Abstract
Aim
At present, the relationship between serum homocysteine (Hcy), fibrinogen (FIB), lipoprotein-a (LPa), and PAD is uncertain, and there has been no meta-analysis to establish the dose–response relationship between their exposure levels and PAD.
Methods and results
Relevant literature published in PubMed, Embase, and Web of Science was retrieved. The robust error meta-regression method was used to assess the linear and non-linear dose–response relationship between exposure level and PAD risk. A total of 68 articles, involving 565,209 participants, were included. Combined with continuous variables, the serum Hcy, FIB, and LPa levels of PAD patients were significantly higher than those of healthy individuals. The odds ratios (ORs) of PAD for individuals with high Hcy, FIB, and LPa levels compared with those with low levels were 1.47, 1.14, and 1.76, respectively. The study also showed that circulating Hcy, FIB, and LPa were significantly elevated in patients with PAD compared with controls. The level of Hcy and the risk of PAD presented a U-shaped distribution. The nonlinear dose–response model showed that each 1 μmol/L increase in serum Hcy increased the risk of PAD by 7%. Similarly, for each 10 mg/dL FIB and 10 mg/dL LPa increases, the risk of PAD increased by 3% and 6%, respectively.
Conclusions
This meta-analysis provided evidence that elevated Hcy, PIB, and LPa levels may increase the risk of PAD, and the risk of PAD increases with the increase in serum exposure within a certain range. By controlling Hcy level, the incidence of PAD may be reduced to control the PAD growing epidemic.
Trial registration number: PROSPERO (CRD42021250501), https://www.crd.york.ac.uk/prospero/
Similar content being viewed by others
Introduction
Peripheral arterial disease (PAD) is characterized by atherosclerosis of the lower limbs. It is the third most common manifestation of atherosclerotic vascular disease after coronary artery disease (CAD) and stroke. PAD affects individuals all over the world [1, 2]. At present, the incidence of PAD is steadily increasing, accounting for 2–10% of the total population and even 20% of patients over 70 years [3, 4]. Although the diagnosis of the ankle/brachial index (ABI) is specific and patients with PAD have a high risk of cardiovascular events, PAD is still often undiagnosed or underestimated [5, 6]. The diagnosis of PAD heralds severe dysfunction, which is characterized by lower limb pain during exercise, called claudication, and severe limb ischemia and limb loss [7, 8]. Symptomatic PAD is usually characterized by intermittent claudication, adversely affecting patients’ quality of life and resulting in functional impairment [9]. Therefore, PAD results in a heavy burden and great pain in the affected families, patients, and even the society as a whole.
Although the etiology and pathogenesis remain unclear, PAD is considered a multifactorial disease; the pathophysiological factors causing peripheral arterial occlusion are complex, and atherosclerosis is the main pathophysiological basis of PAD [10]. The formation of atherosclerotic plaque is based on the gradual accumulation of lipids and inflammatory cells in the arterial wall [11, 12]. Several circulating biomarkers have been proposed to diagnose PAD, especially fibrinogen (FIB), homocysteine (Hcy), lipoprotein a (LPa), C-reactive protein (CRP), D-dimer, and IL-6. Simple, inexpensive, and easy-to-detect markers of inflammation and thrombosis would play an important role in the positive diagnosis or risk stratification of PAD. However, there is no ideal and specific serum biomarker for clinical detection of PAD.
Reducing Hcy level may decrease the risk of PAD, and Hcy level has prognostic significance. However, the association between a mildly elevated Hcy level and risk of PAD is controversial. Although the relationship between circulating FIB and PAD has widely been reported in previous original studies and meta-analyses, inconsistent results have also been obtained in recent studies. For example, Small et al. determined hemostatic factors and their contribution to PAD; they suggested that circulating FIB level was not associated with PAD [13]. Several studies found that increased serum FIB concentration was associated with the presence of symptomatic PAD, independent of traditional and nontraditional cardiovascular risk factors [6, 14]. At present, the relationship between serum apolipoprotein-a (LPa) and PAD is still controversial, and there have been no meta-analyses to evaluate the dose–response relationship between these circulating biomarker levels and PAD.
Evaluating whether there is a dose–response relationship between certain exposure levels and disease outcomes is an important task of epidemiological research. It provides strong evidence for pathogenic inference and can promote the exploration of disease prevention and treatment measures. Therefore, this dose–response meta-analysis evaluated the relationship between serum FIB, Hcy, LPa and PAD, which may be of great significance for identifying the risk of PAD and improving the clinical course of PAD patients. We conducted this updated overall and dose–response meta-analysis to further explore the association between circulating biomarkers and risk of PAD.
Methods
Search strategy
This systematic review was registered at PROSPERO (www.crd.york.ac.uk/PROSPERO) as CRD CRD42021250501 on June 1, 2021. We carried out the meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [15]. The PubMed, Embase, and Web of Science databases were searched by two of the authors until April 20, 2021, without the restriction of language and publication date for eligible studies. Taking the relationship between serum Hcy and PAD as an example, the other two studies on the relationship between exposure levels and PAD can be deduced by analogy. We selected articles that reported on the relationship between homocysteine and PAD. Our search combined keywords and MeSH terms, and the search strategy for all databases is shown in Additional file 1: Table S1. In addition, reference lists of the retrieved articles and reviews on the subject were manually evaluated to identify any other relevant published articles. We did not include abstracts, grey literature, and unpublished studies.
Eligibility criteria and study selection
The eligibility criteria were in accordance with the Population, Intervention/Exposure, Control, Outcomes, and Study design (PICOS) framework. Taking Hcy as an example, PICOS were included based on the following selection criteria:
-
Population: Patients had to have an ankle–brachial index (ABI) value of ≤ 0.90 with intermittent claudication or asymptomatic PAD or chronic limb ischemia, and without diabetes, chronic renal failure, or metabolic syndrome.
-
Intervention/Exposure: The level of fibrinogen had to be measured according to the Clauss method; the level of total homocysteine (free and protein bound) had to be determined by fluorescence polarization immunoassay; and Lp(a) concentration had to be determined by ELISA.
-
Control: Healthy people had to be without PAD. Control individuals had be from the same geographic region, and the same exposure measurement methods had to exist between cases and controls.
-
Outcomes: OR, mean ± SD.
