in adult patients should be further confirmed. Asthma increased daytime sleepiness in OSA patients. More studies are warranted to investigate the effect of asthma on OSA severity and the impact of different OSA severity on the prevalence of asthma. It is strongly recommended that people with moderate-to-severe or difficult-to-control asthma screen for OSA and get the appropriate treatment.
Similar content being viewed by others
Introduction
The prevalence of obstructive sleep apnea (OSA) and asthma is increasing. Nevertheless, OSA and asthma have adverse effects on each other with distinct interactive mechanisms under the upper and lower airway pathologies, in addition to shared comorbidities such as obesity, rhinitis, and gastro-oesophageal reflux [1]. The prevalence of OSA among asthmatic populations has been reported to be 38 to 70%; one of the main reasons is structural and collapsibility changes in the upper airway during sleep related to the use of inhaled corticosteroids (ICS), oral corticosteroids, and systemic corticosteroids [1, 2]. Conversely, 35.1% of clinical populations with OSA report physician-diagnosed asthma, likely due to chronic intermittent hypoxia with consequent inflammation and/or remodeling of the lower airways [3, 4].
A large retrospective cohort study of patients with OSA showed significantly higher rates of all-cause readmission compared with those without OSA (hazard rate = 1.56; 95% confidence interval 1.50–1.62) [5]. Comorbidity with OSA has a negative effect on the prognosis of asthma. As well as being an important test in the diagnosis of asthma, greater variability in lung function is associated with poorly controlled asthma [6]. Nonetheless, the link between OSA and lung function and asthma control is controversial [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26]. Although asthma makes a significant contribution to the risk of OSA, it is unclear if asthma aggravates OSA severity with increased respiratory events and more severe hypoxia [27,28,29,30,31,32,33,34,35,36,37,38,39,40]. A meta-analysis to evaluate the relationship between OSA and asthma severity and vice versa is warranted. This review will focus on the independent interaction of OSA and asthma based on a meta-analysis.
Methods
We conducted a meta-analysis according to PRISMA statement guidelines [41]. The first part, (Part A), examined the effect of OSA on lung function and the risk of asthma in patients with different OSA severity. Part B examined the effect of asthma on PSG and Epworth Sleepiness Scale (ESS), as well as the risk of asthma in patients with different asthma severity (or control status). This meta-analysis has been registered in the PROSPERO database (CRD42021283829).
Search strategy
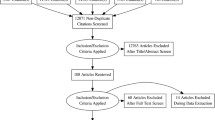
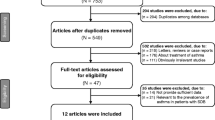
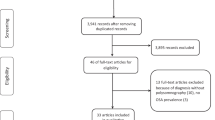
We carried out a systematic search through PubMed, Medline, EMBASE, and Scopus, ClinicalTrials (all searched from inception to September 2022). Index terms such as medical subject headings and free text were utilized to capture a broad range of literature. Index terms were limited to those identified in the title, abstract, and keywords. The detailed strategy listing all search terms used and how they were combined is shown in the Additional file 1: Tables S1–S3) and the selection process is shown in Fig. 1.
Selection criteria
Two researchers independently assessed the articles for eligibility for inclusion. Any disagreements were resolved through discussion, and the senior author was available to arbitrate if necessary.
The inclusion criteria for Part A were as follows: (1) children or adult patients diagnosed with asthma according to accepted guidelines or criterion; (2) cross-sectional or cohort study including OSA and non-OSA groups in asthmatic patients; (3) at least one of the following outcome measurements included: forced expiratory volume in one second %predicted (%FEV1), forced vital capacity % predicted (FVC%), forced expiratory volume in one second/forced vital capacity (FEV1/FVC), forced expiratory flow 25–75% of VC (FEF25-75%), asthma control test (ACT); (4) sufficient data for calculating odds ratio (OR) with 95% confidence interval (CI) of the risk of asthma in OSA with varying severity; (5) English language.
The inclusion criteria for Part B were: (1) children or adult patients diagnosed with OSA by polysomnography (PSG); (2) cross-sectional or cohort study including asthma and non-asthma groups in OSA patients; (3) at least one of the following outcome measurements included: apnea/hypopnea index (AHI), lowest peripheral oxygen saturation (LSpO2), oxygen desaturation index (ODI), arousal index (ArI), Epworth Sleepiness Scale, percent sleeping time in which oxygen saturation was below 90% (T90%); (4) sufficient data for calculating odds ratio (OR) with 95% confidence interval (CI) of the risk of OSA in asthma with varying severity; and (5) English language.
Data extraction
Information was independently extracted from each study by two authors. Tables from baseline included the following variables: first author, publication year, research site (country), gender (male%), mean age and body mass index (BMI) of the study population, study design, smoking condition, severity of OSA or asthma, study population, and quality of the study. The definition of OSA severity was based on the American Academy of Sleep Medicine. And the definition of asthma severity was based on Global Initiative for Asthma (GINA), including moderate-to-severe asthma, uncontrolled asthma, and difficult-to-treat asthma. If the original article did not define asthma severity according to GINA, the characteristics of the asthmatic population were recorded. Other specific variables included the number of asthma patients with and without OSA (Table 1), number of asthma patients graded by severity and OSA patients in each group (Table 2), number of OSA patients with and without asthma (Table 3), number of OSA patients graded by disease severity and patients with asthma in each group (Table 4), subgroup meta-analysis of main outcomes in patients with asthma in OSA group and non-OSA group (Table 5), and subgroup meta-analysis of main outcomes in patients with OSA in asthma group and non-asthma group (Table 6). In forest plot for the subgroup of OSA severity and asthma severity, we macroscopically focused more on the comparison of more severe OSA or asthma with controls, regardless of the specific degree of OSA severity, and the asthma severity or control status.
Statistical methods
All analyses were performed using the comprehensive Meta-Analysis Software (Stata 15.0). The random-effects model was conducted if significant heterogeneity was determined (p < 0.05); otherwise, the fixed-effects model was applied (p ≥ 0.05). We used the I2 index to assess heterogeneity. I2 ≥ 50 was considered moderate-to-high heterogeneity. Subgroup analyses were conducted to determine the sources of heterogeneity when I2 ≥ 50. The pooled weight mean difference (WMD) of each study and 95%CI were used to estimate the following outcomes: %FEV1, FVC%, FEV1/FVC, FEF25–75%, ACT, AHI, LSpO2, ODI, ArI, ESS, and T90%. OR and 95% CI were used to estimate the risk of asthma in OSA with different grades of severity and the risk of OSA in asthma with different degrees of severity. A P-value < 0.05 was considered statistically significant.
