Abstract
Polycystic ovarian syndrome (PCOS) is the most common multifactor heterogeneous endocrine and metabolic disease in women of childbearing age. PCOS is a group of clinical syndromes characterized by reproductive disorders, metabolic disorders, and mental health problems that seriously impact the physical and mental health of patients. At present, new studies suggest that human evolution leads to the body changes and the surrounding environment mismatch adaptation, but the understanding of the disease is still insufficient, the pathogenesis is still unclear. Sirtuin 1 (SIRT1), a member of the Sirtuin family, is expressed in various cells and plays a crucial role in cell energy conversion and physiological metabolism. Pathophysiological processes such as cell proliferation and apoptosis, autophagy, metabolism, inflammation, antioxidant stress and insulin resistance play a crucial role. Moreover, SIRT1 participates in the pathophysiological processes of oxidative stress, autophagy, ovulation disturbance and insulin resistance, which may be a vital link in the occurrence of PCOS. Hence, the study of the role of SIRT1 in the pathogenesis of PCOS and related complications will contribute to a more thorough understanding of the pathogenesis of PCOS and supply a basis for the treatment of patients.
Similar content being viewed by others
Introduction
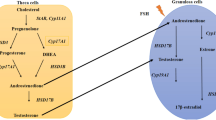
Polycystic ovary syndrome (PCOS) is an endocrine and metabolic disease characterized by hyperandrogenemia, ovulation disorder and ovarian polycystic transformation. Approximately 10–20% of women worldwide suffer from this disease, affecting their quality of life [1,2,3]. Patients with PCOS showed chronic inflammation and oxidative stress. Its metabolic damage is complex, including insulin resistance (IR) and compensatory hyperinsulinemia, which have major effects on muscle and adipose tissue, and are closely connected with other metabolic diseases such as inherent β-cell dysfunction, type 2 diabetes mellitus (T2DM), gestational diabetes mellitus, increased risk factors for cardiovascular disease (hypertension, hyperlipidemia, etc.), obesity and metabolic syndrome (METS) [4,5,6,7]. Nevertheless, existing studies ignore the growing acceptance of evolutionary perspectives, the role of lifestyle and diet, the role of androgens in the origin of PCOS development, the influence of the microbiome, and the reversibility (Fig. 1) of metabolic, biochemical, and endocrine factors of PCOS following lifestyle and other interventions. To some extent, the diagnosis and treatment of the disease are limited, and the prevention and treatment effect is not good.
Sirtuins, a family of nicotinamide adenine dinucleotide + (NAD +)-dependent deacetylases, are the key metabolic receptors of homeostasis in the human body [8]. Silencing information regulator 2-related denzyme 1 (Sirtuin 1, SIRT1) can regulate cell metabolism, senescence, and antioxidant stress by deacetylating transcription factors, coregulatory factors, and histones, to inhibit cell apoptosis, oxidative stress, and inflammation [9,10,11]. The decrease in SIRT1 activity or the inhibition of its related pathway is a common pathological process in nonalcoholic fatty liver, cardiovascular disease, and other metabolic and inflammatory diseases. Previous studies have shown that activating SIRT1 can benefit the treatment of many diseases as a new target [8].
Are SIRT1 and related pathway molecules involved in the pathogenesis of PCOS? What is the specific role of SIRT1 in endocrine, reproductive and metabolic disorders in patients with PCOS? Can the symptoms of PCOS patients be improved by regulating SIRT1 and its pathway molecules? In recent years, extensive research has been devoted to exploring the function of SIRT1 in PCOS, providing new inspiration for the treatment of disease in the future, and offering a scientific basis for clinical application.
Structure and function of SIRT1
Sirtuins 1–7 are widespread and conserved class of NAD + -dependent histone deacetylases in mammals [12]. According to different subcellular localizations, they can be divided into four categories: SIRT1, SIRT2 and SIRT3 belong to class I, SIRT4 belongs to class II, SIRT5 belongs to class III, and SIRT6 and SIRT7 belong to class IV, which can act on different substrates [13]. They can be involved in cell proliferation, metabolism, transcription, apoptosis and cell signal transduction [14, 15].
Human SIRT1 expressed in the nucleus and encoded by the SIRT1 gene is located on chromosome 10q21.3, functioning in the deacetylation of the histone and nonhistone lysine groups of known transcription factors (FOXO, MyoD, p53, PGC-1a) [16]. SIRT1 connects transcriptional regulation with intracellular energetics, coordinates different cellular functions, and goes far beyond simple histone deacetylation [17]. The dysfunction will bring about tissue-specific degenerative changes, which are the pathological basis of many diseases, including cancer, cardiovascular disease, type 2 diabetes, and many other diseases [18, 19]. SIRT1-mediated deacetylation activates liver kinase B1 (LKB1) signals in the cytoplasm and can further add fatty acid oxidation in the liver [20]. SIRT1 is also involved in the balance of cholesterol metabolism in the liver. The process balance disorder may lead to intrahepatic fat accumulation [19,20,21]. SIRT1 can mediate the expression of tumor-related genes, such as apoptosis protein inhibitor (IAP), through nuclear factor kappa B (NF-kB) to participate in tumorigenesis [22]. SIRT1 can modulate mitochondrial function, glucose metabolism, and lipids by activating peroxisome proliferator-activated receptor-gamma coactivator (PGC-1a) gene transcription and regulating peroxisome proliferator-activated receptor (PPAR), nuclear respiratory factor (NRF) and mitochondrial transcription Factor A (TFAM), which are closely related to the occurrence of metabolic syndromes, such as insulin resistance [23].
The relationship of SIRT1 and PCOS
The occurrence of PCOS involves in multiple pathways and lacks common clues, causing different symptoms in patients. Patients with PCOS may be in a state of low-grade inflammation and oxidative stress, often accompanied by clinical manifestations of endocrine, reproductive and metabolic disorders, such as menstrual disorder, hyperandrogenemia, infertility, obesity, insulin resistance, ovarian changes, hirsutism, acne and more [24, 25]. However, the existing evidence shows that there is a significant relationship between PCOS and Sirtuin 1 genetic polymorphism [26]. For the past few years, emerging studies have focused on the role of SIRT1 in the pathophysiological process of PCOS.