-
Study design: Cohort study, case–control study, and cross-sectional study.
Title/abstract screening: The study had to investigate the association between Hcy and PAD.
Full-text review: (1) the study had to be designed as an epidemiological study (e.g., cohort study, case–control study, or cross-sectional study); (2) the research had to report the Hcy levels of cases and controls and the standard deviation that we were able to estimate, or to report odds ratios (ORs), relative risks (RRs), or hazard ratios (HRs) with 95% confidence intervals (95% CIs) for Hcy and PAD; (3) patients had to have asymptomatic PAD, intermittent claudication, or chronic limb ischemia, and they did not have diabetes, chronic renal failure, or metabolic syndrome; (4) control individuals had to be healthy individuals without PAD from the same geographic region and matched with patients with respect to age, gender, and presence of type 2 diabetes mellitus.
Study selection was performed by two independent reviewers (H. W. and P. W.), and any discrepancies were resolved through discussion with a third reviewer (Y. H.).
Data extraction and quality assessment
We extracted the following information from each study: author; country; year; ethnicity; study design; number of control individuals and PAD patients; gender ratio of controls and PAD patients; exposure level; ORs and 95% CIs for the highest vs the lowest level of the exposure variables; ORs and 95% CIs for different levels of the exposure variables; adjusting for confounders. As randomized control trials were not retrieved and only four articles from all the databases involved cross-sectional research, the Newcastle–Ottawa Scale (NOS) guidelines were used to evaluate the quality of the literature in this study. The NOS guidelines-modified studies that achieved six or more stars were considered to be of high quality, otherwise they were marked as low-quality studies.
Statistical analysis
The studies including the serum levels of Hcy, FIB, and LPa of the PAD group and the control group were used to analyze the differences in serum Hcy, FIB, and LPa between patients with PAD and healthy individuals. We combined continuous variables into standard mean difference (SMD) and weighted mean difference (WMD). To find the relationship between the levels of exposures (Hcy, FIB, and LPa) and the risk of PAD, the summarized ORs and 95% CIs were assessed by random-effects models. Because of the low incidence of PAD (approximately 22.4/1000 person-years, with 95% CI of 20.8–24.0) [3], risk ratios were treated as ORs in most studies. We compared the ORs and 95% CIs of the highest level of exposures to the lowest level of exposures.
A potential linear and nonlinear dose–response relationship of Hcy, FIB, and LPa with the risk of PAD was examined by robust error meta-regression (REMR) approach described by Xu and co-workers, namely, we used inverse-variance weighted least squares (WLS) regression with cluster robust error variances [16] and we calculated study-specific slopes (linear trends) and standard error (SE) from the natural logarithms of the reported ORs and CIs across categories of anthropometric measures. The mean levels of Hcy, FIB, and LPa were assigned to the corresponding OR of each study; when these mean levels for this category were not reported, we calculated the average of the upper and the lower cutoff point to estimate the approximate midpoint [17]. When the highest or lowest category was open-ended, we assumed that the open-ended interval length was the same as the adjacent interval when estimating the midpoint [18].). In our data set, as the reference dose of exposures varied from study to study, the data first had to be centered [19]. Taking the average of the lowest dose of each study as the initial value of the exposure dose level, a restricted cubic spline model with knots was used to fit the potential nonlinear dose–response relationship. Thus, we calculated summary ORs and SEs for 1 μmol/L increase in Hcy, 10 mg/dL increase in FIB, and 10 mg/dL increase in LPa concentrations. Then, we run the same process without splines (using the linear dose fit) to calculate the linear trend.
Statistical heterogeneity was assessed using the Q test and I2; P < 0.1 and I2 > 50% indicated high heterogeneity between the studies. If there was a conflict between the Q statistic and the I2 statistic, the I2 statistic prevailed. Potential publication bias was assessed by Egger’s test and Begg’s test, with P < 0.05 indicating publication bias. To judge the robustness of the meta-analysis results, we carried out sensitivity analyses, including changing the effect model, trim-and-fill method, and analyses with excluding one study at a time.
Results
Study selection
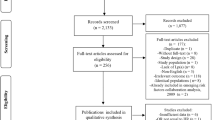
The process of literature screening is presented in Fig. 1. Searching the databases (PubMed, Embase, and Web of Science), a total of 5423 literature records were obtained. After eliminating duplicate articles, the titles and abstracts of 4315 obtained articles were scanned. A total of 3357 references were excluded based on type, correlation, and duplication. Then, the remaining 958 articles were scanned in full text and screened in accordance with the inclusion and exclusion criteria mentioned above. Finally, the 108 articles that met the requirements were evaluated for quality. We excluded the articles with unusable outcome data, unqualified study design or outcome, and data that had not been adjusted for confounders. In the end, a total of 68 papers were included in this study, among which 30, 32, and 18 papers explored the relationships between serum Hcy, FIB, LPa and PAD, respectively. A total of 68 articles, involving 565,206 women and men, published between the year of 1989 and 2021, were included in this meta-analysis.
The detailed characteristics of the included studies are summarized in Table 1 and Additional file 2: Table S2. The quality assessment results are shown in Tables 2 and 3, and studies with more than six stars were considered high-quality studies.
Association of Hcy, FIB, and LPa concentration with PAD
Sixteen articles, with 11,687 participants, were included to compare the serum Hcy levels of PAD patients and healthy individuals. Using the random-effects model, the estimated value of the combined effect size of the SMD point was 0.429 (95% CI: 0.285–0.573, I2 = 81.6%, P < 0.001), i.e., the serum Hcy concentration of PAD patients was significantly higher than that of the controls (Fig. 2A). The point estimate of WMD was 2.252 (95% CI: 1.501–3.002), meaning that the serum Hcy concentration of PAD patients on average was 2.252 μmol/L higher than that of controls (Additional file 3: Fig. S1A). There was no indication of publication bias with Begg’s test (P = 0.096) and Egger’s (P = 0.207). Three studies were added with trim-and-fill method. The results did not change significantly, suggesting that the combined effect size results were robust. We also examined the impact of a single study on the results; the removal of any single study did not make a large change in the SMD, i.e., the result of the combined effect size was robust (Fig. 2B).
