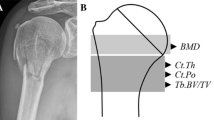
Abstract
Background
The objective of the study was to demonstrate the cortical thickness character in the humeral surgical neck region using 3D cortical bone mapping technique and try to illustrate its morphological changes with age.
Material and methods
Normal individuals, including 11 volunteers younger than 18 years, 87 adult men and 46 adult women, were enrolled. The cortical thickness and height of the surgical neck region was measured with Mimic and 3 Matic software. The height of the region was compared and measured. People with an age of 18–30 years was identified as Group I, people in 31–40 years as Group II, people in 41–50 years as Group III, people in 51–60 years as Group IV, and Group V including people ≥ 61 years.
Results
Compared with the baseline Group I, cortical thickness was significantly decreased by 0.52 mm (P = 0.006) in Group III, by 0.76 mm (P < 0.001) in Group IV, and by 0.77 mm (P < 0.001) in Group V. Age moderately predicted cortical thickness with r = −0.5481. The height of the cortical change region was significantly decreased by 2.25 mm (P = 0.007) in Group II, by 2.98 mm (P < 0.001) in Group III, and by 2.07 mm (P = 0.02) in Group IV. However, no significant decrease was illustrated in Group V (0.57 mm) (P = 0.891). The relation between age and the height of the cortical thickness change region was nonlinear.
Conclusions
This study identified an obvious decrease in cortical thickness with aging, and the height was curve fitted with aging in surgical neck region.
Similar content being viewed by others
Introduction
Proximal humeral fracture (PHF) affects patient health and is the third most common fracture in the elderly [1, 2]. As population demographics continue to change, the incidence of proximal humeral fracture is expected to increase by 50% in 2030 [3]. Osteoporotic fractures are characterized by loss of bone mass and deterioration of bone microarchitecture and have put a great burden on medical expenses [4, 5]. More and more evidences have demonstrated that focal structural weakness might result patients to fragility fractures [6, 7].
It was reported that PHF was caused by impact or muscular pull to the cortical weak region [8]. Many studies have focused on trabecular bone with obvious changes in its structure in osteoporotic disease. Nevertheless, cortical bone, not trabeculae, represents the majority of the mass of the skeletal system [5, 9]. Many recent studies have also reported that bone loss in the radius, femur, and tibia involves cortical bone [10,11,12]. Many anatomical locations and developmental or regional distributions of the bone have been assessed, and the results illustrate that the porosity and thickness of cortical bone, has a great impact on the loss of bone mass, fracture risk [13,14,15,16].
The measurements of cortical bone thickness are studied at many anatomic sites using radiographs or high-resolution peripheral quantitative computerized tomography. As technology advances, Mimics image processing software is also considered a useful method to illustrate the cortical thickness in different anatomic regions [17]. Researches of humerus have demonstrated good results when using cortical bone thickness, and it exhibited that there was an obvious correlation in radiographic cortical bone thickness and local mineral content [18].
Evidence indicates that the cortex is an essential factor in osteoporotic fracture risks, little information is available for the surgical neck region (SNR), which is always considered a weak position. There are a few researches which have analyzed trabecular bone remodeling of proximal humerus, but none of them addressed the problem of cortical thickness changes with Mimics in SNR. Furthermore, a precise definition of the SNR is lacking. The study was to demonstrate the cortical thickness of the humeral SNR using 3D cortical bone mapping and to illustrate its morphological changes with age. We also assessed the relationship between the SNR and proximal epiphyseal plate and formalized precise notation of the SNR.
Material and methods
In the study (NCT04523415), the relevant data of subjects were evaluated retrospectively from 2015.1 to 2019.12. Ethical approval was agreed and obtained from the Regional Ethics Committee of our hospital, and the study was also guaranteed to conduct in accordance with the Declaration of Helsinki. Informed written consent was obtained from patients or their guardian in this study.
Normal individuals with complete radiography and CT data of shoulder were included. Normal healthy volunteers, including 87 men and 46 women without fractures, were collected and then measured the cortical thickness and height of the SNR. All subjects with pathological fractures (tumor, neurological disease) were excluded. Normal healthy volunteers were scanned with computerized tomography machine, and the image matrix size was same (1 mm slices). The threshold values were adjusted and optimized according to the density histograms of the patients.
The subjects enrolled were divided into five different groups: Group I = 18–30 years, Group II = 31–40 years, Group III = 41–50 years, Group IV = 51–60 years, and Group V ≥ 61 years. Individuals younger than 30 years was identified as Group I, and named as the baseline group, and compared with other 4 groups. Subjects younger than 18 years were collected to observe the morphology of the epiphyseal plate (Fig. 1).
Proximal humeral cortical thickness map construction
Normal individuals, including 87 adults men (46.2 ± 13.3 years) and 46 adults women (53.6 ± 16.4 years), were enrolled and then measured the cortical thickness and height of the SNR. 11 subjects younger than 18 years was also collected to observe the morphology of epiphyseal plate. The DICOM images were downloaded through PACS and then were reconstructed to identify the fracture pattern. The data were then constructed with Mimics (21.0). In this study, Hounsfield units of 1600 (maximum) and 226 (minimum) were identified as the upper and lower threshold of the bone.
The 3D proximal humerus images were obtained, and exported directly to 3 Matic (12.0). The cortical thickness was measured with the function of the wall thickness analysis tool. The minimum threshold was identified as 0.33 mm, and the maximum thickness was 10 mm. The cortical thickness can be examined and observed integrally from the constructed cortical thickness map, and its relationship with SNR is also demonstrated.