SIRT1 and oxidative stress
Oxidative stress is the imbalance between oxidants and antioxidants and the production of excessive reactive oxygen species (ROS) [27]. An increasing number of studies have shown that active oxygen will be overproduced, the level of biomarkers of circulating oxidative stress will increase, and the antioxidant capacity will gradually decrease in patients with PCOS [28,29,30]. In addition, PCOS patients also have mitochondrial dysfunction, Redox potential imbalance and increased oxidative stress levels are observed in cumulus cells [31]. Currently, SIRT1 has been found to be able to protect against PCOS by reducing the expression of oxidative stress markers and methylglyoxal (MG), which is closely related to glycosylation stress, and improving mitochondrial disorders [32].
P53, forkhead box O (FOXO) and nuclear factor NF-kappa B (NF-κB) are the core targets of SIRT1-mediated redox state alteration [33]. P53, a transcription factor, can activate antioxidant defense-related genes, such as superoxide dismutase 2 (SOD2) and glutathione peroxidase (GPX1). FOX03a induces an antioxidant response by upregulating catalase [34, 35]. SIRT1 can also stabilize antioxidation by upregulating nuclear factor erythroid 2 (NRF2) by deacetylating nuclear Factor E2-related Factor 2 and promoting the expression of SOD, catalase (CAT) and glutathione (GSH) [36]. Advanced glycation end-products (AGEs) have been shown to bind to the multiligand receptor for advanced glycation end products (RAGE) to activate important intracellular signaling pathways and induce the production of oxidative stress-related factors and proinflammatory cytokines [37]. Increasing ROS levels and the inflammatory response aggravate endocrine and metabolic disorders in PCOS [38]. In a PCOS mouse model, MG accumulation can lead to the imbalance of SIRT1, decreasing the expression of protective factors related to mitochondria (PGC1 α, MtTFA, TOMM20) [39]. In contrast, the balance of SIRT1 was confirmed to have a protective effect on mitochondria and further protect cells from oxidative stress.
SIRT1 and autophagy
Autophagy is a type of cell death recently identified in PCOS ovarian cells, characterized by the phagocytosis of cytoplasmic material into two-membranous vesicles (autophagosomes) and subsequent degradation in lysosomes [40]. Excessive autophagy is the self-destruction of cells when they are subjected to oxidative damage, which can be manifested by mitochondrial dysfunction or structural changes [41, 42]. The degree of autophagy in PCOS patients, rat ovarian tissue and PCOS cell model was significantly increased. For example, Chuyue Zhang et al. found that high migration framework 1(HMGB1) can induce increased autophagy in granulosa cells of PCOS patients, thus aggravating insulin resistance [43]. Other indicators of significant change are mitochondrial membrane potential, mtDNA content, and decreased protein level of the autophagy substrate p62; however, the number of autophagosomes and the levels of the autophagy markers Beclin1 and LC3B-II increased [44, 45].
SIRT1 can regulate the deacetylation of LC3, an important autophagy mediator, suggesting that SIRT1 plays an important role in the regulation of autophagy [46]. Previous studies have also demonstrated that SIRT1–FOXO1 plays a critical role in the regulation of autophagy [47]. Giovanna Di Emidio et al. found that SIRT1 expression and adenosine monophosphate-activated protein kinase (AMPK) activation were significantly enhanced in the ovary in the established dehydroepiandrosterone (DHEA)-induced PCOS mouse model, suggesting that SIRT1 may regulate PCOS ovarian autophagy through activation of AMPK [48]. In addition, activation of SIRT1 inhibits PTEN-induced putative kinase 1 in granulosa cells (GCs) of PCOS patients, thereby protecting mitochondria from damage, reducing the level of ovarian autophagy, and improving oxidative stress [48]. In conclusion, when activated by external factors, SIRT1 can prevent autophagy and mitochondrial damage by inhibiting autophagy-related molecules, thus promoting the body's antioxidant effect and protecting mitochondria and cells from the adverse effects of oxidative stress.
SIRT1 and ovulation disorders
Ovulation disorders account for approximately 30% of infertility, and are usually characterized by irregular menstruation (less menstruation) or no menstruation (amenorrhea) [49]. In the reproductive system, Xian Qin et al. found that ovarian reserve was positively correlated with an increase in SIRT1 expression in mice, suggesting that SIRT1 can delay ovarian aging [50]. Other experiments have proven that SIRT1 can inhibit FOXO1 acetylation to promote the decomposition of the Fox01–ATG7 complex, reduce the autophagic death of GCs under oxidative stimulation, and delay the senescence of oocytes [51, 52]. Likewise, in a rat model, SIRT1 activation can not only suppress the expression of androgen receptor and decrease the level of androgen but also keep down p66Shc expression, thus maintaining TGF-β, α-SMA and CTGF expression and reforming the structural fibrosis of the ovary [53]. On all accounts, SIRT1 has great potential in ameliorating ovulation disorders in PCOS, and more in-depth research on its mechanism is needed.
SIRT1 and insulin resistance (IR)
Worldwide, 1 in 6 to 20 women of reproductive age (5 to 20%) who exhibit hyperandrogenemia in PCOS are affected by insulin resistance (IR) or hyperinsulinemia [54]. IR is a pathological metabolic state in which the ability of the body to use glucose decreases to compensate and maintain normal blood sugar levels and increase insulin secretion, resulting in hyperinsulinemia. SIRT1 positively regulates insulin secretion in pancreatic β-cells [55]. Moreover, increased expression of SIRT1 improved insulin sensitivity, especially under insulin-resistant conditions [56].
Studies have shown that the levels of AMPK (the key regulator of the mitochondrial response to energy deprivation) and SIRT1 in the ovaries of PCOS rats are significantly lower than the levels of AMPK of the control group, and they are in an obvious IR state, which is the same as in PCOS mice [57,58,59]. However, when the expression of AMPK and SIRT1 is significantly increased, it can reduce blood sugar and protect microvascular endothelial cells from glucose toxicity [57,58,59,60]. In other studies, a potent small molecule activator of SIRT1 reduced blood glucose and improved insulin sensitivity in mice with diet-induced obesity [61]. AMPK and Sirtuins are present in all eukaryotic cells and may have coexisted during evolution [62, 63]. AMPK enhances SIRT1 expression by regulating nicotinamide activity, and SIRT1 also activates AMPK [64, 65]. In conclusion, the AMPK–SIRT1 pathway may be the molecular mechanism of IR in PCOS and may serve as a therapeutic target for developing potential therapies to improve the metabolism and reproductive function of PCOS.