There were 21 studies, with 17,998 participants, exploring the difference in serum FIB level between patients with PAD and healthy individuals. The estimated value of the combined effect size of the SMD point was 0.631 (95% CI: 0.506–0.757, I2 = 78.4%, P < 0.001), indicating that the serum FIB concentration of PAD patients was significantly higher than that of the controls (Fig. 2C). The point estimate of WMD was 39.071 (95% CI: 30.120–48.022), indicating that the serum FIB concentration of PAD patients on average was 39.071 mg/dL higher than that of controls (Additional file 3: Fig. S1B). No significant publication bias was observed with Begg’s test (P = 0.284) and Egger’s test (P = 0.279). Four studies were added with trim-and-fill method; the SMD did not change significantly, suggesting that the combined effect size results were robust. Examination of the impact of a single study on the result revealed that the removal of any single study did not make a large change in the SMD (Fig. 2D).
Five studies, with 2,533 participants, were included to compare serum LPa levels between patients with PAD and controls. The estimated value of the combined effect size of the SMD point was 0.420 (95% CI: 0.231–0.609, I2 = 75.4%, P = 0.003), meaning that the serum LPa concentration of PAD patients was significantly higher than that of the controls (Fig. 2E). The point estimate of WMD was 39.071 (95% CI: 30.120–48.022), indicating that the serum LPa concentration of PAD patients on average was 39.071 mg/dL higher than that of controls (Additional file 3: Fig. S1C). No significant publication bias was found by Begg’s test (P = 0.806) and Egger’s (P = 0.503). Two studies were added with trim-and-fill method and the SMD did not change significantly, suggesting that the combined effect size results were robust. Examining the impact of a single study on the result revealed that the removal of any single study did not make a large change in the SMD (Fig. 2F).
Elevated Hcy, FIB, Lpa, and risk of PAD
Data extracted from 13 articles (21,630 participants) that compared the relative risk of PAD between individuals at the top level of Hcy and those at the bottom level of Hcy yielded a summary OR of 1.470 (95% CI: 1.274–1.696, I2 = 78.9%, P < 0.001; in Fig. 3A). No significant publication bias was found by Begg’s test (P = 0.913). We examined the impact of a single study on the results; removing two studies [14, 20] made the result exceed the confidence interval, but it did not reverse the result, and removing other studies did not change the results significantly (Fig. 3B).
Prevalence of PAD in patients with homocysteine levels homocysteine ≥ 10 μmol/L vs those with less than 10 μmol/L A and two studies were added with trim-and-fill method B for homocysteine levels and PAD. Distribution of fibrinogen levels upper and lower status in controls and PAD patients and odds ratios (C), and trim-and-fill method analysis (D). Prevalence of PAD in patients with upper lipoprotein-a levels vs those with lower lipoprotein-a levels E and trim-and-fill method analysis (F)
Twelve studies, including 303,710 participants, were used to evaluate the risk of PAD in individuals with the highest FIB levels compared with those with the lowest FIB levels. The summary OR was 1.142 (95% CI: 1.005–1.237, I2 = 79.0%, P < 0.001; in Fig. 3C). There was no indication of publication bias with Begg’s test (P = 0.541). We examined the impact of a single study on the results; removal of two studies [21, 22] made the result larger than the confidence interval, but it did not reverse the result, and removing other studies did not change the results significantly (Fig. 3D).
Nine studies, including 5,764 participants, were used to evaluate the risk of PAD in individuals with high FIB levels; the summary OR was 1.755 (95% CI: 1.438–2.143, I2 = 83.6%, P < 0.001; in Fig. 3E). There was no evidence of publication bias with Begg’s test (P = 0.118). The result of the combined effect size was robust, because there was no significant change in the result after applying the trim-and-fill method, and the removal of any single study did not significantly change the result of the combined effect size (Fig. 3F).
Dose–response analysis
Six studies (34,898 participants) were included to analyze the dose–response relationship between Hcy and PAD. The data were fitted using an RCS with three knots (at 7, 9, and 12) allowing for a potential nonlinear relationship. The regression parameter estimates of the first spline and the second spline were − 0.4353 (β1) and 0.4180 (β2), respectively (Table 4, Additional file 4: Table S3 and Additional file 5: Table S4). An increase in the Hcy concentration of 1 μmol/L resulted in a 7% increase in the risk of PAD (P = 0.398). The level of Hcy and the risk of PAD presented a U-shaped distribution (Fig. 4A). When the concentration of Hcy was higher than 11.7 μmol/L, the risk of PAD increased sharply. We used the same process to calculate the linear trend over the entire range of doses, and the risk of PAD increased 3% per each 1 μmol/L increase of Hcy concentration (Fig. 4B).
Linear and nonlinear dose–response analyses of circulating Hcy levels, FIB level and LPa level and risk of AAA. The nonlinear dose–response analysis of homocysteine, per 1 μmol/L (A), fibrinogen level, per 10 mg/dL (B), and lipoprotein-a level, per 1 mg/dL C and risk of PAD. The linear dose–response analysis of homocysteine, per 1 μmol/L (D), fibrinogen level, per 10 mg/dL (E), and lipoprotein-a level, per 1 mg/dL F and risk of PAD
Five studies (37,933 participants) were used to analyze the dose–response relationship between FIB and PAD. An RCS was created (with three knots at 268, 318, and 370 of the dose distribution), which generated two splines, and these were then employed for the potential nonlinear dose-specific modeling. The regression parameter estimates of the first spline and the second spline were 0.0005 (β1) and 0.0020 (β2), respectively (Table 4, Additional file 4: Table S3 and Additional file 5: Table S4). The risk of PAD increased by 3% for each 10 mg/dL increase of FIB concentration (P = 0.882). Nonlinear dose–response analysis showed that the risk of PAD continued to increase as FIB increased (Fig. 4C). However, the nonlinear dose–response relationship also showed a flat curve over the typical range of FIB concentrations, suggesting that higher risks were associated with higher concentrations. Under the linear model, the risk of PAD increased by 3% for the same increment (Fig. 4D).