Quality assessment
Agency for Healthcare Research and Quality (AHRQ) [42] was used to assess the quality and risk of bias of included cross-sectional studies. The Newcastle–Ottawa scale (NOS) [43] scoring system was used to assess the quality and risk of bias of included longitudinal studies. The publication bias of included studies was assessed by funnel plot, Meta-regression Egger’s test [44]. Sensitivity analyses were conducted to assess the stability of the results.
Results
Study selection
According to our research strategy, 1646 articles were retrieved initially and after excluding duplicates, 882 articles remained. After the abstract screening, 752 articles that did not meet the inclusion criterion were excluded. After reading the full text of the remaining 130 articles, a further 99 were excluded: 89 due to a lack of appropriate data, 8 conference abstracts without available data, 1 clinical study protocol, and 1 article that was retracted. 34 eligible studies were included with a total sample size of 27,722 subjects [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40]. In Part A, 13 studies [7,8,9,10,11,12,13,14,15,16,17,18,19] examined the effect of OSA on lung function and ACT in asthma with 1,111 subjects (Table 1), and 7 studies [20,21,22,23,24,25,26] examined the relationship between asthma and varying severity of OSA with 15,266 subjects (Table 2). In Part B, 10 studies [9, 22, 25, 27,28,29,30,31,32,33] examined the effect of asthma on PSG and ESS in OSA patients with 9,666 subjects (Table 3), and 11 studies [10, 13,14,15, 34,35,36,37,38,39,40] examined the relationship between OSA and varying severity of asthma with 1869 subjects (Table 4). Other characteristics of the included studies are presented in each table.
The effect of OSA on lung function and ACT in asthmatic patients
In 13 studies (N = 1111) that fulfilled the inclusion criterion, OSA was not significantly associated with aggravation of lung function. The initial meta-analysis indicated that OSA had no influence on %FEV1 (pooled WMD = − 2.29, 95%CI − 4.91–0.33, I2 = 75.9%), FVC% (pooled WMD = 0.01, 95%CI − 1.92–1.94, I2 = 0%), FEV1/FVC (pooled WMD = − 0.82, 95%CI − 3.08–1.44, I2 = 69.0%), or FEF25–75% (pooled WMD = − 5.15, 95%CI − 12.72–2.42, I2 = 27%) (Table 5). The result of ACT scoring revealed that the comorbidity of OSA tended to make asthma more difficult to control (pooled WMD = − 0.37, 95%CI − 0.74–0.00, P = 0.05) (Table 5, Additional file 1: Fig. S1). OSA did not significantly affect the result of FEF25–75% (pooled WMD = − 5.15, 95%CI − 12.72–2.42, P = 0.183) (Additional file 1: Fig. S2). Nonetheless, further analysis suggested that OSA increased fractional exhaled nitric oxide (FeNO) in patients with asthma (pooled WMD = 4.37, 95%CI 0.05–8.69, P = 0.047) (Additional file 1: Fig. S3).
The results for %FEV1 and FVC% revealed high heterogeneity so a subgroup analysis was performed to determine the sources of heterogeneity. Analysis after subgrouping subjects by age (children and adults) revealed that %FEV1 and FEV1/FVC decreased by 4.33 (pooled WMD = − 4.33, 95%CI − 7.82–− 0.83, P = 0.015, between-group P value = 0.210) (Fig. 2) and 4.75 (pooled WMD = − 4.75, 95%CI − 8.55– − 0.95, P = 0.014, between-group P value = 0.035) (Fig. 2), respectively, in children with asthma complicated by OSA but no such effect was seen in adults (the heterogeneity evaluated by I2 for a subgroup is shown in Table 5). Analysis based on the severity of OSA (AHI ≥ 1 or 5/h, AHI ≥ 15/h) revealed that %FEV1 was slightly decreased in patients with asthma complicated by OSA (AHI ≥ 1 or 5/h) (pooled WMD = − 3.04, 95%CI − 5.68–− 0.40, P = 0.024, between-group P value = 0.015) (Fig. 3) but tended to increase (pooled WMD = 7.69, 95%CI − 0.54–15.93, P = 0.067) in patients with AHI ≥ 15/h. Interestingly, FEV1/FVC significantly improved (pooled WMD = 4.83, 95%CI 0.05–9.61, P = 0.047, between-group P value = 0.017) in patients with AHI ≥ 15/h, but was not significantly affected when OSA complicated with asthma (AHI ≥ 1 or 5/h) (Fig. 3). We also conducted a subgroup analysis based on the severity of asthma. There were no statistically significant differences when patients with asthma but no additional conditions (routine group) were compared with an uncontrolled group (defined according to the criterion of GINA) (Table 5).
Since the pooled results of %FEV1 and FEV1/FVC in adult patients with asthma were inconsistent with high heterogeneity, more subgroup analyses were performed to address this. When evaluating %FEV1 and FEV1/FVC, the results of subgroup analysis based on OSA severity were similar to those of the all age group, containing 2 same articles in a group of AHI ≥ 15/h [7, 18]. However, the decline in %FEV1 (pooled WMD = − 2.34, 95%CI − 5.78–1.10, P = 0.182, I2 = 81.1%, between-group P value = 0.028) and FEV1/FVC (pooled WMD = − 0.79, 95%CI − 3.26–0.79, P = 0.535, I2 = 72.6%, between-group P value = 0.041) showed no significant differences in asthmatic patients with AHI > 5/h (Additional file 1: Table S4). There were no significant differences among subgroups regarding severity of asthma, age, male%, and BMI, with remaining high heterogeneity (all between-group P values > 0.05, Additional file 1: Table S4).
