Abstract
Purpose
Helical tomotherapy (HT) is a viable method for delivering total body irradiation (TBI) when preparing patients for allogenic stem cell or bone-marrow transplantation. TBI can be planned to reduce the amount of radiation delivered to organs at risk, such as the lungs, with the aim of decreasing toxicity. However, it is important for the ribcage to receive the prescribed radiation dose in preparation for bone-marrow transplantation. In this retrospective study, we analyzed radiation dose coverage of the lungs and ribcage in patients who underwent TBI delivered by HT to achieve lung dose sparing.
Methods
Thirty-five patients were included in the analysis and divided into three groups based on their prescribed radiation dose (4, 8, or 12 Gy). HT was performed using a rotating gantry to reduce radiation to the lungs. Dosimetric parameters for the lungs and ribcage as well as dose-volume histograms were calculated.
Results
The mean lung D95 was 60.97%, 54.77%, and 37.44% of the prescribed dose for patients receiving 4 Gy, 8 Gy, and 12 Gy, respectively. Ribcage coverage was most optimal for patients receiving 4 Gy, with a D95 of 91.27% and mean homogeneity index of 1.17, whereas patients receiving 12 Gy had a mean D95 of 78.65% and homogeneity index of 1.37, which is still within the range recommended by treatment guidelines.
Conclusions
Using HT to achieve lung tissue sparing is a viable approach to minimizing pulmonic complications in patients undergoing TBI. As this planning adjustment does not compromise the dose and quality of coverage received by the ribcage, it is a feasible tool within conditioning regimens for allogeneic bone-marrow transplantation.
Similar content being viewed by others
Introduction
Alongside chemotherapy, total body irradiation (TBI) is a major component of the conditioning regimen in preparation for allogenic bone-marrow transplantation (BMT) in patients with diseases, such as acute lymphatic leukemia (ALL) or acute myeloid leukemia (AML) [1, 2]. TBI helps eradicate radiosensitive malignant cells and is immunosuppressive, which increases the likelihood of donor transplant acceptance by the patient’s body. However, the toxicity of TBI can limit its use in conditioning regimens, especially considering organs at risk (OAR), such as the lungs [3], which can be vulnerable to complications including interstitial pneumonia [4]. To reduce the risk of such side effects, approaches such as using lung blocks to reduce the amount of radiation reaching the lungs can be employed [5]. However, lung blocks can also severely reduce the dose of radiation delivered to the mediastinum and ribs, which is important in the preparation for allogenic BMT.
Our institution delivers TBI using helical tomotherapy (HT), which minimizes differences between planned and delivered doses and increases the overall homogeneity of irradiation across the body [6,7,8]. Lung sparing in TBI reduces the risk of pulmonic side effects and long-term problems resulting from radiation-induced lung damage [9]. However, when using HT to reduce the dose delivered to the lungs, it is of utmost importance to prohibit excessive reductions in the dose delivered to the ribcage, which can lead to an increased risk of relapse of the original disease [10]. A previous simulation study suggests that HT may be a feasible method of delivering the prescribed dose to the ribcage and planning target volume (PTV) while reducing lung irradiation [11]. Here, we analyzed data from 35 patients at our institution who underwent TBI using HT as part of their bone-marrow conditioning regimen. We demonstrate the feasibility of using HT to reduce irradiation of lung tissue, thereby minimizing risks of side effects or long-term health issues while maintaining the radiation dose prescribed to the ribcage to lessen the likelihood of relapse.
Materials and methods
As our data were obtained for routine quality assurance, which is standard of care in our institution, and are in line with requirements of the German radiation protection law, ethical approval was not required.
Patients
We retrospectively analyzed data from all patients undergoing TBI in our institution between 2012 and 2020 whose treatment plan and delivery involved sparing of lung tissue.
Treatment planning
Two computed tomography (CT) scans were performed for each patient to delineate and plan for TBI using TomoTherapy® Hi-ART II. Because TomoTherapy® Hi-ART II has a maximum couch shift of 135 cm, table rotation was needed between upper body and lower body CT scans, thus requiring two treatment plans that were merged following further technical considerations [12]. As the junction between scans is located at the thighs, only data from the upper body scan were analyzed in this study. CT scans were acquired in cranio-caudal alignment with a slice thickness of 5 mm.