Five studies (247,709 participants) were included to analyze the dose–response relationship between LPa and PAD. There were three knots (at 2, 19, and 74 across the reported dose distribution) using RCSs. Estimated regression parameters were 0.007384 for β1 and − 0.002629 for β2 (Table 4, Additional file 4: Table S3 and Additional file 5: Table S4). An LPa increment of 1 mg/dL resulted in a 6% increase in the risk of PAD. Nonlinear dose–response analysis (Fig. 4E) showed a consistently increasing risk with increased LPa. Given the linear relationship (Fig. 4F), the linear trend (for 10 mg/dL increase) was 1.06, that is, the risk of PAD increased by 6% for each 10 mg/dL increase in LPa concentration.
Subgroup analysis
Subgroup analysis was performed based on study design, and studies were divided into prospective and retrospective studies. Retrospective studies include case–control studies and cross-sectional studies, while prospective studies are cohort studies. Due to limitations in the data included in the study, we performed subgroup analyses only to assess the risk between Hcy, FIB, and LPa and PAD. As shown in Fig. 5A, whether it is a combination of prospective studies or retrospective studies, the results show that HCY is a risk factor for PAD. When evaluating the relationship between FIB and PAD risk, combined prospective research results show that FIB is a risk factor for PAD, while combined retrospective studies have no effective results, as shown in Fig. 5B. As for the relationship between LPa and PAD risk, both prospective studies and retrospective studies have shown that LPa is a risk factor for PAD, as shown in Fig. 5C.
Discussion
PAD commonly results from progressive narrowing of arteries in the lower extremities due to atherosclerosis. Previous studies have shown that PAD is associated with a significantly elevated risk of cardiovascular disease morbidity and mortality [23, 24]. PAD is also a common macrovascular complication of T2DM, which not only may contribute to initiation and aggravation of diabetic foot ulcer but is also an efficient predictor of cardiovascular mortality and morbidity.
Main implications
In the present study, we found that the serum homocysteine levels of PAD patients were significantly higher than those of healthy individuals. In addition, the risk of PAD in individuals with high serum homocysteine was 1.47 times higher than that of the corresponding low-level population. Meanwhile, Hcy was significantly higher (pooled mean difference 2.25 μmol/L; 95% CI: 1.50–3.00, P < 0.0001) in patients with PAD compared with controls. Previously, a meta-analysis of 14 relevant studies showed that Hcy was significantly elevated (pooled mean difference + 4.31 μmol/L; 95% CI: 1.71–6.31, P < 0.0001 with significant heterogeneity) in patients with PAD compared with controls [25]. However, that study only reported the pooled relative risk of PAD when comparing the highest Hcy category group with the lowest Hcy category group and failed to explore the quantitative dose–response association between Hcy levels and risk of PAD. The present study is the first systematic dose–response meta-analysis of serum homocysteine levels and the risk of PAD. Regarding the nonlinear dose–response relationship between serum Hcy levels and PAD, we found that when the concentration of Hcy was higher than 11.7 μmol/L, every increase of 1 μmol/L in serum concentration of Hcy increased the risk of PAD by 7%. More importantly, we found that the relationship between serum Hcy level and the risk of PAD showed a U-shaped curve distribution. When the Hcy concentration was lower than 11.7 μmol/L, Hcy was not considered a risk factor for PAD; in contrast, when the Hcy concentration was higher than 11.7 μmol/L, the risk of PAD increased sharply with the increase of Hcy level. This conclusion is supported by the previously reported relationship between PAD and Hcy. [26]. Our findings may have important implications for public health. Elevated Hcy level may be a modifiable risk factor for PAD.
According to the results of our meta-analysis, controlling and maintaining a healthy fibrinogen level should be recommended to benefit health. Here, the risk of PAD in individuals with high serum fibrinogen levels was 1.14 times higher than that of the corresponding low-level population. Kremers et al. identified fibrinogen as a promising biomarker that represents different pathophysiological processes implicated in lower extremity PAD; in that meta-analysis, increased fibrinogen levels have been associated with an increased relative risk of mortality of 2.08 [27]. However, the study designs included in that meta-analysis were inconsistent, including prospective nested case–control studies, prospective cohort studies, and case–control studies. Thus, here we performed a meta-analysis focusing only on prospective cohort studies to investigate the dose–response association between the FIB level and risk of PAD. We also found that the relationship between serum FIB levels and the risk of PAD presented an approximately J-shaped curve distribution. When the FIB concentration was higher than 319.7 mg/dL, every 10 mg/dL increase in serum FIB concentration increased the risk of PAD by 3%. When the FIB concentration was lower than 319.7 mg/dL, the risk of PAD did not change significantly with the increase of FIB level; in contrast, when the FIB concentration was higher than 319.7 mg/dL, the risk of PAD increased sharply with the increase in FIB level.
We found that the serum lipoprotein-a (LPa) levels of PAD patients were significantly higher than those of healthy individuals. In addition, the risk of PAD in individuals with high serum LPa levels was 1.76 times higher than that of the corresponding low-level population. The dose–response relationship between serum LPa level and PAD indicated that as the LPa level increased, the risk of PAD increased. In addition, every 10 mg/dL increase in serum LPa concentration increased the risk of PAD by 6%.
Potential mechanism
The pathogenesis of PAD is complex, involving a variety of genetic and environmental factors related to atherosclerosis and thrombosis, and the interaction between them [28]. The most common cause of PAD is atherosclerosis [29]. Atherosclerotic plaque causes narrowing or occlusion of the arteries, thereby reducing blood flow to the affected limb [29]. Thrombin-mediated conversion of plasma fibrinogen to fibrin, forming a relatively insoluble clot, is the final step in the clotting cascade. Fibrin has been shown to be a stable component of atherosclerotic plaque and to promote its growth [30]. In addition, FIB is also a determinant of inflammation biomarkers, acute phase reactants, and blood viscosity. Hyperhomocysteinemia has been reported to reduce thrombus permeability and solubility sensitivity, and the current study confirmed that Hcy is associated with certain fibrin clot markers in patients with PAD, despite a number of potential confounding factors [31]. In addition, Hcy is involved in various pathological processes, such as endothelial dysfunction, oxidative stress, and vascular remodeling, which further aggravate the impairment of vasodilation in PAD patients during exercise, resulting in motor dysfunction [32]. These mechanisms may explain how Hcy increases the risk of PAD. LPa is the main carrier of oxidized phospholipids in plasma, and it induces the activation of monocytes. The increased migration of these monocytes between endothelial cells leads to the production of proinflammatory cytokines and other cellular effects that contribute to the progression of atherosclerotic disease [33,34,35]. At present, the pathophysiological effects of serum Hcy, FIB, LPa, and PAD are still not fully understood, and more studies on the correlation mechanism are needed in the future.