The risk of asthma in patients with different OSA severity
When exploring the association of asthma with OSA severity, we found that the risk of asthma was lower in patients with more severe OSA with low heterogeneity (pooled OR = 0.87, 95%CI 0.763 to 0.998, I2 = 0%, P = 0.047) in a large number of subjects (N = 15,266). The result suggested that people with more severe OSA are less likely to be complicated with asthma. The characteristics of included OSA patients and the forest plot are shown in Table 2 and Fig. 4, respectively.
The effect of asthma on lung function and the ESS score in OSA patients
A review of 10 studies (N = 9666) that fulfilled the inclusion criterion revealed that asthma had no significant effect on OSA severity when assessed mainly by parameters of PSG (Table 6). There was an insignificant association with AHI (pooled WMD = 0.47, 95%CI − 1.59–2.52, I2 = 65.9%), LSaO2 (pooled WMD = 0.07, 95%CI − 1.28–1.09, I2 = 47.2%), ODI (pooled WMD = − 0,56, 95%CI − 1.68–0.56, I2 = 30.2%), ArI (pooled WMD = − 0.94, 95%CI − 2.14–0.26, I2 = 46%) and T90% (pooled WMD = 0.02, 95%CI − 0.42–0.47, I2 = 0.0%), regardless of subgroup analyses by age (children and adults) or severity of OSA (AHI ≥ 5/h, AHI ≥ 15/h). In addition, our study suggested that comorbidity of asthma may exacerbate daytime sleepiness assessed by ESS (pooled WMD = 0.60, 95%CI 0.16–1.04, P = 0.007) (Additional file 1: Fig. S4).
We also conducted several subgroup analyses based on age, male%, and BMI for adult OSA patients. The results showed that there were no significant differences among subgroups (Additional file 1: Table S5).
The risk of OSA in patients with different asthma severity
The risk of OSA was significantly higher in more severe or more difficult-to-control asthma in 7 of the 11 studies (Total N = 1869). Assessment of asthma severity included frequency of severe exacerbations over 12 months, symptoms during the day or night, activity limitation, and frequency of SABA reliever use for symptoms [6, 45]. The meta-analysis suggested that more severe or more difficult-to-control asthma is a significant risk factor for OSA (pooled OR = 4.36, 95%CI 2.49–7.64, I2 = 74.2%, P < 0.001) (Fig. 5A).
Then, we conducted a subgroup analysis based on BMI, the I2 for asthmatic patients with mean BMI ≥ 27 dramatically decreased to 0% (pooled OR = 2.51, 95%CI 1.95–3.24, P < 0.001). Nevertheless, the subgroup of asthmatic patients with mean BMI < 27 still remained a high heterogeneity, presenting a stronger association with OSA (pooled OR = 12.81, 95%CI 3.2–51.37, I2 = 68.7%, P < 0.001) (Fig. 5B). Characteristics of included studies are shown in Table 4.
Risk of bias and sensitivity analysis
AHRQ score for cross-sectional and NOS score for cohort studies are listed in Table 1–4. There were no studies considered low quality according to the criterion and no publication bias was observed in the funnel plot or Egger’s test for (a) the relationship between OSA and %FEV1 in asthmatic patients; (b) the relationship between asthma and AHI in OSA patients; (c) the relationship between OSA and asthma severity in asthmatic patients; and (d) the relationship between asthma and OSA severity in asthmatic patients. (P = 0.875, P = 0.650, P = 0.084, and P = 0.535, respectively, Fig. 6, Additional file 1: Fig. S5). The meta-regression revealed that age, gender, and BMI did not affect the statistical significance of the pooled results, showing the association between OSA and more severe or difficult-to-control asthma (P = 0.203, P = 0.826, P = 0.672, respectively). Moreover, sensitivity analyses of two parts in the meta-analysis confirmed that the result was stable with no statistical significance compared with the estimate (Additional file 1: Fig. S6).
A The relationship between OSA and %FEV1 in asthma patients; B The relationship between asthma and AHI in OSA patients; C The relationship between OSA and asthma severity in asthma patients; D The relationship between asthma and OSA severity in asthma patients. The funnel plot analysis of publication bias. OSA: obstructive sleep apnea; %FEV1: forced expiratory volume in one second %predicted; AHI: apnea hypopnea index
Discussion
To the best of our knowledge, this is the first meta-analysis to investigate the effect of OSA on lung function in asthmatic patients (Part A) and the effect of asthma on PSG in OSA patients (Part B), with a large amount of subjects. The results of Part A suggested that in asthmatic patients complicated with OSA, lung function may be aggravated with decreased %FEV1, especially in children. Nevertheless, the %FEV1 tended to decrease in adult asthma patients complicated with OSA, but did not reach statistical significance. OSA also had a negative effect on asthma symptoms and airway inflammation, as shown by increased ACT and FeNO. OSA did not affect small airway function assessed by FEF25–75%. Interestingly, the risk of asthma appeared to be lower in patients with more severe OSA with pooled OR = 0.87. Results of Part B indicated that more severe asthma or more difficult-to-control asthma was associated with OSA with pooled OR = 4.36, independent of age, gender, and BMI. And when OSA patients complicated with asthma, daytime sleepiness was aggravated with increased ESS score, but there was no positive impact on parameters of PSG (AHI, LSpO2, ODI, ArI, and T90%) with extremely low heterogeneity.
The effect of OSA on asthma
Previous studies have reported that OSA is one of the most important pathophysiological mechanisms related to the worsening of asthma symptoms and control, in addition to shared risk factors and comorbidities [46]. OSA causes chronic systemic inflammation with the activation and release of cytokine and inflammatory mediators such as TNF-a, interleukin-6, vascular endothelial growth factor, pentane, 8-isoprostane, C-reactive protein, leptin and matrix metallopeptidase-9 [47,48,49,50,51]. Many studies have found that OSA could lead to increased sputum neutrophils, which are linked to increased type 1 airway inflammation, airway remodeling, steroid resistance, and increased disease severity in asthma [52, 53]. The meta-analysis indicated that OSA slightly increased FeNO in asthma patients. FeNO is a biomarker widely used to assess airway inflammation and is higher in patients with asthma characterized by type 2 airway inflammation [54]. Nonetheless, the previous study revealed that chronic intermittent hypoxia for OSA reduced type 2 airway inflammation with decreased interleukin-5 and interleukin-13 in an animal model [55]. The measurement of FeNO led to inconsistent results that OSA patients had similar or slightly higher levels of FeNO compared to healthy controls [56]. The specific mechanism and clinical significance of increasing FENO in asthma patients complicated with OSA need to be further explored. In addition, bronchial hyperresponsiveness is closely associated with nocturnal pulmonary blood pooling caused by increasing negative intrathoracic pressures, chronic intermittent hypoxia mediated through vagal neural receptor activation that accompanies the Muller maneuvers of obstructive events and stimulation of the carotid body [1, 36].