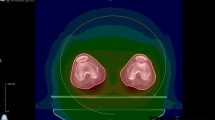
An Eclipse treatment planning system (Varian Medical System, Palo Alto, CA) was used for all delineations and planning to optimize preparation for treatment. The contouring of OARs was performed according to current institutional and international standards [13]. The PTV was also contoured. Special care was taken to accurately contour the lungs and ribcage so as to evaluate dose sparing to the lungs as well as the homogeneity and correct delivery of radiation to the ribcage according to a previously described method used at our institution [12]. The lungs were delineated using a standardized template that covers internal lung tissue up to 1 cm below the lung surface, and ribcage contouring was individually adjusted for each patient (Fig. 1). A 2.5 cm field width, pitch of 0.390, and planned modulation factor of 2.7 were used as setup planning parameters.
During TBI, patient position was fixed using a vacuum cushion for the body and fixation mask for the head to reduce movement, which could alter delivery of the planned irradiation. The beam-on time was 32.42 min on average (standard deviation (SD), 6.5 min) to deliver HT to the whole body. However, as additional time was needed for preparation and further considerations, each patient was given a 90 min timeslot.
We analyzed the efficiency of radiation dose reduction to the lungs and the resultant radiation dose delivered the ribcage during TBI. Patients were divided into three groups by their prescribed dose (4 Gy (2 × 2 Gy), 8 Gy (4 × 2 Gy), or 12 Gy (6 × 2 Gy), because the percentile dose reduction varies among these doses due to overall lower toxicity of lung tissue when irradiated with 4 Gy compared with 12 Gy [14, 15]. We also generated dose volume histograms (DVHs) and calculated the homogeneity index (HI) to examine ribcage dose exposure relative to the achieved lung dose reduction in each group. HI was calculated using the formula proposed by Kataria et al. (HI = D5/D95) [16]. Statistical analysis was performed using SPSS v26.0 (IBM, Armonk, New York, USA).
Results
We analyzed data from 35 patients (18 women and 17 men) with an average age of 40.2 years (SD, 15.9; range, 13–72). Patients were being treated for AML (n = 9), ALL (n = 21), mixed phenotype acute leukemia (n = 2), mast cell leukemia (n = 2), diffuse large B-cell lymphoma (n = 1), or anaplastic large cell lymphoma (n = 1). Fifteen patients were planned to receive 12 Gy, 15 to receive 8 Gy, and four to receive 4 Gy.
Considering the lungs, patients in the 4 Gy group had a D95 of 60.97 ± 22.68% (mean ± SD) and D5 of 101.63 ± 3.62% relative to the prescribed dose (Table 1, Fig. 2), resulting in actual doses of 2.44 Gy over 95% of the lung volume and 4.07 Gy over 5% of the lung volume, respectively. Patients in the 8 Gy and 12 Gy groups had a D95 of 54.77 ± 13.20% and 37.44 ± 10.28% relative to the prescribed dose, resulting in doses of 4.38 Gy and 4.49 Gy over 95% of the lung volume, respectively.
Considering the ribcage, patients in the 4 Gy group had a D95 of 91.27 ± 6.16% relative to the prescribed dose, whereas patients in the 8 Gy and 12 Gy groups had a D95 of 89.14 ± 9.32% and 78.65 ± 11.59%, respectively (Table 2). However, patients in the 4 Gy, 8 Gy, and 12 Gy groups had similar a D5 of 106.01 ± 2.13%, 105.60 ± 2.10%, and 105.82 ± 2.75%, respectively.
The HI ranged from 1.17 ± 0.09 in the 4 Gy group to 1.37 ± 0.20 in the 12 Gy group (Table 3).
The difference between lung and ribcage doses are visualized in a DVH showing an example of the effect of lung sparing (Fig. 3a). By contrast, a simulation of a plan without lung sparing shows significant dose differences (Fig. 3b). In addition, a dose cross profile in coronal projection for an example TBI plan demonstrates the steep decrease in the dose received by the lungs relative to that received by the ribcage, mediastinum, and PTV (Fig. 4).