Advantages and limitations
Our meta-analysis has several strengths. To our knowledge, this is the first systematic dose–response meta-analysis of serum exposure levels of Hcy, FIB, and LPa and the risk of PAD, and the results obtained have a mutual collaborative relationship with previous research conclusions. Compared with the sample size limit of a single study, this meta-analysis included a total of 68 articles, with a total sample size of 565,209 patients, including > 56,754 PAD cases. The total sample size of the studies on the relationship between Hcy, FIB, LPa and PAD reached 65,886, 320,756, and 255,456, respectively. A large number of cases allowed us to determine the relationship between exposure levels and PAD risk. In addition, the 68 included articles were of high quality (all studies scored ≥ 6 stars). The method used in this study was the REMR method. Compared with previous studies using Generalized Least Squares (GLST) as a dose–response meta-analysis method, the REMR method eliminated the bias generated by the GLST method using the non-intercept model, resulting in better error estimation and a better visual fit to the data [16]. Moreover, our meta-analysis included prospective cohort studies, which may effectively avoid the possibility of a reverse relation and enhance the possibility of an etiological hypothesis. Finally, all the included studies have a relatively high quality, and the main results were robust after sensitivity analyses and Egger’s test.
Several potential limitations should be mentioned in this meta-analysis. First, according to previous reports, the relationship between exposure levels and the risk of PAD may vary slightly by gender and region [36,37,38], but we did not perform the subgroup analysis based on sex and region due to the limited data. Second, although the included studies adjusted for potential risk factors of PAD, residual confounders might exist because of the observational nature of the data. In addition, there was a large heterogeneity among the included studies, but the results of the sensitivity analysis showed that the combined effect size results were robust. Of course, the three exposure factors in this study are inherently controversial as risk factors for PAD, which may have led to high heterogeneity between the studies. Moreover, differences in the measurement of exposure levels may have also increased the heterogeneity. However, overall, the results of this study were stable, and the direction of exposure factors on PAD had never changed.
Conclusions
Based on the above results, it can be reasonably concluded that serum Hcy, FIB, and LPa are related to the risk of PAD, and within a certain range of their serum levels, the risk of PAD increases with the increase in the serum level. These three exposure factors are expected to become serum biomarkers of PAD. Individuals with high serum Hcy, FIB, and LPa levels should be highly concerned about the risk of PAD, and early screening and appropriate treatment are crucial. By controlling the Hcy level, the incidence of PAD may be reduced to control the growing epidemic. Therefore, our research is of great significance for the prevention of PAD and for improving the diagnosis of the disease. Certainly, further observational studies with large sample sizes are needed to verify our results, and more studies are also needed to explore the underlying physiological and pathological mechanisms.
Availability of data and materials
The data sets used or analyzed during the current study are available from the corresponding author on request.
Code availability
Microsoft Excel 2010; Microsoft, Redmond, WA, USA. STATA meta.ado module (version 15.1; COLLEGE Station, TX, USA). This paper is not based on a previous communication to a society or meeting.
Abbreviations
- PAD:
-
Peripheral artery disease
- Hcy:
-
Homocysteine
- FIB:
-
Fibrinogen
- LPa:
-
Lipoprotein(a)
- OR:
-
Odds ratio
- REMR:
-
Robust error meta-regression
- SD:
-
Standard error
- CI:
-
Confidence interval
- CRP:
-
C-reactive protein
- CVD:
-
Cardiovascular disease
- DM:
-
Diabetes mellitus
- ABI:
-
Ankle brachial index
- BMI:
-
Body mass index
- SBP:
-
Systolic blood pressure
References
Fowkes FGR, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382(9901):1329–40.
Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116(9):1509–26.
Kalbaugh CA, Kucharska-Newton A, Wruck L, et al. Peripheral artery disease prevalence and incidence estimated from both outpatient and inpatient settings among medicare fee-for-service beneficiaries in the atherosclerosis risk in communities (ARIC) study. JAHA. 2017;6(5):e003796.
Heiss C, Pitcher A, Jf Belch J, et al. The year in cardiology: aorta and peripheral circulation. The year in cardiology 2019. Cardiol Croat. 2020;15(7–8):214–27.
Conte SM, Vale PR. Peripheral arterial disease. Heart Lung Circ. 2018;27(4):427–32.
Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004;110(6):738–43.
Hiatt WR, Armstrong EJ, Larson CJ, et al. Pathogenesis of the limb manifestations and exercise limitations in peripheral artery disease. Circ Res. 2015;116(9):1527–39.
Hamburg NM, Balady GJ. Exercise rehabilitation in peripheral artery disease: functional impact and mechanisms of benefits. Circulation. 2011;123(1):87–97.
Hicken G, Ameli M. Quality of life in patients with intermittent claudication. Eur J Vasc Endovasc Surg. 1996;12(4):511–2.
Aboyans V, Ricco J-B, Bartelink M-L, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Kardiol Pol. 2017;75(11):1065–160.
Marchio P, Guerra-Ojeda S, Vila JM, et al. Targeting early atherosclerosis: a focus on oxidative stress and inflammation. Oxid Med Cell Longev. 2019. https://doi.org/10.1155/2019/8563845.
Ruparelia N, Choudhury R. Inflammation and atherosclerosis: what is on the horizon? Heart. 2020;106(1):80–5.
Small AM, Huffman JE, Klarin D, et al. Mendelian randomization analysis of hemostatic factors and their contribution to peripheral artery disease-brief report. Arterioscler Thromb Vasc Biol. 2021;41(1):380–6.
Meijer WT, Grobbee DE, Hunink MG, et al. Determinants of peripheral arterial disease in the elderly: the Rotterdam study. Arch Intern Med. 2000;160(19):2934–8.
Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7): e1000097.
Xu C, Doi SA. The robust error meta-regression method for dose–response meta-analysis. Int J Evid Based Healthc. 2018;16(3):138–44.
Berlin JA, Longnecker MP, Greenland S. Meta-analysis of epidemiologic dose-response data. Epidemiology. 1993;4:218–28.