%FEV1 and FEV1/FVC are the most common spirometric measurements applied to identify both the presence and degree of airflow obstruction [6]. In the subgroup of varying severity of OSA, two included studies [7, 18] showed that patients with asthma and moderate-severe OSA had better lung function (%FEV1, FEV1/FVC) than those with AHI < 15/h without heterogeneity. Careful analysis of two studies revealed that baseline %FEV1, age, BMI, and gender did not differ between patients with and without OSA. Nonetheless, Oyama and his colleagues showed that 15/20 patients with AHI ≥ 15/h were at asthma treatment stage 4 compared with 4/16 patients with AHI < 15/h [18]. Similarly, another study showed that baseline %FEV1 in asthmatic patients with severe OSA was higher than that of patients with moderate OSA (77.1 ± 25.3 vs 65.3 ± 24.6), and medication was similar except for leukotriene antagonist (61.8% vs 30.3%) [12]. When prescribed as monotherapy or as add-on therapy to ICS, leukotriene antagonists can increase FEV1 [57]. Therefore, the conflicting results may be because asthma is more severe or difficult to control in the presence of severe OSA, requiring higher levels of asthmatic treatment that presents a better baseline lung function. In addition, OSA was associated with more rapid lung function decline per year in patients with asthma and OSA severity had an essential effect on the rate of decline [12, 58]. Guidelines advise that %FEV1 < 60% determines that a patient is at risk of future asthma exacerbations [6]. OSA is strongly connected to asthma exacerbations with decreased lung function.
Although a significant decline in baseline %FEV1 was observed among children asthmatic patients complicated with OSA, it was not seen in adult patients with high heterogeneity. Some studies indicated that airway hyperresponsiveness is a strong risk factor for low lung function, mainly seen in airway parameters (FEV1, FEV1/FVC) in children [59, 60]. This may be the reason children with asthma and OSA had a greater reduction in lung function compared with adults in our meta-analysis with increasing airway hyperresponsiveness in OSA. According to pathophysiology mechanisms and the result of our study that %FEV1 tented to decline in adult asthma in OSA, a cohort study controlling many confounding factors such as age, male%, BMI and asthma treatment stage is recommended to investigate the effect of OSA on lung function in asthmatic patients. Blood and urine samples can also be collected for proteomic studies to explore new potential biomarkers, the pathophysiological mechanisms, and the specific differences between children and adults [61]. Poor asthmatic control will slow development and reduce the basal level of future lung function [62,63,64].
Although more severe OSA aggravated asthma control, the meta-analysis came out that it was not associated with the risk of OSA (OR = 0.87, P = 0.047, I2 = 0%). In the two included studies with the largest population (N = 8400), AHI was slightly lower in asthmatic than in non-asthmatic patients with OSA [25, 32] (26.2 vs 23.0 events/h, P < 0.001; 12.6 vs 11.3 events/h, P < 0.001). In terms of baseline characteristics, those without asthma were more likely to be male and older than those with asthma. Advancing age and male gender will increase the risk and worsen OSA severity [65]. This may suggest that patients with asthma were mainly female and younger, and more likely to appear in the moderate OSA group. Therefore, age and gender were the important confounding factors to account for OR = 0.87, and there is no clear evidence that coexisting with more severe OSA reduces the prevalence of asthma.
Smoking condition is another potential confounding factor of inconsistent lung functions in the adult group. In the study of Wang et al. and Lu et al., the results showed that the group with more smokers had worse average lung function in %FEV1 [12, 14]. Smokers with asthma may present an accelerated rate of decline in lung function and may develop persistent airflow obstruction due to airway remodeling, increasing the use of rescue medication and the number of hospitalizations and emergency room visits [66,67,68]. Additionally, secondhand smoke is an important risk factor for childhood asthma, asthma-like syndrome, and wheezing [69]. Despite tobacco smoking negatively affecting asthma control, OSA is strongly associated with poor control of asthma independent of smoking and other known asthma aggravators [70, 71]. Since the most included original article did not demonstrate detailed information, we failed to undertake a subgroup analysis or meta-regression on smoking conditions. In conclusion, physicians should be alert for the presence of comorbid OSA in patients with asthma, especially those who have low %FEV1, preventing accelerated airway remodeling and reduction in lung function.
The effect of asthma on OSA
The result of Part B in our meta-analysis suggested that more severe asthma or more difficult-to-control asthma was associated with OSA with pooled OR = 4.36. The reasonable explanations may be OSA aggravates asthma, or severe asthma leads to OSA. A large prospective cohort study with 8-year follow-up intervals suggested that patients with asthma experienced a higher risk of OSA than those without asthma (49% vs 28%, P < 0.001), and asthma duration was an important predictor of OSA occurrence [72]. Many studies consistently consider that ICS and oral corticosteroids, both common therapies for asthma, also constitute a high risk for OSA occurrence with peri-pharyngeal fat deposition and upper airway myopathy [73, 74]. Asthma may disrupt the balance of forces to maintain pharyngeal airway patency, for example, increased negative intrathoracic pressure may lead to higher pharynx collapsibility during asthma attacks [75, 76]. Collett and colleagues also suggested that asthma could promote a reduction in the surface area of the upper airway with persistent airway mucosal inflammation [77].
Although many studies identified a mechanism by which asthma aggravates OSA, asthma was not obviously associated with increased respiratory events or worsened nocturnal hypoxia (T90% and LSpO2) in OSA in our meta-analysis (Table 6). A potential reason for the discrepancy in meta-analysis is that low arousal threshold is more frequent in patients with OSA and co-existing asthma, compared to those who have OSA alone [33]. Asthma is associated with respiratory muscle weakness and greater instability of the respiratory drive, and asthma nocturnal symptoms may result in sleep fragmentation and less total sleep time [78,79,80]. These pathological mechanisms may contribute to the low arousal threshold in OSA, presented as lower AHI [81]. Sleep structure disorders raised by various asthma severity might account for the inaccurate acquisition of PSG parameters. Most of the original studies in this section did not address the severity of asthma, so we did not perform a subgroup analysis. A recent study suggests that it is more important to consider mean oxygen saturation during sleep when assessing OSA in asthma patients, which is associated with asthma symptoms and lung function [82].