Discussion
Due to its positive contribution to bone-marrow conditioning regimens, TBI is regularly used when preparing patients for allogenic BMT [17]. In combination with chemotherapy, TBI improves the long-term survival of patients with diseases, such as ALL or AML [18]. Given patients’ improved outcomes and lower relapse rate after TBI, it becomes increasingly important to reduce toxicity to OARs to prevent acute side-effects and long-term negative consequences. Lung tissue is particularly vulnerable to TBI, which can lead to interstitial pneumonitis, radiation pneumonitis, or pulmonary infection [13,14,15]. Thus, HT can be used to safely deliver radiation to patients undergoing BMT by reducing the dose received by lung tissue. Previous stimulation studies and studies using phantoms suggest that even with substantial dose sparing to lung tissue, all relevant structures in close proximity may be irradiated with the prescribed dose. Structures such as the ribcage are of particular importance, as they are one of the main targets of TBI or total marrow irradiation [7, 12].
Using data from actual patients treated at our institution, we examined the extent to which TBI delivered via HT reduces the dose of radiation received by lung tissue and the ribcage, as a substantial reduction in ribcage dose coverage could decrease the advantage of lung dose sparing. We found that the mean D95 for the lungs, indicating the minimum dose received by 95% of the lung volume, was reduced to 60.97% in the 4 Gy group, which was the smallest dose reduction observed across the three groups. This result could partially be explained by a lesser accumulation of tissue dose sparing due to only two treatment sessions but could also be an outcome of treatment planning. For patients in the 8 Gy and 12 Gy groups, mean D95 dropped to 54.77% and 37.44%, resulting in minimum doses of 4.38 Gy and 4.49 Gy, respectively. Shinde et al. propose a mean dose of < 8 Gy when delivering radiation to the lungs to reduce the possibility of pulmonary infection and radiation pneumonitis [14]. This outcome was achieved for all three groups in the present study, indicating that the risk of pulmonary toxicity was meaningfully reduced. However, the maximum D5 was close to the prescribed dose in all groups (101.63% for the 4 Gy group, 100.15% for the 8 Gy group, and 96.77% for the 12 Gy group), indicating a steep dose gradient. A complete vertical dose gradient is technically impossible, as some toxic effects, especially in higher dose areas, cannot be completely prevented. However, this is an expected result and does not reduce the feasibility of lung dose reduction.
To further support the viability of our approach, we also evaluated ribcage dose coverage. In the 4 Gy and 8 Gy groups, mean D95 was approximately 90%, which is still acceptably high, whereas mean D50 and D5 remained at the prescribed dose levels, ensuring good overall coverage of the ribcage. However, mean D95 was 78.65% in the 12 Gy group, which calls into question whether the relapse rate of these patients was elevated in comparison to that reported in the literature. It should be noted that although this variation is minor, it should be carefully considered in future analyses of patient relapse rates and toxic side effects.
In addition, mean HI was 1.17 for the 4 Gy group, 1.20 for the 8 Gy group, and 1.37 for the 12 Gy group. These values are well inside the range proposed by the Radiation Therapy Oncology Group, which recommends a maximum HI of < 2 [12]. Therefore, even our maximum observed value of 1.76 in the 12 Gy group is still acceptable.
The need for dose reduction in patients receiving 4 Gy is questionable given that this dose may have minor toxic effects [19]. That is, a marginal reduction in lung tissue toxicity may not warrant the additional time needed to plan for lung sparing. Further studies are needed to determine toxicity levels and treatment success after lung sparing, although previous studies suggest positive outcomes [10].
This study addresses the challenge of sparing sensitive lung tissue while maintaining the prescribed dose to the ribcage during TBI. Although we demonstrate the technical effectiveness of this approach, further studies are needed to ensure that it does not increase the risk of disease recurrence or relapse. Additional research is also warranted to determine whether similar methods are feasible for planning and delineating other OARs, such as the liver, spleen, or kidneys. Furthermore, whereas 3D planning methods are already well-established for delivering TBI, our approach to reducing the dose to the lungs while maintaining the prescribed level to the ribcage could also be implemented when developing and establishing 4D planning approaches in the future.