Aune D, Saugstad OD, Henriksen T, et al. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta-analysis. JAMA. 2014;311(15):1536–46.
Crippa A, Orsini N. Dose-response meta-analysis of differences in means. BMC Med Res Methodol. 2016;16(1):1–10.
Eller P, Schgoer W, Mueller T, et al. Hepatic lipase polymorphism and increased risk of peripheral arterial disease. J Intern Med. 2005;258(4):344–8.
Sartori M, Favaretto E, Legnani C, et al. Thrombophilic risk factors and peripheral arterial disease severity. Thromb Haemost. 2010;104(07):71–7.
Ceasovschih A, Sorodoc V, OnofreiAursulesei V, et al. Biomarker utility for peripheral artery disease diagnosis in real clinical practice: a prospective study. Diagnostics (Basel). 2020;10(9):723.
Newman AB, Shemanski L, Manolio TA, et al. Ankle-arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. The Cardiovascular Health Study Group. Arterioscler Thromb Vasc Biol. 1999;19(3):538–45.
Mlacak B, Blinc A, Pohar M, et al. Peripheral arterial disease and ankle-brachial pressure index as predictors of mortality in residents of Metlika County. Slovenia Croat Med J. 2006;47(2):327–34.
Khandanpour N, Loke YK, Meyer FJ, et al. Homocysteine and peripheral arterial disease: systematic review and meta-analysis. Eur J Vasc Endovasc Surg. 2009;38(3):316–22.
Graham IM, Daly LE, Refsum HM, et al. Plasma homocysteine as a risk factor for vascular disease: the European Concerted Action Project. JAMA. 1997;277(22):1775–81.
Kremers B, Wübbeke L, Mees B, et al. Plasma biomarkers to predict cardiovascular outcome in patients with peripheral artery disease: a systematic review and meta-analysis. Arterioscler Thromb Vasc Biol. 2020;40(9):2018–32.
Voetsch B, Loscalzo J. Genetic determinants of arterial thrombosis. Arterioscler Thromb Vasc Biol. 2004;24(2):216–29.
Morley RL, Sharma A, Horsch AD, et al. Peripheral artery disease. BMJ. 2018;360:j5842.
Bini A, Fenoglio JJ Jr, Mesa-Tejada R, et al. Identification and distribution of fibrinogen, fibrin, and fibrin (ogen) degradation products in atherosclerosis. Use of monoclonal antibodies. Arteriosclerosis. 1989;9(1):109–21.
Undas A, Brozek J, Jankowski M, et al. Plasma homocysteine affects fibrin clot permeability and resistance to lysis in human subjects. Arterioscler Thromb Vasc Biol. 2006;26(6):1397–404.
Zaric BL, Obradovic M, Bajic V, et al. Homocysteine and hyperhomocysteinaemia. Curr Med Chem. 2019;26(16):2948–61.
Coassin S, Kronenberg F. Mechanistic insights into lipoprotein (a): from infamous to ‘inflammous.’ Eur Heart J. 2020;41(24):2272–4.
Boffa MB, Koschinsky ML. Oxidized phospholipids as a unifying theory for lipoprotein (a) and cardiovascular disease. Nat Rev Cardiol. 2019;16(5):305–18.
Van Der Valk FM, Bekkering S, Kroon J, et al. Oxidized phospholipids on lipoprotein (a) elicit arterial wall inflammation and an inflammatory monocyte response in humans. Circulation. 2016;134(8):611–24.
Liu M, Fan F, Liu B, et al. Joint effects of plasma homocysteine concentration and traditional cardiovascular risk factors on the risk of new-onset peripheral arterial disease. Diabetes Metab Syndr Obes. 2020;13:3383.
Rong D, Liu J, Jia X, et al. Hyperhomocysteinaemia is an independent risk factor for peripheral arterial disease in a Chinese Han population. Atherosclerosis. 2017;263:205–10.
Forbang NI, Criqui MH, Allison MA, et al. Sex and ethnic differences in the associations between lipoprotein (a) and peripheral arterial disease in the Multi-Ethnic Study of Atherosclerosis. J Vasc Surg. 2016;63(2):453–8.
Mueller T, Marschon R, Dieplinger B, et al. Factor V Leiden, prothrombin G20210A, and methylenetetrahydrofolate reductase C677T mutations are not associated with chronic limb ischemia: the Linz Peripheral Arterial Disease (LIPAD) study. J Vasc Surg. 2005;41(5):808–15.
Weragoda J, Seneviratne R, Weerasinghe MC, et al. Risk factors of peripheral arterial disease: a case control study in Sri Lanka. BMC Res Notes. 2016;9(1):1–8.
Bertoia ML, Pai JK, Cooke JP, et al. Plasma homocysteine, dietary B vitamins, betaine, and choline and risk of peripheral artery disease. Atherosclerosis. 2014;235(1):94–101.
Mohamad T, Veeranna V, Shenoy M, et al. Predictors of combined peripheral arterial disease and coronary artery disease in a population cohort representative of the current us population (sub-analysis of nhanes study). J Am Coll Cardiol. 2011;57(14S):E1499–E1499.
Sabino A, Fernandes AP, Lima LM, et al. Polymorphism in the methylenetetrahydrofolate reductase (C677T) gene and homocysteine levels: a comparison in Brazilian patients with coronary arterial disease, ischemic stroke and peripheral arterial obstructive disease. J Thromb Thrombolysis. 2009;27(1):82–7.
Garofolo L, Barros N Jr, Miranda F Jr, et al. Association of increased levels of homocysteine and peripheral arterial disease in a Japanese-Brazilian population. Eur J Vasc Endovasc Surg. 2007;34(1):23–8.
Guallar E, Silbergeld EK, Navas-Acien A, et al. Confounding of the relation between homocysteine and peripheral arterial disease by lead, cadmium, and renal function. Am J Epidemiol. 2006;163(8):700–8.
Allison MA, Criqui MH, Mcclelland RL, et al. The effect of novel cardiovascular risk factors on the ethnic-specific odds for peripheral arterial disease in the Multi-Ethnic Study of Atherosclerosis (MESA). J Am Coll Cardiol. 2006;48(6):1190–7.