Overview of the role of SIRT1 in the treatment of PCOS
Multiple lines of evidence now suggest that in the modern world, there are maladaptive reactions in humans to rapidly changing nutritional, physiological, psychological and cultural environments, which lead to pathological responses to IR, hyperandrogens, enhanced energy storage and ovulation [66, 67]. SIRT1 is conserved throughout evolutionary history, as a cellular metabolic energy sensor, right back to the beginning of eukaryotic organisms. Sirtuins constitute a family of metabolic sensor proteins that translate changes in NAD+levels into adaptive responses and play an important regulatory role in lipid glucose metabolism and mitochondrial activity [68]. SIRT1 is an NAD-dependent histone deacetylase that is activated when there are low cellular energy levels that result in an elevated NAD + to NADH ratio, which occurs between meals and during fasting and leads to the activation of multiple catabolic pathways, inhibition of anabolic pathways (with activation of AMP kinase and inhibition of mTOR), and activation of cellular processes, such as autophagy (as discussed in Sect. 3.2). Previous research established that the treatment strategy of activating SIRT1 can be applied to the treatment and life management of patients with PCOS to further improve symptoms. To date, the application of SIRT1 in PCOS treatment is in the exploratory stage, which basically includes the following aspects: 1. Lifestyle and dietary intervention; 2. Supplement of dietary polyphenols; and 3. Pharmaceutical management.
Lifestyle and dietary intervention in the management of PCOS
The activation of SIRT1 and the subsequent cellular changes are, therefore, dependent on nutritional energy intake and activity levels, which highlights the central role of lifestyle factors, such as diet and exercise in the pathogenesis of PCOS (as elaborated on in the 2018 International Guidelines) [69]. Data from several studies suggest that regular exercise and a whole food diet can regulate SIRT1 activity and have effects on weight loss and metabolic and clinical biomarkers [70]. Appropriate sun exposure can promote the synthesis of vitamin D, improve metabolic parameters, and increase SIRT1 activity [71, 72]. A number of other ways of activating SIRT1 by changing diet and lifestyle have been investigated, including increased intake of docosahexaenoic acid (DHA), polyphenols, extra virgin olive oil, and moderate cold stimulation [73,74,75,76]. Some of these factors are potentially important components of a healthy lifestyle and need further clinical investigation.
The role of dietary polyphenols in the management of PCOS
Polyphenols undergo intensive biotransformation by the gastrointestinal microbiota, and less than 5% of ingested polyphenols are estimated to reach the circulation intact [77]. A large number of microbial polyphenol metabolites can be detected in plasma compared with extremely low levels of the parent compounds. Despite their low bioavailability, numerous studies have reported significant biological effects related to dietary polyphenols in women with PCOS. These effects include resveratrol, quercetin and curcumin. The anti-inflammatory and oxidative stress effects of polyphenols can effectively reduce the incidence of chronic diseases, such as obesity, diabetes and cardiovascular diseases in the population. Supplementing natural compounds through diet or other means can effectively reduce the adverse effects of related diseases [19]. A large number of experiments have proven that bioactive substances such as polyphenols can play a protective role by regulating SIRT1 expression and activity in vivo, which is a potential way to treat or prevent metabolism-related diseases [78, 79]. The activation of SIRT1 to improve symptoms in PCOS patients not only provides a new target for treatment but also further validates the pathogenesis of PCOS.
Resveratrol
Resveratrol can remove ROS, inhibit cyclooxygenase (COX), and activate anti-inflammatory and antioxidant stress pathways through SIRT1 [80]. In a rat control experiment, resveratrol (20 mg/kg/d) decreased body weight and ovarian weight, reduced the levels of testosterone, luteinizing hormone (LH), LH/follicle stimulating hormone (FSH), tumor necrosis factor (TNF)-α and tissue antiMüllerian hormone (AMH), and affected the maintenance of follicular formation [81]. Another set of rat models of PCOS induced by high androgens (dehydroepiandrosterone and dihydrotestosterone) found that resveratrol significantly reduced ovarian oxidative stress levels, inhibited phosphorylation of p66Shc, inhibited fibrotic factor activation, and improved ovarian morphology [53].In clinical trials of women with PCOS, resveratrol has been shown to improve ovarian volume, high-quality oocyte rate, high-quality embryo rate, androgen and gonadotropin concentrations, angiogenic factor levels, and endoplasmic reticulum stress levels in PCOS patients [82].In two randomized controlled trials of patients with nonalcoholic fatty liver disease and obesity, resveratrol combined with a low-calorie diet or exercise significantly reduced body weight and improved serum levels of total cholesterol (TC), high density lipoprotein cholesterol (HDL-C), very low density lipoprotein cholesterol (VLDL-C), urea, creatinine, and albumin compared with diet control or exercise alone. PCOS is suggested to be able to be used in weight management and treatment of metabolic disorders [83, 84].
Quercetin
Quercetin may exert anti-inflammatory, antiapoptotic, antioxidant and anticancer effects mainly through the SIRT1/AMPK axis and can enhance oocyte and embryo quality in the ovary [85, 86]. For the past few years, tests on the therapeutic effects of quercetin on PCOS and ovarian cancer have been carried out. In a letrozole-induced rat PCOS model, the expression of AMPK and RT-1 in ovarian tissue was upregulated in the quercetin (100 mg/kg) treatment group, and the PCOS-related estrus cycle, lipid profile, serum testosterone, estradiol and progesterone levels, and IR disorders were improved. The changes in adiponectin, adiponectin and resistin in adipose tissue induced by PCOS were also reversed to a certain extent [85]. After quercetin treatment, the following changes occurred in the rats with PCOS induced by dihydrotestosterone: the activity of progesterone, metabolic enzymes and antioxidant enzymes was significantly increased, and DHEA-induced morphological changes related to polycystic ovaries were alleviated [87]. Quercetin also significantly decreased the expression of testosterone (T), estradiol (E(2)), LH, Bax, IL-1β, IL-6 and TNF-α, increased the expression of FSH and Bcl-2, and inhibited the expression of AR. By affecting the binding of androgen receptor (AR) to specific sequences of (C-type natriuretic peptide) CNP and (natriuretic peptide receptor 2) NPR2 gene promotors, the expression of CNP/NPR2 genes and proteins is regulated to restore oocyte maturation and ovulation [88]. Controlled trials in overweight or obese PCOS patients have shown that quercetin can significantly reduce serum testosterone, luteinizing hormone, and serum inhibin levels and expression and improve insulin resistance [89]. Quercetin can also enhance the expression of adiponectin receptor transcripts in PCOS patients and effectively improve adiponectin-mediated insulin resistance and hormone metabolism disorder [90, 91].