Daytime sleepiness is one of the most important features of OSA evaluated by the ESS score. The results of the meta-analysis indicated that comorbidity with asthma slightly increased ESS in patients with OSA (WMD = 0.60, P = 0.007, I2 = 0%), which is consistent with the previous study. The risk of daytime sleepiness is 50% higher in asthmatic patients compared to those without asthma, and allergic rhinitis, a common comorbidity of asthma, is independently associated with sleepiness [83]. Impaired sleep quality correlates with worse asthma control and quality of life with daytime sleepiness [84]. Most patients included in studies were suspected or diagnosed with OSA, and sleep physicians may not have investigated patients sufficiently to determine the presence or absence of asthma [39]. We believe that there should be a more pronounced difference in ESS between asthmatics and non-asthmatics and that asthma may have been underdiagnosed on self-report questionnaires due to the similarity of nocturnal asthmatic symptoms to those of OSA.
Limitations
This study has some important limitations. In the analysis of the relationship between OSA and lung function in asthmatic patients, we failed to lower the high heterogeneity with different subgroup analyses. It is possible that coexisting OSA has a negative effect on asthma with different phenotypes and endotypes. We failed to collect sufficient data on peak expiratory flow, a predictive indicator for exacerbation of asthma. Although comorbidity with asthma did not show a significant effect on PSG in OSA, the included patients with asthma were not grouped according to severity. Whether severe or uncontrolled asthma affects OSA severity requires further study. In the included studies, mismatches in age, gender, BMI, and treatment status between the single disease group and comorbidity group may have contributed to risk bias for meta-analysis, with high heterogeneity in adult patients. OSA and asthma are closely associated with shared comorbidities such as rhinitis, obesity, smoking condition, and gastroesophageal reflux disease, and would have affected our final results [46]. Finally, asthma is a heterogeneous disease (allergic, non-allergic, eosinophilic, neutrophilic, etc.), and the specific relationship between endotypes of asthma and OSA is unknown. Taken together, a large-scale cohort study is needed to investigate the association between OSA and asthma by figuring out the confounding factors derived from phenotypes and endotypes.
Conclusion
In this meta-analysis, OSA was associated with more severe or more difficult-to-control asthma with decreased %FEV1 and increased airway inflammation, especially in children. %FEV1 tended to decrease in adult asthma patients complicated with OSA, but did not reach statistical significance. The differences between children and adults should be investigated by clinical research and proteomic studies. Asthma increased daytime sleepiness but did not significantly worsen OSA severity despite a lack of a subgroup for severity of asthma. More studies are warranted to investigate the effect of asthma on OSA severity and the impact of different OSA severity on the prevalence of asthma. Overall, clinicians should perform early detection, diagnosis and therapy for OSA in patients with asthma, in order to slow down the rate of airway remodeling and the decline in lung function.
Availability of data and materials
The data sets used or analyzed during the current study are available from the corresponding author on request.
Abbreviations
- OSA:
-
Obstructive sleep apnea
- ICS:
-
Inhaled corticosteroids
- %FEV1 :
-
Forced expiratory volume in one second %predicted
- FVC%:
-
Forced vital capacity % predicted
- FEV1/FVC:
-
Forced expiratory volume in one second/forced vital capacity
- FEF25-75%:
-
Forced expiratory flow 25–75% of VC
- ACT:
-
Asthma control test
- PSG:
-
Polysomnography
- AHI:
-
Apnea/hypopnea index
- LSpO2:
-
Lowest peripheral oxygen saturation
- ODI:
-
Oxygen desaturation index
- ArI:
-
Arousal index
- ESS:
-
Epworth sleepiness scale
- T90%:
-
Percent sleeping time in which oxygen saturation was below 90%
- WMD:
-
Weight mean difference
- AHRQ:
-
Agency for healthcare research and quality
- NOS:
-
Newcastle–Ottawa scale
- GINA:
-
The global initiative for asthma
References
Wang R, Mihaicuta S, Tiotiu A, et al. Asthma and obstructive sleep apnoea in adults and children—an up-to-date review. Sleep Med Rev. 2022;61:101564.
Teodorescu M, Xie A, Sorkness CA, et al. Effects of inhaled fluticasone on upper airway during sleep and wakefulness in asthma: a pilot study. J Clin Sleep Med. 2014;10:183e93.
Alharbi M, Almutairi A, Alotaibi D, et al. The risk of asthma in patients with obstructive sleep apnoea. Prim Care Respir J. 2009;18:328–30.
Althoff MD, Ghincea A, Wood LG, et al. Asthma and three colinear comorbidities: obesity, OSA, and GERD. J Allergy Clin Immunol Pract. 2021;9(11):3877–84.
Hirayama A, Goto T, Faridi MK, et al. Association of obstructive sleep apnea with all-cause readmissions after hospitalization for asthma exacerbation in adults aged 18–54 years: a population-based study, 2010–2013. J Asthma. 2021;58(9):1176–85.
Global Strategy for Asthma Prevention and Treatment. 2018 update. https://ginasthma.org/wp-content/uploads/2020/04/GINA-2020-full-report_-final-_wms.pdf. Accessed Nov 2022
Ciftci TU, Ciftci B, Guven SF, et al. Effect of nasal continuous positive airway pressure in uncontrolled nocturnal asthmatic patients with obstructive sleep apnea syndrome. Respir Med. 2005;99(5):529–34.
Kheirandish-Gozal L, Dayyat EA, Eid NS, et al. Obstructive sleep apnea in poorly controlled asthmatic children: effect of adenotonsillectomy. Pediatr Pulmonol. 2011;46(9):913–8.
Teng Y-K, Chiang L-C, Lue K-H, et al. Poor sleep quality measured by polysomnography in non-obese asthmatic children with or without moderate to severe obstructive sleep apnea. Sleep Med. 2014;15(9):1062–7.