Mueller T, Dieplinger B, Gegenhuber A, et al. Serum total 8-iso-prostaglandin F2α: a new and independent predictor of peripheral arterial disease. J Vasc Surg. 2004;40(4):768–73.
Van Den Bosch MA, Bloemenkamp DG, Willem PTM, et al. Hyperhomocysteinemia and risk for peripheral arterial occlusive disease in young women. J Vasc Surg. 2003;38(4):772–8.
Bloemenkamp DG, Van Den Bosch MA, Mali WP, et al. Novel risk factors for peripheral arterial disease in young women. Am J Med. 2002;113(6):462–7.
Bloemenkamp D, Mali WTM, Tanis B, et al. The relation between Helicobacter pylori and atherosclerosis cannot be explained by a high homocysteine concentration. Eur J Clin Invest. 2002;32(8):549–55.
Stricker H, Soldati G, Balmelli T, et al. Homocysteine, vitamins and gene mutations in peripheral arterial disease. Blood Coag Fibrinol. 2001;12(6):469–75.
Loncar R, Hrboka V, Tabakovic-Loncar V, et al. Screening of plasma homocysteine in peripheral arterial disease. Ann Med. 2001;33(1):48–54.
Bunout D, Petermann M, Hirsch S, et al. Low serum folate but normal homocysteine levels in patients with atherosclerotic vascular disease and matched healthy controls. Nutrition. 2000;16(6):434–8.
Todesco L, Angst C, Litynski P, et al. Methylenetetrahydrofolate reductase polymorphism, plasma homocysteine and age. Eur J Clin Invest. 1999;29(12):1003–9.
Aronow WS, Ahn C. Association between plasma homocysteine and peripheral arterial disease in older persons. Coron Artery Dis. 1998;9(1):49–50.
Malinow M, Kang S, Taylor L, et al. Prevalence of hyperhomocyst (e) inemia in patients with peripheral arterial occlusive disease. Circulation. 1989;79(6):1180–8.
Taylor LM Jr, Defrang RD, Harris EJ Jr, et al. The association of elevated plasma homocyst (e) ine with progression of symptomatic peripheral arterial disease. J Vasc Surg. 1991;13(1):128–36.
Celebi S, Berkalp B, Amasyali B. The association between thrombotic and inflammatory biomarkers and lower-extremity peripheral artery disease. Int Wound J. 2020;17(5):1346–55.
Mancho CR, Martinez-Aguilar E, Gomez-Rodriguez V, et al. C0162: procoagulant phospolipid activity in microparticles is associated to cardiovascular risk factors and disease severity in peripheral artery disease. Thromb Res. 2014;133:S94.
Grenon SM, Cohen BE, Smolderen K, et al. Peripheral arterial disease, gender, and depression in the Heart and Soul Study. J Vasc Surg. 2014;60(2):396–403.
Undas A, Nowakowski T, Ciesla-Dul M, et al. Abnormal plasma fibrin clot characteristics are associated with worse clinical outcome in patients with peripheral arterial disease and thromboangiitis obliterans. Atherosclerosis. 2011;215(2):481–6.
De Bruijne EL, Gils A, Rijken DC, et al. High thrombin activatable fibrinolysis inhibitor levels are associated with an increased risk of premature peripheral arterial disease. Thromb Res. 2011;127(3):254–8.
Reich LM, Heiss G, Boland LL, et al. Ankle—brachial index and hemostatic markers in the Atherosclerosis Risk in Communities (ARIC) study cohort. Vasc Med. 2007;12(4):267–73.
Mangiafico RA, Sarnataro F, Mangiafico M, et al. Impaired cognitive performance in asymptomatic peripheral arterial disease: relation to C-reactive protein and D-dimer levels. Age Ageing. 2006;35(1):60–5.
Kaperonis E, Liapis C, Kakisis J, et al. Inflammation and Chlamydia pneumoniae infection correlate with the severity of peripheral arterial disease. Eur J Vasc Endovasc Surg. 2006;31(5):509–15.
Wildman RP, Muntner P, Chen J, et al. Relation of inflammation to peripheral arterial disease in the national health and nutrition examination survey, 1999–2002. Am J Cardiol. 2005;96(11):1579–83.
Nasir K, Guallar E, Navas-Acien A, et al. Relationship of monocyte count and peripheral arterial disease: results from the National Health and Nutrition Examination Survey 1999–2002. Arterioscler Thromb Vasc Biol. 2005;25(9):1966–71.
Bozkurt AK, Koksal C, Ercan M. The altered hemorheologic parameters in thromboangiitis obliterans: a new insight. Clin Appl Thromb Hemost. 2004;10(1):45–50.
Smith FB, Connor JM, Lee AJ, et al. Relationship of the platelet glycoprotein PlA and fibrinogen T/G+ 1689 polymorphisms with peripheral arterial disease and ischaemic heart disease. Thromb Res. 2003;112(4):209–16.
Makin AJ, Chung NA, Silverman SH, et al. Thrombogenesis and endothelial damage/dysfunction in peripheral artery disease: Relationship to ethnicity and disease severity. Thromb Res. 2003;111(4–5):221–6.
Giunta R, Coppola A, Luongo C, et al. Ozonized autohemotransfusion improves hemorheological parameters and oxygen delivery to tissues in patients with peripheral occlusive arterial disease. Ann Hematol. 2001;80(12):745–8.
Blann A, Bignell A, Mccollum C. von Willebrand factor, fibrinogen and other plasma proteins as determinants of plasma viscosity. Atherosclerosis. 1998;139(2):317–22.
Poredoš P, Žižek B. Plasma viscosity increase with progression of peripheral arterial atherosclerotic disease. Angiology. 1996;47(3):253–9.
Herren T, Stricker H, Haeberli A, et al. Fibrin formation and degradation in patients with arteriosclerotic disease. Circulation. 1994;90(6):2679–86.
Fabsitz R, Sidawy A, Go O, et al. Prevalence of peripheral arterial disease and associated risk factors in American Indians: the Strong Heart Study. Am J Epidemiol. 1999;149(4):330–8.
Okraska-Bylica A, Wilkosz T, Słowik L, et al. Altered fibrin clot properties in patients with premature peripheral artery disease. Pol Arch Intern Med. 2012. https://doi.org/10.20452/pamw.1535.