Curcumin
Other substances that enhance SIRT1 activity include curcumin [19], which is one of the main polyphenol compounds in turmeric, with antioxidant, anti-inflammatory, anticancer, antiarthritis, antiasthma, antimicrobial, antiviral and antifungal properties and has potential benefits for the treatment of female reproductive diseases [92].Previous studies focused on the therapeutic potential and mechanism of curcumin on PCOS by constructing curcumin nanoparticles: The use of curcumin (Cur) coated with arginine (Arg) and N-acetylhistidine (Nache)-modified chitosan (ARG-Cs-Nache/Cur) nanoparticles (NPs) in estradiol valerate (EV)-induced PCOS rats reversed multiple symptoms of PCOS [93]. Curcumin nanocapsules can improve insulin resistance and lipid profiles in conjunction with metformin in PCOS patients [94]. Other related studies have also shown that curcumin can improve the metabolic disorders of PCOS patients, which is beneficial to their weight control and reduces serum inflammatory markers [95, 96] and may be a safe and effective supplement for improving PCOS-associated hyperandrogenemia and hyperglycemia [97].
Current studies indeed show that the antioxidant and anti-inflammatory effects of dietary polyphenols can be applied to the treatment of metabolic and inflammatory diseases, such as PCOS, and most of the substances can be ingested through food, which further demonstrates the important role of diet in disease management [19]. However, the mechanism of action of dietary polyphenols is still not thoroughly studied: whether it plays a role mainly through the activation of the SIRT1 pathway, the dose/dosage form required for its application in disease treatment and auxiliary programs, and safety still needs much clinical trial data.
Pharmaceutical management of PCOS
Metformin can significantly improve insulin resistance and contribute to weight loss in PCOS patients [98]. In some studies, the mechanism of metformin alone or in combination with bioactive substances and other drugs to improve symptoms of patients has been further clarified: In rat experiments, it was found that the SIRT1 and AMPK immune reactivity were significantly increased and showed an increasing trend after metformin alone or in combination with resveratrol and exenatide, and the ovarian morphology and related metabolic indicators were significantly improved in PCOS rats. These results suggest that activation of SIRT1 may be an important pathway for metformin, exenatide, tayin-35 and other drugs and bioactive substances to treat PCOS patients [57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100]. However, the regulatory pathways and molecular mechanisms of SIRT1 activation by these pathways have not been fully studied. In the absence of a bridge between SIRT1 and the activator, increased SIRT1 activity can only be determined by downstream signals. Exploring the direct-action target of SIRT1 by molecular biology or histology research, and the development of high bioavailability, high specificity and clear SIRT1 target activator is the direction of future research.
In addition to the more in-depth study of the above drugs, new therapeutic drugs are being developed. Examples include glucagon-like peptide-1 receptor (GLP-1) agonists and sodium–glucose cotransporter 2 (SGLT-2) inhibitors [101]. Liraglupeptide (Lira) is a glucagon-like peptide-1 receptor agonist (GLP-1) that improves insulin sensitivity, reduces the risk of cardiovascular disease (CVD), leads to weight loss, and improves nonalcoholic fatty liver disease [102,103,104]. Lira has been shown to induce the expression of adenosine monophosphate activated protein kinase-α (AMPK-α) and SIRT-1 proteins and promote brown adipocyte differentiation and anti-inflammatory effects, thereby improving insulin sensitivity, reducing inflammation, and inducing adaptive thermogenesis [105]. In addition, sodium–glucose cotransporter 2 (SGLT-2) inhibitors, such as licogliflozin increase insulin sensitivity and ameliorate hyperinsulinemia and hyperandrogenemia in women with PCOS [106,107,108]. Unfortunately, there is currently a lack of studies on the interaction between SGLT2 inhibitors and SIRT1, which is also a new direction for future research.
Conclusions
As a key hub of steady-state cellular energy metabolism in the human body, SIRT1 is not only related to the occurrence of cardiovascular and cerebrovascular diseases, such as fatty liver, but also is closely related to the occurrence and development of PCOS. In addition to the current relatively recognized pathogenesis of PCOS, oxidative stress/autophagy/hyperandrogenia/insulin resistance, this paper also considers the correlation between the generation of PCOS and the ancient evolutionary theory to further explore new views on the diet and lifestyle of the modern world and new treatment methods.
SIRT1 may protect PCOS patients, mainly through oxidative stress, inhibition of granular cell autophagy, improvement of mitochondrial dysfunction, abnormal improvement of ovulation disorders (enhanced quality of oocyte and embryo), improvement of the hormone metabolism disorder (lower testosterone levels), and a certain degree in improvement of its complications: obesity and lipid metabolic disorder. However, the current research direction of SIRT1 and PCOS exists only in the aspects of antioxidant stress, ovulation disorders, autophagy abnormalities, insulin resistance, etc., and many aspects remain to be explored. In addition, the current research level is relatively superficial, and it is only speculative based on the experimental results of existing studies, such as the clear treatment mechanism of SIRT1 and AMPK pathways in insulin resistance. The relationship between the SIRT1 expression level and the nutritional status of the human body and cells, the interaction mechanism between SIRT1 and new drug therapy, and the balance mechanism of its inhibition and promotion of autophagy need to be further studied and determined.
SIRT1-related PCOS treatment strategies are mainly to promote the activity of SIRT1 to exert the protective effects of antioxidant stress and anti-inflammatory pathways on PCOS patients. Specific plans include adjustment of diet and living habits, rational intake of bioactive substances and the use of drugs. However, the specific mechanism of enhancing SIRT1 activity in each scheme is still unclear, and there is a lack of clear molecular connection between dietary polyphenols and SIRT1 activity.
In the future, bioinformatics tools can be used to predict and verify molecular interactions and improve the drug action network. Specific dosage forms/dosages of dietary polyphenols in treatment regimens still need to include extensive data from animal and clinical trials, such as whether there is a difference in efficacy when nanotechnology is applied to drugs, such as quercetin. If natural compounds are used as treatment options, there is a lack of long-term observation and research on long-term patients' pregnancy and fetal safety. Further exploration of treatment plans will improve the understanding of SIRT1 and PCOS diseases, for example, the correlation between melatonin and biological rhythm, the correlation between circadian rhythm and PCOS, and whether these correlations affect autophagy in PCOS patients and further affect the disease phenotype. A fuller and more comprehensive understanding can help us search for SIRT1 modulators with high bioavailability and specificity and provide new efficient targets for the treatment and management of endocrine and metabolic diseases, such as PCOS.
Availability of data and materials
Not applicable.
References
Picton HM, Balen AH. Transgenerational PCOS transmission. Nat Med. 2019;25(12):1818–20.