Conclusions
To improve the outcomes of patients undergoing TBI in preparation for allogeneic BMT, toxic effects on lung tissue can be reduced using HT to delineate, contour, and plan irradiation with lung dose sparing. The results of our study suggest that this approach does not compromise the quality of ribcage dose coverage, as dose homogeneity and overall coverage were consistent with guideline recommendations. These findings can inform clinicians’ planning and preparation for BMT to achieve optimal treatment outcomes.
Availability of data and materials
All data relevant to this publication have been included into the manuscript’s body.
Abbreviations
- ALL:
-
Acute lymphoblastic leukaemia
- AML:
-
Acute myeloblastic leukaemia
- BMT:
-
Bone-marrow transplantation
- CT:
-
Computed tomography
- HI:
-
Homogeneity index
- ICRU:
-
International Commission on Radiation Units and Measurements
- JT:
-
Junctional target
- PTV:
-
Planning target volume
- RTOG:
-
Radiation Therapy Oncology Group
- TBI:
-
Total body irradiation
References
Sabloff M, Tisseverasinghe S, Babadagli ME, Samant R. Total body irradiation for hematopoietic stem cell transplantation: what can we agree on? Curr Oncol. 2021;28(1):903–17. https://doi.org/10.3390/curroncol28010089.
Wong JYC, Filippi AR, Dabaja BS, Yahalom J, Specht L. Total body irradiation: guidelines from the international lymphoma radiation oncology group (ILROG). Int J Radiat Oncol Biol Phys. 2018;101(3):521–9. https://doi.org/10.1016/j.ijrobp.2018.04.071.
Travis EL, Peters LJ, McNeill J, Thames HD Jr, Karolis C. Effect of dose-rate on total body irradiation: lethality and pathologic findings. Radiother Oncol. 1985;4(4):341–51. https://doi.org/10.1016/s0167-8140(85)80122-5.
Keane TJ, Van Dyk J, Rider WD. Idiopathic interstitial pneumonia following bone marrow transplantation: the relationship with total body irradiation. Int J Radiat Oncol Biol Phys. 1981;7(10):1365–70. https://doi.org/10.1016/0360-3016(81)90032-8.
Bailey DW, Wang IZ, Lakeman T, Hales LD, Singh AK, Podgorsak MB. TBI lung dose comparisons using bilateral and anteroposterior delivery techniques and tissue density corrections. J Appl Clin Med Phys. 2015;16(2):5293. https://doi.org/10.1120/jacmp.v16i2.5293.
Peñagarícano JA, Chao M, Van Rhee F, Moros EG, Corry PM, Ratanatharathorn V. Clinical feasibility of TBI with helical tomotherapy. Bone Marrow Transplant. 2011;46(7):929–35. https://doi.org/10.1038/bmt.2010.237.
Hui SK, Kapatoes J, Fowler J, et al. Feasibility study of helical tomotherapy for total body or total marrow irradiation. Med Phys. 2005;32(10):3214–24. https://doi.org/10.1118/1.2044428.
Cleuziou JP, Desgranges C, Henry I, Jaumot M, Chartier P, Sihanath R, Carré M, Bulabois CE, Cahn JY, Pasteris C, Balosso J, Gabelle-Flandin I, Verry C, Giraud JY. Total body irradiation using helical tomotherapy: set-up experience and in-vivo dosimetric evaluation. Cancer Radiother. 2021;25(3):213–21. https://doi.org/10.1016/j.canrad.2020.07.009.
Hui SK, Kapatoes J, Fowler J, Henderson D, Olivera G, Manon RR, Gerbi B, Mackie TR, Welsh JS. Feasibility study of helical tomotherapy for total body or total marrow irradiation. Med Phys. 2005;32(10):3214–24. https://doi.org/10.1118/1.2044428.