Li S-B, Yang F, Jing L, et al. Correlation between plasma lipoprotein-associated phospholipase A2 and peripheral arterial disease. Exp Ther Med. 2013;5(5):1451–5.
Leng G, Lee A, Fowkes F, et al. The relationship between cigarette smoking and cardiovascular risk factors in peripheral arterial disease compared with ischaemic heart disease: the Edinburgh Artery Study. Eur Heart J. 1995;16(11):1542–8.
Mustonen P, LepaNtalo M, Lassila R. Physical exertion induces thrombin formation and fibrin degradation in patients with peripheral atherosclerosis. ATVB. 1998;18(2):244–9.
Kheirkhah A, Lamina C, Rantner B, et al. Elevated levels of serum PCSK9 in male patients with symptomatic peripheral artery disease: The CAVASIC study. Atherosclerosis. 2021;316:41–7.
Tunstall-Pedoe H, Peters SA, Woodward M, et al. Twenty-year predictors of peripheral arterial disease compared with coronary heart disease in the Scottish Heart Health Extended Cohort (SHHEC). J Am Heart Assoc. 2017;6(9): e005967.
Tmoyan N, Ezhov M, Afanasieva O, et al. Lipoprotein (a) is a marker of peripheral artery disease in the absence of coronary heart disease: P1411. Eur Heart J. 2017. https://doi.org/10.1093/eurheartj/ehx502.P1411.
Laschkolnig A, Kollerits B, Lamina C, et al. Lipoprotein (a) concentrations, apolipoprotein (a) phenotypes, and peripheral arterial disease in three independent cohorts. Cardiovasc Res. 2014;103(1):28–36.
Ye Z, Ali Z, Klee GG, et al. Associations of candidate biomarkers of vascular disease with the ankle–brachial index and peripheral arterial disease. Am J Hypertens. 2013;26(4):495–502.
Bertoia ML, Pai JK, Lee J-H, et al. Oxidation-specific biomarkers and risk of peripheral artery disease. J Am Coll Cardiol. 2013;61(21):2169–79.
Bérard AM, Bedel A, Le Trequesser R, et al. Novel risk factors for premature peripheral arterial occlusive disease in non-diabetic patients: a case-control study. PLoS ONE. 2013;8(3): e37882.
Gurdasani D, Sjouke B, Tsimikas S, et al. Lipoprotein (a) and risk of coronary, cerebrovascular, and peripheral artery disease: the EPIC-Norfolk prospective population study. Arterioscler Thromb Vasc Biol. 2012;32(12):3058–65.
Volpato S, Vigna GB, Mcdermott MM, et al. Lipoprotein (a), inflammation, and peripheral arterial disease in a community-based sample of older men and women (the InCHIANTI study). Am J Cardiol. 2010;105(12):1825–30.
Pradhan AD, Shrivastava S, Cook NR, et al. Symptomatic peripheral arterial disease in women: nontraditional biomarkers of elevated risk. Circulation. 2008;117(6):823–31.
Ix JH, Allison MA, Denenberg JO, et al. Novel cardiovascular risk factors do not completely explain the higher prevalence of peripheral arterial disease among African Americans: the San Diego Population Study. J Am Coll Cardiol. 2008;51(24):2347–54.
Dieplinger B, Lingenhel A, Baumgartner N, et al. Increased serum lipoprotein (a) concentrations and low molecular weight phenotypes of apolipoprotein (a) are associated with symptomatic peripheral arterial disease. Clin Chem. 2007;53(7):1298–305.
Vigna G, Volpato S, Zuliani G, et al. Th-P15: 171 Lipoprotein (A) and peripheral arterial disease by ankle/brachial index: data from the Inchianti study. Atherosclerosis. 2006;3(7):530.
Sofi F, Lari B, Rogolino A, et al. Thrombophilic risk factors for symptomatic peripheral arterial disease. J Vasc Surg. 2005;41(2):255–60.
Diehm C, Lange S, Trampisch HJ, et al. Relationship between lipid parameters and the presence of peripheral arterial disease in elderly patients. Curr Med Res Opin. 2004;20(12):1873–5.
Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein (a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA. 2001;285(19):2481–5.
Sutton-Tyrrell K, Evans RW, Meilahn E, et al. Lipoprotein (a) and peripheral atherosclerosis in older adults. Atherosclerosis. 1996;122(1):11–9.
Trinder M, Uddin MM, Finneran P, et al. Clinical utility of lipoprotein (a) and LPA genetic risk score in risk prediction of incident atherosclerotic cardiovascular disease. JAMA cardiology. 2021;6(3):287–95.
Acknowledgements
The authors thank LetPub (Accdon LLC) for editing and proofreading the manuscript prior to submission. We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Funding
This work was supported by the Fundamental Research Funds for the Central Universities (Grant Number: DUT22YG107), the National Natural Science Foundation of China (grant number: 81600370), and the China Postdoctoral Science Foundation (Grant Number: 2018M640270).
Author information
Authors and Affiliations
Contributions
Hecheng Wang, Pengpeng Wu, and Yanshuo Han conceived, designed, performed the meta-analysis, and drafted the manuscript; Pengpeng Wu, Hao Zhang, Jian Zhang, and Yanshuo Han analyzed the data; Hecheng Wang, and Yanshuo Han revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Search strategy.
Additional file 2: Table S2.
Characteristics of individual studies on Hcy, FIB, LPa and risk of PADs.
Additional file 3: Figure S1
. Weighted mean difference (WMDs) and 95% confidence intervals (CIs) of the selected studies and the pooled Hcy levels (A), FIB level (B) and LPa level C in patients with PAD and control subjects.
Additional file 4: Table S3.
Table of relative risks (95% confidence intervals) from the nonlinear dose–response analysis of Hcy, FIB, LPa and risk of PAD.
Additional file 5: Table S4.
Table of relative risks (95% confidence intervals) from the linear dose–response analysis of Hcy, FIB, LPa and risk of PAD.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, H., Wu, P., Jiang, D. et al. Relationship between serum homocysteine, fibrinogen, lipoprotein-a level, and peripheral arterial disease: a dose–response meta-analysis. Eur J Med Res 27, 261 (2022). https://doi.org/10.1186/s40001-022-00870-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-022-00870-1