Zidan M, Daabis R, Gharraf H. Overlap of obstructive sleep apnea and bronchial asthma: effect on asthma control. Egypt J Chest Dis Tuberc. 2015;64(2):425–30.
Taillé C, Rouvel-Tallec A, Stoica M, Danel C, Dehoux M, Marin-Esteban V, Pretolani M, Aubier M, d’Ortho MP. Obstructive sleep apnoea modulates airway inflammation and remodelling in severe asthma. PLoS ONE. 2016;11(3):e0150042.
Wang TY, Lo YL, Lin SM, Huang CD, Chung FT, Lin HC, Wang CH, Kuo HP. Obstructive sleep apnoea accelerates FEV1 decline in asthmatic patients. BMC Pulm Med. 2017;17(1):55.
Shaker A. Study of obstructive sleep apnea (OSA) in asthmatics. Egypt J Chest Dis Tuberc. 2017;66(2):293–8.
Lu H, Fu C, Li W, Jiang H, Wu X, Li S. Screening for obstructive sleep apnea syndrome in asthma patients: a prospective study based on Berlin and STOP-Bang questionnaires. J Thorac Dis. 2017;9(7):1945–58.
Nguyen-Hoang Y, Nguyen-Thi-Dieu T, Duong-Quy S. Study of the clinical and functional characteristics of asthmatic children with obstructive sleep apnea. J Asthma Allergy. 2017;10:285–92.
Ng SSS, Chan TO, To KW, Chan KKP, Ngai J, Yip WH, Lo RLP, Ko FWS, Hui DSC. Continuous positive airway pressure for obstructive sleep apnoea does not improve asthma control. Respirology. 2018;23(11):1055–62.
He Z, Armoni Domany K, Nava-Guerra L, Khoo MCK, Difrancesco M, Xu Y, Mcconnell K, Hossain MM, Amin R. Phenotype of ventilatory control in children with moderate to severe persistent asthma and obstructive sleep apnea. Sleep. 2019;42(9):zsz130.
Oyama B, Tsuburai T, Tsuruoka H, Nishida K, Usuba A, Hida N, Inoue T, Komase Y, Mineshita M, Miyazawa T. Complicating effects of obstructive sleep apnea syndrome on the severity of adult asthma. J Asthma. 2020;57(11):1173–8.
Lin JL, Feng XK, Zhang DM, Sun HY. Clinical features and risk factors in patients with asthma complicated with obstructive sleep apnea-hypopnea syndrome: a hospital-based study. Sleep Breath. 2021;25(1):339–45.
ten Brinke A, Sterk PJ, Masclee AA, Spinhoven P, Schmidt JT, Zwinderman AH, Rabe KF, Bel EH. Risk factors of frequent exacerbations in difficult-to-treat asthma. Eur Respir J. 2005;26(5):812–8.
Julien JY, Martin JG, Ernst P, Olivenstein R, Hamid Q, Lemière C, Pepe C, Naor N, Olha A, Kimoff RJ. risk of obstructive sleep apnea-hypopnea in severe versus moderate asthma. J Allergy Clin Immunol. 2009;124(2):371–6.
Teodorescu M, Polomis DA, Teodorescu MC, Gangnon RE, Peterson AG, Consens FB, Chervin RD, Jarjour NN. Association of obstructive sleep apnea risk or diagnosis with daytime asthma in adults. J Asthma. 2012;49(6):620–8.
Byun MK, Park SC, Chang YS, Kim YS, Kim SK, Kim HJ, Chang J, Ahn CM, Park MS. Associations of moderate to severe asthma with obstructive sleep apnea. Yonsei Med J. 2013;54(4):942–8.
Wang Y, Liu K, Hu K, Yang J, Li Z, Nie M, Dong Y, Huang H, Chen J. Impact of obstructive sleep apnea on severe asthma exacerbations. Sleep Med. 2016;26:1–5.
Megersa S, Chala G, Fikremariam K. Determinants of asthma attack among adult asthmatic patients attending at public hospitals of west Shoa zone, Oromia regional state, Ethiopia, 2021: case-control study. J Asthma Allerg. 2022;15:1143–54.
Al-Lawati F, Al-Mubaihsi SM, Jayakrishnan B, Rizvi S, Al-Abri MA. Obstructive sleep apnea in patients with severe asthma: risk and association between severity and asthma control. Ann Thorac Med. 2022;17(2):118–23.
Bonay M, Nitenberg A, Maillard D. Should flow-volume loop be monitored in sleep apnea patients treated with continuous positive airway pressure? Respir Med. 2003;97(7):830–4.
Alharbi M, Almutairi A, Alotaibi D, Alotaibi A, Shaikh S, Bahammam AS. The risk of asthma in patients with obstructive sleep apnoea. Prim Care Respir J. 2009;18(4):328–30.
Ramagopal M, Mehta A, Roberts DW, Wolf JS, Taylor RJ, Mudd KE, Scharf SM. Asthma as a predictor of obstructive sleep apnea in urban African-American children. J Asthma. 2009;46(9):895–9.
Greenberg-Dotan S, Reuveni H, Tal A, Oksenberg A, Cohen A, Shaya FT, Tarasiuk A, Scharf SM. Increased risk of obstructive lung disease in patients with obstructive sleep apnea. Sleep Breath. 2014;18(1):69–75.
Zaffanello M, Gasperi E, Tenero L, Piazza M, Pietrobelli A, Sacchetto L, Antoniazzi F, Piacentini G. Sleep-disordered breathing in children with recurrent wheeze/asthma: a single centre study. Children (Basel). 2017;4(11):97.
Bonsignore MR, Pepin JL, Anttalainen U, Schiza SE, Basoglu OK, Pataka A, Steiropoulos P, Dogas Z, Grote L, Hedner J, McNicholas WT, Marrone O. ESADA study group clinical presentation of patients with suspected obstructive sleep apnea and self-reported physician-diagnosed asthma in the ESADA cohort. J Sleep Res. 2018;27(6):12729.
Sundbom F, Janson C, Malinovschi A, Lindberg E. Effects of coexisting asthma and obstructive sleep apnea on sleep architecture, oxygen saturation, and systemic inflammation in women. J Clin Sleep Med. 2018;14(2):253–9.