Brutocao C, Zaiem F, Alsawas M, Morrow AS, Murad MH, Javed A. Psychiatric disorders in women with polycystic ovary syndrome: a systematic review and meta-analysis. Endocrine. 2018;62(2):318–25.
McCartney CR, Marshall JC. CLINICAL PRACTICE polycystic ovary syndrome. N Engl J Med. 2016;375(1):54–64.
Legro RS, Kunselman AR, Dunaif A. Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. Am J Med. 2001;111(8):607–13.
Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2018;33(9):1602–18.
Sex TVG. Microbes, and polycystic ovary syndrome. Trends Endocrinol Metab. 2019;30(1):54–65.
Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010;8:41.
Tatone C, Di Emidio G, Barbonetti A, Carta G, Luciano AM, Falone S, et al. Sirtuins in gamete biology and reproductive physiology: emerging roles and therapeutic potential in female and male infertility. Hum Reprod Update. 2018;24(3):267–89.
Chen C, Zhou M, Ge Y, Wang X. SIRT1 and aging related signaling pathways. Mech Ageing Dev. 2020;187: 111215.
Silvestre MF, Viollet B, Caton PW, Leclerc J, Sakakibara I, Foretz M, et al. The AMPK-SIRT signaling network regulates glucose tolerance under calorie restriction conditions. Life Sci. 2014;100(1):55–60.
Kong X, Guan J, Li J, Wei J, Wang R. P66 (Shc)-SIRT1 regulation of oxidative stress protects against cardio-cerebral vascular disease. Mol Neurobiol. 2017;54(7):5277–85.
Hong JY, Lin H. Sirtuin modulators in cellular and animal models of human diseases. Front Pharmacol. 2021;12: 735044.
Frye RA. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Commun. 2000;273(2):793–8.
Wang M, Lin H. Understanding the function of mammalian sirtuins and protein lysine acylation. Annu Rev Biochem. 2021;90:245–85.
Kosciuk T, Wang M, Hong JY, Lin H. Updates on the epigenetic roles of sirtuins. Curr Opin Chem Biol. 2019;51:18–29.
Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305(5686):1010–3.
Kiyak Caglayan E, Engin-Ustun Y, Gocmen AY, Polat MF, Aktulay A. Serum sirtuin 1 levels in patients with polycystic ovary syndrome. J Obstet Gynaecol. 2015;35(6):608–11.
Saboori S, Koohdani F, Nematipour E, Yousefi Rad E, Saboor-Yaraghi AA, Javanbakht MH, et al. Beneficial effects of omega-3 and vitamin E coadministration on gene expression of SIRT1 and PGC1alpha and serum antioxidant enzymes in patients with coronary artery disease. Nutr Metab Cardiovasc Dis. 2016;26(6):489–94.
Iside C, Scafuro M, Nebbioso A, Altucci L. SIRT1 activation by natural phytochemicals: an overview. Front Pharmacol. 2020;11:1225.
Lee BB, Kim Y, Kim D, Cho EY, Han J, Kim HK, et al. Metformin and tenovin-6 synergistically induces apoptosis through LKB1-independent SIRT1 down-regulation in non-small cell lung cancer cells. J Cell Mol Med. 2019;23(4):2872–89.
Wang HY, Fang HJ, Wang Q, Liang CM, Hu H. [Impact of electroacupuncture on liver lipid metabolism and hepatic Sirt1 and PPARgamma expression in abdominal obese rats]. Zhen Ci Yan Jiu. 2019;44(7):492–6.
Yao H, Hwang JW, Sundar IK, Friedman AE, McBurney MW, Guarente L, et al. SIRT1 redresses the imbalance of tissue inhibitor of matrix metalloproteinase-1 and matrix metalloproteinase-9 in the development of mouse emphysema and human COPD. Am J Physiol Lung Cell Mol Physiol. 2013;305(9):L615-624.
Suh JH, Sieglaff DH, Zhang A, Xia X, Cvoro A, Winnier GE, et al. SIRT1 is a direct coactivator of thyroid hormone receptor beta1 with gene-specific actions. PLoS ONE. 2013;8(7): e70097.
Neven ACH, Laven J, Teede HJ, Boyle JA. A summary on polycystic ovary syndrome: diagnostic criteria, prevalence, clinical manifestations, and management according to the latest international guidelines. Semin Reprod Med. 2018;36(1):5–12.
Tosatti JAG, Alves MT, Candido AL, Reis FM, Araujo VE, Gomes KB. Influence of n-3 fatty acid supplementation on inflammatory and oxidative stress markers in patients with polycystic ovary syndrome: a systematic review and meta-analysis. Br J Nutr. 2021;125(6):657–68.
Jamshidi M, Mohammadi Pour S, Bahadoram M, Mahmoudian-Sani MR, Saeedi Boroujeni A. Genetic polymorphisms associated with polycystic ovary syndrome among Iranian women. Int J Gynaecol Obstet. 2021;153(1):33–44.
Mohammadi M. Oxidative stress and polycystic ovary syndrome: a brief review. Int J Prev Med. 2019. https://doi.org/10.4103/ijpvm.IJPVM_576_17.
Hyderali BN, Mala K. Oxidative stress and cardiovascular complications in polycystic ovarian syndrome. Eur J Obstet Gynecol Reprod Biol. 2015;191:15–22.
Papalou O, Victor VM, Diamanti-Kandarakis E. Oxidative stress in polycystic ovary syndrome. Curr Pharm Des. 2016;22(18):2709–22.
Murri M, Luque-Ramirez M, Insenser M, Ojeda-Ojeda M, Escobar-Morreale HF. Circulating markers of oxidative stress and polycystic ovary syndrome (PCOS): a systematic review and meta-analysis. Hum Reprod Update. 2013;19(3):268–88.
Zhao H, Zhao Y, Li T, Li M, Li J, Li R, et al. Metabolism alteration in follicular niche: the nexus among intermediary metabolism, mitochondrial function, and classic polycystic ovary syndrome. Free Radic Biol Med. 2015;86:295–307.
Di Emidio G, Santini SJ, D’Alessandro AM, Vetuschi A, Sferra R, Artini PG, et al. SIRT1 participates in the response to methylglyoxal-dependent glycative stress in mouse oocytes and ovary. Biochim Biophys Acta Mol Basis Dis. 2019;1865(6):1389–401.
Chang HC, Guarente L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol Metab. 2014;25(3):138–45.
Sablina AA, Budanov AV, Ilyinskaya GV, Agapova LS, Kravchenko JE, Chumakov PM. The antioxidant function of the p53 tumor suppressor. Nat Med. 2005;11(12):1306–13.