Gruen A, Ebell W, Wlodarczyk W, Neumann O, Kuehl JS, Stromberger C, Budach V, Marnitz S. Total Body Irradiation (TBI) using helical tomotherapy in children and young adults undergoing stem cell transplantation. Radiat Oncol. 2013;15(8):92. https://doi.org/10.1186/1748-717X-8-92.
Wilhelm-Buchstab T, Leitzen C, Schmeel LC, Simon B, Koch D, Schmeel FC, Schoroth F, Garbe S, Röhner F, Wolf D, Schüller H, Schild HH, Müdder T. Total body irradiation: Significant dose sparing of lung tissue achievable by helical tomotherapy. Z Med Phys. 2020;30(1):17–23.
Köksal M, Baumert J, Schoroth F, et al. Helical versus static approaches to delivering tomotherapy to the junctional target for patients taller than 135 cm undergoing total body irradiation. Eur J Med Res 2022;27:265. https://doi.org/10.1186/s40001-022-00886-7
Hodapp N. Der ICRU-Report 83: Verordnung, Dokumentation und Kommunikation der fluenzmodulierten Photonenstrahlentherapie (IMRT) [The ICRU Report 83: prescribing, recording and reporting photon-beam intensity-modulated radiation therapy IMRT)]. Strahlenther Onkol. 2012 Jan;188(1):97–9. German. https://doi.org/10.1007/s00066-011-0015-x. PMID: 22234506.
Shinde A, Yang D, Frankel P, Liu A, Han C, Del Vecchio B, Schultheiss T, Cheng J, Li R, Kim D, Radany EH, Hui S, Somlo G, Rosenthal J, Stein A, Forman S, Wong JYC. Radiation-related toxicities using organ sparing total marrow irradiation transplant conditioning regimens. Int J Radiat Oncol Biol Phys. 2019;105(5):1025–33. https://doi.org/10.1016/j.ijrobp.2019.08.010.
Carruthers SA, Wallington MM. Total body irradiation and pneumonitis risk: a review of outcomes. Br J Cancer. 2004;90(11):2080–4. https://doi.org/10.1038/sj.bjc.6601751.
Kataria T, Sharma K, Subramani V, Karrthick KP, Bisht SS. Homogeneity index: an objective tool for assessment of conformal radiation treatments. J Med Phys. 2012;37(4):207–13. https://doi.org/10.4103/0971-6203.103606.
Cahu X, Labopin M, Giebel S, et al. Impact of conditioning with TBI in adult patients with T-cell ALL who receive a myeloablative allogeneic stem cell transplantation: a report from the acute leukemia working party of EBMT. Bone Marrow Transplant. 2016;51(3):351–7. https://doi.org/10.1038/bmt.2015.278.
Dejonckheere, CS, Böhner, AMC, Schmitz, E, et al. Peripheral blood kinetics following total body irradiation and allogeneic hematopoietic stem cell transplantation: Timing matters. Cancer Med. 2022;00:1–5. https://doi.org/10.1002/cam4.5452.
Schröder C, Buchali A, Windisch P, Vu E, Basler L, Zwahlen DR, Förster R. Impact of low-dose irradiation of the lung and heart on toxicity and pulmonary function parameters after thoracic radiotherapy. Cancers (Basel). 2020;13(1):22. https://doi.org/10.3390/cancers13010022.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
MK formulated the research goals and aims, designed the study, and was responsible for research planning and execution, including providing mentorship external to the core team. JB performed the data collection. JB and FS performed the statistical analyses. MK, GS, JB and FS analysed the data. MK and JB drafted the initial manuscript. All authors reviewed the drafted manuscript for critical content. All authors approved the final version of the manuscript and attest to the validity and legitimacy of the data as well as its interpretation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
As this analysis was conducted for routine quality assurance in line with requirements of the German radiation protection law, ethical approval was not required.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Köksal, M., Baumert, J., Schoroth, F. et al. Lung sparing and ribcage coverage in total body irradiation delivered by helical tomotherapy. Eur J Med Res 27, 287 (2022). https://doi.org/10.1186/s40001-022-00918-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-022-00918-2