Shrestha B, Mukhtar O, Kandel S, Bhattrai B, Dattar P, Amgai B, Mandal A, Alhafidh O, Thapa S, Khalid M, Gayam V, Ting B, Enriquez DA, Quist J, Schmidt MF. Polysomnographic variables in alternate overlap syndrome: data from sleep heart health study. J Community Hosp Intern Med Perspect. 2019;9(2):108–12.
Antonaglia C, Passuti G, Giudici F, et al. Low arousal threshold: a common pathophysiological trait in patients with obstructive sleep apnea syndrome and asthma. Sleep Breath. 2022. https://doi.org/10.1007/s11325-022-02665-4.
Robichaud-Hallé L, Beaudry M, Fortin M. Obstructive sleep apnea and multimorbidity. BMC Pulm Med. 2012;12:60.
Gutierrez MJ, Zhu J, Rodriguez-Martinez CE, Nino CL, Nino G. Nocturnal phenotypical features of obstructive sleep apnea (OSA) in asthmatic children. Pediatr Pulmonol. 2013;48(6):592–600.
Pinto JA, Ribeiro DK, Cavallini AF, Duarte C, Freitas GS. Comorbidities associated with obstructive sleep apnea: a retrospective study. Int Arch Otorhinolaryngol. 2016;20(2):145–50.
Tamanyan K, Walter LM, Davey MJ, Nixon GM, Horne RS, Biggs SN. Risk factors for obstructive sleep apnoea in Australian children. J Paediatr Child Health. 2016;52(5):512–7.
Tveit RL, Lehmann S, Bjorvatn B. risk of several somatic diseases depends on the presence and severity of obstructive sleep apnea. PLoS ONE. 2018;13(2):e0192671.
Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1.
Norris SL, Atkins D, Bruening W, et al. Observational studies in systematic [corrected] reviews of comparative effectiveness: AHRQ and the effective health care program. J Clin Epidemiol. 2011;64(11):1178–86.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Thornton A, Lee P. Publication bias in meta-analysis: its causes and consequences. J Clin Epidemiol. 2000;53:20716.
Aggarwal AN, Agarwal R. The new ATS/ERS guidelines for assessing the spirometric severity of restrictive lung disease differ from previous standards. Respirology. 2007;12(5):759–62.
Tiotiu A, Novakova P, Baiardini I, et al. Manifesto on united airways diseases (UAD): an Interasma (global asthma association—GAA) document. J Asthma. 2022;59(4):639–54.
Camargo CA Jr, Weiss ST, Zhang S, Willett WC, Speizer FE. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch Intern Med. 1999;159:2582–8.
Vernooy JH, Ubags ND, Brusselle GG, et al. Leptin as regulator of pulmonary immune responses: involvement in respiratory diseases. Pulm Pharmacol Ther. 2013;26(4):464–72.
Salerno FG, Carpagnano E, Guido P, et al. Airway inflammation in patients affected by obstructive sleep apnea syndrome. Respir Med. 2004;98:25–8.
Yokoe T, Minoguchi K, Matsuo H, et al. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation. 2003;107:1129–34.
Shamsuzzaman AS, Winnicki M, Lanfranchi P, et al. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation. 2002;105:2462–4.
Teodorescu M, Broytman O, Curran-Everett D, et al. Obstructive sleep apnea risk, asthma burden, and lower airway inflammation in adults in the severe asthma research program (SARP) II. J Allergy Clin Immunol Pract. 2015;3(4):566-75.e1.
Taillé C, Rouvel-Tallec A, Stoica M, et al. Obstructive sleep apnoea modulates airway inflammation and remodelling in severe asthma. PLoS ONE. 2016;11(3):0150042.
Israel E, Reddel HK. Severe and difficult-to-treat asthma in adults. N Engl J Med. 2017;377(10):965–76.
Ohta S, Tanaka A, Jinno M, et al. Exposure to intermittent hypoxia inhibits allergic airway inflammation in a murine model of asthma. Sleep Breath. 2020;24(2):523–32.
Finamore P, Scarlata S, Cardaci V, et al. Exhaled breath analysis in obstructive sleep apnea syndrome: a review of the literature. Medicina. 2019;55(9):538.
Miligkos M, Bannuru RR, Alkofide H, Kher SR, Schmid CH, Balk EM. Leukotriene-receptor antagonists versus placebo in the treatment of asthma in adults and adolescents: a systematic review and meta-analysis. Ann Intern Med. 2015;163(10):756–67.
Emilsson ÖI, Sundbom F, Ljunggren M, et al. Association between lung function decline and obstructive sleep apnoea: the ALEC study. Sleep Breath. 2021;25(2):587–96.
McGeachie MJ, Yates KP, Zhou X, et al. Patterns of growth and decline in lung function in persistent childhood asthma. N Engl J Med. 2016;374(19):1842–52.
Belgrave DCM, Granell R, Turner SW, et al. Lung function trajectories from pre-school age to adulthood and their associations with early life factors: a retrospective analysis of three population-based birth cohort studies. Lancet Respir Med. 2018;6(7):526–34.
Feliciano A, Torres VM, Vaz F, et al. Overview of proteomics studies in obstructive sleep apnea. Sleep Med. 2015;16(4):437–45.
Morgan WJ, Stern DA, Sherrill DL, et al. Outcome of asthma and wheezing in the first 6 years of life: follow-up through adolescence. Am J Respir Crit Care Med. 2005;172(10):1253–8.
Berry CE, Billheimer D, Jenkins IC, et al. A distinct low lung function trajectory from childhood to the fourth decade of life. Am J Respir Crit Care Med. 2016;194(5):607–12.
Duijts L, Granell R, Sterne JA, Henderson AJ. Childhood wheezing phenotypes influence asthma, lung function and exhaled nitric oxide fraction in adolescence. Eur Respir J. 2016;47(2):510–9.
Senaratna CV, Perret JL, Lodge CJ, et al. risk of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. 2017;34:70–81.
Klein DK, Silberbrandt A, Frøssing L, et al. Impact of former smoking exposure on airway eosinophilic activation and autoimmunity in patients with severe asthma. Eur Respir J. 2022;60(4):2102446. https://doi.org/10.1183/13993003.02446-2021.