Hasegawa K, Wakino S, Yoshioka K, Tatematsu S, Hara Y, Minakuchi H, et al. Sirt1 protects against oxidative stress-induced renal tubular cell apoptosis by the bidirectional regulation of catalase expression. Biochem Biophys Res Commun. 2008;372(1):51–6.
Ding YW, Zhao GJ, Li XL, Hong GL, Li MF, Qiu QM, et al. SIRT1 exerts protective effects against paraquat-induced injury in mouse type II alveolar epithelial cells by deacetylating NRF2 in vitro. Int J Mol Med. 2016;37(4):1049–58.
Ott C, Jacobs K, Haucke E, Navarrete Santos A, Grune T, Simm A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014;2:411–29.
Merhi Z, Kandaraki EA, Diamanti-Kandarakis E. Implications and future perspectives of AGEs in PCOS pathophysiology. Trends Endocrinol Metab. 2019;30(3):150–62.
Emidio GD, Placidi M, Rea F, Rossi G, Falone S, Cristiano L, et al. Methylglyoxal-dependent glycative stress and deregulation of SIRT1 functional network in the ovary of PCOS mice. Cells. 2020. https://doi.org/10.3390/cells9010209.
Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6(4):463–77.
Dadakhujaev S, Jung EJ, Noh HS, Hah YS, Kim CJ, Kim DR. Interplay between autophagy and apoptosis in TrkA-induced cell death. Autophagy. 2009;5(1):103–5.
Salehi R, Mazier HL, Nivet AL, Reunov AA, Lima P, Wang Q, et al. Ovarian mitochondrial dynamics and cell fate regulation in an androgen-induced rat model of polycystic ovarian syndrome. Sci Rep. 2020;10(1):1021.
Zhang C, Hu J, Wang W, Sun Y, Sun K. HMGB1-induced aberrant autophagy contributes to insulin resistance in granulosa cells in PCOS. FASEB J. 2020;34(7):9563–74.
Yi S, Zheng B, Zhu Y, Cai Y, Sun H, Zhou J. Melatonin ameliorates excessive PINK1/Parkin-mediated mitophagy by enhancing SIRT1 expression in granulosa cells of PCOS. Am J Physiol Endocrinol Metab. 2020;319(1):E91–101.
Li D, You Y, Bi FF, Zhang TN, Jiao J, Wang TR, et al. Autophagy is activated in the ovarian tissue of polycystic ovary syndrome. Reproduction. 2018;155(1):85–92.
Kitada M, Ogura Y, Koya D. The protective role of Sirt1 in vascular tissue: its relationship to vascular aging and atherosclerosis. Aging (Albany NY). 2016;8(10):2290–307.
Mei ZG, Huang YG, Feng ZT, Luo YN, Yang SB, Du LP, et al. Electroacupuncture ameliorates cerebral ischemia/reperfusion injury by suppressing autophagy via the SIRT1-FOXO1 signaling pathway. Aging (Albany NY). 2020;12(13):13187–205.
Di Emidio G, Falone S, Vitti M, D’Alessandro AM, Vento M, Di Pietro C, et al. SIRT1 signalling protects mouse oocytes against oxidative stress and is deregulated during aging. Hum Reprod. 2014;29(9):2006–17.
Hamilton-Fairley D, Taylor A. Anovulation. BMJ. 2003;327(7414):546–9.
Qin X, Du D, Chen Q, Wu M, Wu T, Wen J, et al. Metformin prevents murine ovarian aging. Aging (Albany NY). 2019;11(11):3785–94.
Yang Q, Dai S, Luo X, Zhu J, Li F, Liu J, et al. Melatonin attenuates postovulatory oocyte dysfunction by regulating SIRT1 expression. Reproduction. 2018;156(1):81–92.
Shen M, Cao Y, Jiang Y, Wei Y, Liu H. Melatonin protects mouse granulosa cells against oxidative damage by inhibiting FOXO1-mediated autophagy: Implication of an antioxidation-independent mechanism. Redox Biol. 2018;18:138–57.
Wang D, Wang T, Wang R, Zhang X, Wang L, Xiang Z, et al. Suppression of p66Shc prevents hyperandrogenism-induced ovarian oxidative stress and fibrosis. J Transl Med. 2020;18(1):84.
Azziz R, Carmina E, Chen Z, Dunaif A, Laven JS, Legro RS, et al. Polycystic ovary syndrome. Nat Rev Dis Primers. 2016;2:16057.
Kitada M, Koya D. SIRT1 in type 2 diabetes: mechanisms and therapeutic potential. Diabetes Metab J. 2013;37(5):315–25.
Kong XX, Wang R, Liu XJ, Zhu LL, Shao D, Chang YS, et al. Function of SIRT1 in physiology. Biochemistry (Mosc). 2009;74(7):703–8.
Tao X, Cai L, Chen L, Ge S, Deng X. Effects of metformin and exenatide on insulin resistance and AMPKalpha-SIRT1 molecular pathway in PCOS rats. J Ovarian Res. 2019;12(1):86.
Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, et al. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A. 2002;99(25):15983–7.
Tao X, Chen L, Cai L, Ge S, Deng X. Regulatory effects of the AMPKalpha-SIRT1 molecular pathway on insulin resistance in PCOS mice: An in vitro and in vivo study. Biochem Biophys Res Commun. 2017;494(3–4):615–20.
Arunachalam G, Samuel SM, Marei I, Ding H, Triggle CR. Metformin modulates hyperglycaemia-induced endothelial senescence and apoptosis through SIRT1. Br J Pharmacol. 2014;171(2):523–35.
Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450(7170):712–6.
Canto C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009;20(2):98–105.
Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8(10):774–85.
Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458(7241):1056–60.
Rogacka D, Audzeyenka I, Rychlowski M, Rachubik P, Szrejder M, Angielski S, et al. Metformin overcomes high glucose-induced insulin resistance of podocytes by pleiotropic effects on SIRT1 and AMPK. Biochim Biophys Acta Mol Basis Dis. 2018;1864(1):115–25.
Parker J, O’Brien C, Hawrelak J, Gersh FL. Polycystic ovary syndrome: an evolutionary adaptation to lifestyle and the environment. Int J Environ Res Public Health. 2022. https://doi.org/10.3390/ijerph19031336.
Dumesic DA, Padmanabhan V, Chazenbalk GD, Abbott DH. Polycystic ovary syndrome as a plausible evolutionary outcome of metabolic adaptation. Reprod Biol Endocrinol. 2022;20(1):12.
Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012;13(4):225–38.
Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Clin Endocrinol (Oxf). 2018;89(3):251–68.
Hooshmand-Moghadam B, Eskandari M, Golestani F, Rezae S, Mahmoudi N, Gaeini AA. The effect of 12-week resistance exercise training on serum levels of cellular aging process parameters in elderly men. Exp Gerontol. 2020;141: 111090.
An BS, Tavera-Mendoza LE, Dimitrov V, Wang X, Calderon MR, Wang HJ, et al. Stimulation of Sirt1-regulated FoxO protein function by the ligand-bound vitamin D receptor. Mol Cell Biol. 2010;30(20):4890–900.
Koka S, Aluri HS, Xi L, Lesnefsky EJ, Kukreja RC. Chronic inhibition of phosphodiesterase 5 with tadalafil attenuates mitochondrial dysfunction in type 2 diabetic hearts: potential role of NO/SIRT1/PGC-1alpha signaling. Am J Physiol Heart Circ Physiol. 2014;306(11):H1558-1568.
Mishra JS, Zhao H, Hattis S, Kumar S. Elevated glucose and insulin levels decrease DHA transfer across human trophoblasts via SIRT1-dependent mechanism. Nutrients. 2020. https://doi.org/10.3390/nu12051271.
Menendez JA, Joven J, Aragones G, Barrajon-Catalan E, Beltran-Debon R, Borras-Linares I, et al. Xenohormetic and anti-aging activity of secoiridoid polyphenols present in extra virgin olive oil: a new family of gerosuppressant agents. Cell Cycle. 2013;12(4):555–78.
Bruckbauer A, Zemel MB, Thorpe T, Akula MR, Stuckey AC, Osborne D, et al. Synergistic effects of leucine and resveratrol on insulin sensitivity and fat metabolism in adipocytes and mice. Nutr Metab (Lond). 2012;9(1):77.
Gerhart-Hines Z, Dominy JE Jr, Blattler SM, Jedrychowski MP, Banks AS, Lim JH, et al. The cAMP/PKA pathway rapidly activates SIRT1 to promote fatty acid oxidation independently of changes in NAD(+). Mol Cell. 2011;44(6):851–63.
Tremellen K, Pearce K. Dysbiosis of Gut Microbiota (DOGMA)—a novel theory for the development of polycystic ovarian syndrome. Med Hypotheses. 2012;79(1):104–12.
Mansur AP, Roggerio A, Goes MFS, Avakian SD, Leal DP, Maranhao RC, et al. Serum concentrations and gene expression of sirtuin 1 in healthy and slightly overweight subjects after caloric restriction or resveratrol supplementation: a randomized trial. Int J Cardiol. 2017;227:788–94.
Naseri L, Khazaei MR, Khazaei M. Potential therapeutic effect of bee pollen and metformin combination on testosterone and estradiol levels, apoptotic markers and total antioxidant capacity in a rat model of polycystic ovary syndrome. Int J Fertil Steril. 2021;15(2):101–7.
Roggerio A, Strunz CMC, Pacanaro AP, Leal DP, Takada JY, Avakian SD, et al. Gene expression of Sirtuin-1 and endogenous secretory receptor for advanced glycation end products in healthy and slightly overweight subjects after caloric restriction and resveratrol administration. Nutrients. 2018. https://doi.org/10.3390/nu10070937.
Furat Rencber S, Kurnaz Ozbek S, Eraldemir C, Sezer Z, Kum T, Ceylan S, et al. Effect of resveratrol and metformin on ovarian reserve and ultrastructure in PCOS: an experimental study. J Ovarian Res. 2018;11(1):55.
Shojaei-Zarghani S, Rafraf M. Resveratrol and markers of polycystic ovary syndrome: a systematic review of animal and clinical studies. Reprod Sci. 2021. https://doi.org/10.1007/s43032-021-00653-9.
Asghari S, Asghari-Jafarabadi M, Somi MH, Ghavami SM, Rafraf M. Comparison of calorie-restricted diet and resveratrol supplementation on anthropometric indices, metabolic parameters, and serum Sirtuin-1 levels in patients with nonalcoholic fatty liver disease: a randomized controlled clinical trial. J Am Coll Nutr. 2018;37(3):223–33.
Batista-Jorge GC, Barcala-Jorge AS, Silveira MF, Lelis DF, Andrade JMO, de Paula AMB, et al. Oral resveratrol supplementation improves metabolic syndrome features in obese patients submitted to a lifestyle-changing program. Life Sci. 2020;256: 117962.
Mihanfar A, Nouri M, Roshangar L, Khadem-Ansari MH. Therapeutic potential of quercetin in an animal model of PCOS: possible involvement of AMPK/SIRT-1 axis. Eur J Pharmacol. 2021;900: 174062.
Rashidi Z, Khosravizadeh Z, Talebi A, Khodamoradi K, Ebrahimi R, Amidi F. Overview of biological effects of quercetin on ovary. Phytother Res. 2021;35(1):33–49.
Olaniyan OT, Bamidele O, Adetunji CO, Priscilla B, Femi A, Ayobami D, et al. Quercetin modulates granulosa cell mRNA androgen receptor gene expression in dehydroepiandrosterone-induced polycystic ovary in Wistar rats via metabolic and hormonal pathways. J Basic Clin Physiol Pharmacol. 2020. https://doi.org/10.1515/jbcpp-2019-0076.
Zheng S, Chen Y, Ma M, Li M. Mechanism of quercetin on the improvement of ovulation disorder and regulation of ovarian CNP/NPR2 in PCOS model rats. J Formos Med Assoc. 2021. https://doi.org/10.1016/j.jfma.2021.08.015.
Khorshidi M, Moini A, Alipoor E, Rezvan N, Gorgani-Firuzjaee S, Yaseri M, et al. The effects of quercetin supplementation on metabolic and hormonal parameters as well as plasma concentration and gene expression of resistin in overweight or obese women with polycystic ovary syndrome. Phytother Res: PTR. 2018;32(11):2282–9.
Rezvan N, Moini A, Gorgani-Firuzjaee S, Hosseinzadeh-Attar M. Oral quercetin supplementation enhances adiponectin receptor transcript expression in polycystic ovary syndrome patients: a randomized placebo-controlled double-blind clinical trial. Cell J. 2018;19(4):627–33.
Rezvan N, Moini A, Janani L, Mohammad K, Saedisomeolia A, Nourbakhsh M, et al. Effects of quercetin on adiponectin-mediated insulin sensitivity in polycystic ovary syndrome: a randomized placebo-controlled double-blind clinical trial. Horm Metab Res. 2017;49(2):115–21.