Bellou V, Gogali A, Kostikas K. Asthma and tobacco smoking. J Pers Med. 2022;12(8):1231.
Jiménez-Ruiz CA, Andreas S, Lewis KE, et al. Statement on smoking cessation in COPD and other pulmonary diseases and in smokers with comorbidities who find it difficult to quit. Eur Respir J. 2015;46(1):61–79.
He Z, Wu H, Zhang S, et al. The association between secondhand smoke and childhood asthma: a systematic review and meta-analysis. Pediatr Pulmonol. 2020;55(10):2518–31.
Teodorescu M, Polomis DA, Hall SV, et al. Association of obstructive sleep apnea risk with asthma control in adults. Chest. 2010;138(3):543–50.
Özden Mat D, Firat S, Aksu K, Aksu F, Duyar SŞ. Obstructive sleep apnea is a determinant of asthma control independent of smoking, reflux, and rhinitis. Allergy Asthma Proc. 2021;42(1):e25–9.
Teodorescu M, Barnet JH, Hagen EW, Palta M, Young TB, Peppard PE. Association between asthma and risk of developing obstructive sleep apnea. JAMA. 2015;313(2):156–64.
Henao MP, Kraschnewski JL, Bolton MD, Ishmael F, Craig T. Effects of inhaled corticosteroids and particle size on risk of obstructive sleep apnea: a large retrospective cohort study. Int J Environ Res Publ Health. 2020;17(19):7287. https://doi.org/10.3390/ijerph17197287.
Yigla M, Tov N, Solomonov A, Rubin AH, Harlev D. Difficult-to-control asthma and obstructive sleep apnea. J Asthma. 2003;40:865–71.
Van de Graaff WB. Thoracic influence on upper airway patency. J Appl Physiol 1985. 1988;65(5):2124–31.
Ioachimescu OC, Teodorescu M. Integrating the overlap of obstructive lung disease and obstructive sleep apnoea: OLDOSA syndrome. Respirology. 2013;18(3):421–31.
Collett PW, Brancatisano AP, Engel LA. Upper airway dimensions and movements in bronchial asthma. Am Rev Respir Dis. 1986;133:1143–9.
Sériès F, Roy N, Marc I. Effects of sleep deprivation and sleep fragmentation on upper airway collapsibility in normal subjects. Am J Respir Crit Care Med. 1994;150(2):481–5.
Ragnoli B, Pochetti P, Raie A, Malerba M. Interrelationship between obstructive sleep apnea syndrome and severe asthma: from endo-phenotype to clinical aspects. Front Med (Lausanne). 2021;8:640636.
Issa FG, Sullivan CE. Respiratory muscle activity and thoracoabdominal motion during acute episodes of asthma during sleep. Am Rev Respir Dis. 1985;132(5):999–1004.
Edwards BA, Eckert DJ, McSharry DG, et al. Clinical predictors of the respiratory arousal threshold in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2014;190(11):1293–300.
Sundbom F, Janson C, Ljunggren M, et al. Asthma and asthma-related comorbidity: effects on nocturnal oxygen saturation. J Clin Sleep Med. 2022;18(11):2635–41.
Kavanagh J, Jackson DJ, Kent BD. Sleep and asthma. Curr Opin Pulm Med. 2018;24(6):569–73.
Janson C, De Backer W, Gislason T, et al. Increased risk of sleep disturbances and daytime sleepiness in subjects with bronchial asthma: a population study of young adults in three European countries. Eur Respir J. 1996;9(10):2132–8.
Acknowledgements
We thank Professor Nanshan Zhong from the State Key Laboratory of Respiratory Disease for his assistance with the literature search.
Funding
This research project was supported by the Basic Research Project (Dengfeng hospital) jointly Funded by Guangzhou City and the School (No.202201020586), Independent Project of State Key Laboratory of Respiratory Diseases (SKLRD-Z-202005), and the Natural Science Foundation of Guangdong Province China (No. 2019A1515010981).
Author information
Authors and Affiliations
Contributions
Study design, literature review, data analysis, statistical analysis: DW, YZ, RK, XZ; Data management, data analysis, drafting manuscript: DW, YZ, RK, XZ, SZ, YT, SL, YL, ZZ; Manuscript revision, intellectual revisions, mentorship: SZ, XS,YR, NZ; DW, YZ, RK, XZ, SZ, XS,YR, ZD, NZ takes responsibility for the content of the manuscript including the data and the analysis. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
Non-financial disclosure. All authors declared no competing interests. All authors have seen and approved the manuscript. The authors have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Search Medline via PubMed (up to September 2022). Table S2. Search Embase via Ovid (up to September 2022). Table S3. Search Scopus (up to September 2022). Table S4. Subgroup meta-analysis of main outcomes in adults patients with asthma in OSA group and non-OSA group. Table S5. Subgroup meta-analysis of main outcomes in adults patients with OSA in asthma group and non-asthma group. Fig S1. The meta-analysis of asthma control trial (ACT) in the relationship between OSA and asthma control status. Fig S2. The meta-analysis of forced expiratory flow (25–75% of VC) (FEF25–75%) in the relationship between OSA and lung function in asthma patients. Fig S3. The meta-analysis of fractional exhaled nitric oxide (FeNO) in asthma patients with or without OSA. Fig S4. The meta-analysis of Epworth Sleepiness Scale (ESS) in OSA patients with or without asthma. Fig S5. The Egger’s test for publication bias. (a): The relationship between OSA and %FEV1 in asthma patients(b): The relationship between asthma and AHI in OSA patients(c): The relationship between OSA and asthma severity in asthma patients(d): The relationship between asthma and OSA severity in asthma patients.Fig S6. The sensitivity analyses for assessing the stability of the results. (a): The relationship between OSA and %FEV1 in asthma patients(b): The relationship between asthma and AHI in OSA patients(c): The relationship between OSA and asthma severity in asthma patients(d): The relationship between asthma and OSA severity in asthma patients.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, D., Zhou, Y., Chen, R. et al. The relationship between obstructive sleep apnea and asthma severity and vice versa: a systematic review and meta-analysis. Eur J Med Res 28, 139 (2023). https://doi.org/10.1186/s40001-023-01097-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-023-01097-4