Kamal DAM, Salamt N, Yusuf ANM, Kashim M, Mokhtar MH. Potential health benefits of curcumin on female reproductive disorders: a review. Nutrients. 2021. https://doi.org/10.3390/nu13093126.
Raja MA, Maldonado M, Chen J, Zhong Y, Gu J. Development and evaluation of curcumin encapsulated self-assembled nanoparticles as potential remedial treatment for PCOS in a female rat model. Int J Nanomedicine. 2021;16:6231–47.
Sohrevardi SM, Heydari B, Azarpazhooh MR, Teymourzadeh M, Simental-Mendía LE, Atkin SL, et al. Therapeutic effect of curcumin in women with polycystic ovary syndrome receiving metformin: a randomized controlled trial. Adv Exp Med Biol. 2021;1308:109–17.
Chien Y, Chang C, Wu M, Chen C, Horng Y, Wu H. Effects of curcumin on glycemic control and lipid profile in polycystic ovary syndrome: systematic review with meta-analysis and trial sequential analysis. Nutrients. 2021. https://doi.org/10.3390/nu13020684.
Heshmati J, Golab F, Morvaridzadeh M, Potter E, Akbari-Fakhrabadi M, Farsi F, et al. The effects of curcumin supplementation on oxidative stress, Sirtuin-1 and peroxisome proliferator activated receptor γ coactivator 1α gene expression in polycystic ovarian syndrome (PCOS) patients: a randomized placebo-controlled clinical trial. Diabetes Metab Syndr. 2020;14(2):77–82.
Heshmati J, Moini A, Sepidarkish M, Morvaridzadeh M, Salehi M, Palmowski A, et al. Effects of curcumin supplementation on blood glucose, insulin resistance and androgens in patients with polycystic ovary syndrome: a randomized double-blind placebo-controlled clinical trial. Phytomedicine: Int J Phytother Phytopharmacol. 2021;80: 153395.
Udesen PB, Glintborg D, Sorensen AE, Svendsen R, Nielsen NLS, Wissing MLM, et al. Metformin decreases miR-122, miR-223 and miR-29a in women with polycystic ovary syndrome. Endocr Connect. 2020;9(11):1075–84.
Tao X, Zhang X, Ge SQ, Zhang EH, Zhang B. Expression of SIRT1 in the ovaries of rats with polycystic ovary syndrome before and after therapeutic intervention with exenatide. Int J Clin Exp Pathol. 2015;8(7):8276–83.
Zhang S, Tu H, Yao J, Le J, Jiang Z, Tang Q, et al. Combined use of Diane-35 and metformin improves the ovulation in the PCOS rat model possibly via regulating glycolysis pathway. Reprod Biol Endocrinol. 2020;18(1):58.
Cena H, Chiovato L, Nappi RE. Obesity, polycystic ovary syndrome, and infertility: a new avenue for GLP-1 receptor agonists. J Clin Endocrinol Metab. 2020. https://doi.org/10.1210/clinem/dgaa285.
Siamashvili M, Davis SN. Update on the effects of GLP-1 receptor agonists for the treatment of polycystic ovary syndrome. Expert Rev Clin Pharmacol. 2021;14(9):1081–9.
Ma R, Ding X, Wang Y, Deng Y, Sun A. The therapeutic effects of glucagon-like peptide-1 receptor agonists and metformin on polycystic ovary syndrome: a protocol for systematic review and meta-analysis. Medicine (Baltimore). 2021;100(23): e26295.
Torres Fernandez ED, Huffman AM, Syed M, Romero DG, Yanes Cardozo LL. Effect of GLP-1 receptor agonists in the cardiometabolic complications in a rat model of postmenopausal PCOS. Endocrinology. 2019;160(12):2787–99.
Zhou JY, Poudel A, Welchko R, Mekala N, Chandramani-Shivalingappa P, Rosca MG, et al. Liraglutide improves insulin sensitivity in high fat diet induced diabetic mice through multiple pathways. Eur J Pharmacol. 2019;861: 172594.
Marinkovic-Radosevic J, Cigrovski Berkovic M, Kruezi E, Bilic-Curcic I, Mrzljak A. Exploring new treatment options for polycystic ovary syndrome: review of a novel antidiabetic agent SGLT2 inhibitor. World J Diabetes. 2021;12(7):932–8.
Elkind-Hirsch KE, Chappell N, Seidemann E, Storment J, Bellanger D. Exenatide, dapagliflozin, or phentermine/topiramate differentially affect metabolic profiles in polycystic ovary syndrome. J Clin Endocrinol Metab. 2021;106(10):3019–33.
Tan S, Ignatenko S, Wagner F, Dokras A, Seufert J, Zwanziger D, et al. Licogliflozin versus placebo in women with polycystic ovary syndrome: a randomized, double-blind, phase 2 trial. Diabetes Obes Metab. 2021;23(11):2595–9.
Acknowledgements
This work was supported by the Key Research and Development Program of Hubei Province (2020BCB023); National Natural Science Foundation of China (Grant Number 81860276, 82071655); China Medical Association Clinical Medical Research Special Fund Project (Grant Number 17020310700); the Fundamental Research Funds for the Central Universities (Grant Number 2042020kf1013); Educational and Teaching Reform Research Project (Grant Number 413200095); Graduate credit course projects (grant number 413000206).
Funding
This work was supported by the Key Research and Development Program of Hubei Province (2020BCB023); National Natural Science Foundation of China (Grant Number 81860276, 82071655); China Medical Association Clinical Medical Research Special Fund Project (Grant Number 17020310700); the Fundamental Research Funds for the Central Universities (Grant Number 2042020kf1013); Educational and Teaching Reform Research Project (Grant Number 413200095); Graduate credit course projects (Grant Number 413000206).
Author information
Authors and Affiliations
Contributions
MW and RG are the main writers of the review, completing the collection and analysis of relevant literature and the writing of the first draft of the paper. FD, DY, YZ, WT participated in the analysis and arrangement of the literature. YJ participated in collating of first draft. JZ participated in the initial topic discussion, and undertook the revision of the key content of article with BL. Besides, BL was also provided some help for polishing with JZ. YC was in charge of the project, and guides the writing of the thesis. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential Conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wu, M., Zhang, J., Gu, R. et al. The role of Sirtuin 1 in the pathophysiology of polycystic ovary syndrome. Eur J Med Res 27, 158 (2022). https://doi.org/10.1186/s40001-022-00746-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-022-00746-4