Cortical thickness and height
The line located immediately below the lesser tuberosity was selected to calculate the cortical thickness. Three different points were chosen: adjacent to the bicipital groove (anterolateral), middle of the greater tuberosity and posterolateral point (Fig. 2). The valid height which was identified as the distance between the proximal and distal directions) of SNR was also compared (Fig. 2). Valid height represents the minimum height of the cortical change region with same color (cortical thickness) in this 3D bone map.
Parameter measurement method in the cortical change region. A The line around at the base of the lesser tuberosity was chosen as the plane to measure the cortical thickness of the surgical neck region. Three points were selected adjacent to the bicipital groove (anterolateral), middle of the cortical change region and posterolateral point. B The valid height of the cortical change region was measured. Valid height represents the minimum height of the cortical change region with same color (cortical thickness) in this 3D bone map
Statistical analysis
Relevant data are presented as the means ± standard deviation. Comparisons between the age groups were performed by one-way ANOVA with the S–N–K test, and Tukey test was utilized to determine the significant differences among different groups. For all analyses in this research, significance was identified as P < 0.05 level. All analyses were conducted using SPSS 22.0. Spearman correlations were calculated, and linear or nonlinear correlation coefficients were used and calculated to assess the correlation between ages and other parameters. The following correlation parameters were established. When |r| ≥ 0.8, it means a high correlation was noted. For 0.5 ≤ |r| < 0.8 means a moderate correlation. For 0.3 ≤ |r| < 0.5, it means a low correlation. When |r| < 0.3, there was no correlation in two variables.
According to the general requirements of statistics, taking α = 0.05, β = 0.1, the pre-experiment illustrate that the mean ± standard deviation of the height of the epiphyseal plate (the main index) in five different age groups was 7.89 ± 0.55 mm, 5.03 ± 0.53 mm, 5.01 ± 0.39 mm, 5.39 ± 0.65 mm, 7.03 ± 0.65 mm. The above parameters were substituted into PASS11 software, and concluded that the minimum number of cases to be completed in each group is 15 cases.
Results
There was no cortical change region (substituted by epiphysis) in subjects younger than 18 years. The grown subjects were divided depending on different ages into 5 groups as follows: Group I was identified as the baseline group and included 16 normal individuals between 18 and 30 years of age with a mean age of 26.1 ± 3.6 years. Group II included 27 normal individuals between 31 and 40 years old with an average age of 34.9 ± 2.8 years. Group III included 29 normal individuals between 41 and 50 years with a mean age of 45.4 ± 3.1 years. Group IV included 24 normal individuals between 51 and 60 years with a mean age of 55.8 ± 3.0 years. 37 normal individuals ≥ 61 years old with a mean age of 66.9 ± 5.1 years was included Group V (Table 1).
Cortical thickness measurement of the cortical change region
Compared with the baseline Group (Group I), the cortical thickness in Group II (31–40 years) was decreased by 0.37 mm (the CI was 2.32–2.64, − 13%) (P = 0.104), but the result was not significant. Cortical thickness was significantly decreased by 0.52 mm (the CI was 2.07–2.58, − 18.3%) (P = 0.006) in Group III (40–50 years), by 0.76 mm (the CI was 1.90–2.22, − 26.7%) (P < 0.001) in Group IV (51–60 years), and by 0.77 mm (the CI was 2.22–2.40, − 27%) (P < 0.001) in Group V (above 60 years) (Figs. 3A, 4A). Age moderately predicted cortical thickness with r = − 0.5481, and the linear correlation equation was as follows: y = − 0.01533X + 3.69 (Fig. 5A).
Cortical change region parameters in different age groups. A Box plot of cortical thickness comparison in different groups. The results show a significant difference between the age groups 18–30 and 41–100 years. B The box plot of valid height in different groups. All results illustrate a significant difference between the age groups 18–30 and 31–60 years
The height measurement of the cortical change region
Compared with the baseline Group I, the height of the cortical change region was significantly decreased by 2.25 mm (CI 4.82–6.72, − 28.1%) (P = 0.007) in Group II (31–40 years), by 2.98 mm (CI 4.27–5.82, − 37.2%) (P < 0.001) in Group III (41–50 years), and by 2.07 mm (CI 5.13–6.75, − 25.8%) (P = 0.02) in Group IV (51–60 years). However, no significant decrease was illustrated in Group V (0.57 mm) (CI 6.86–8.05, − 7.1%) (P = 0.891) (Figs. 3B, 4B). The relation between age and the height of the cortical change region was nonlinear, and the nonlinear regression equation is as follows: y = 0.003921X2− 0.3687X + 14.19 (Fig. 5B).
Discussion
Trabecular bone loss is a characteristic of osteoporosis and has promoted research and thinking into the structural basis of bone fragility in recent decades. Approximately 70% of appendicular bone loss originates from cortices, and the main bone loss occurs by intracortical remodeling that cavitates the cortex [12, 15]. The present study is the first of its kind to illustrate the cortical thickness at various ages in proximal humerus within a 3D bone cortical thickness map. It was illustrated that the SNR had a thinner cortical thickness and decreased with aging. The height of the SNR was curve fitted with different ages and was still observed across adolescent ages. The phenomenon of cortical height reduction in Group I, II, III was possibly caused by delayed maturation of epiphyseal plate which was still not studied in previous research. It was concluded that the SNR was a region that exhibited a different cortical thickness compared with the adjacent structure as well as demonstrated a potential relationship with or even originated from the closed epiphyseal plate in adolescents which need to be testified with further study.
Loss of cancellous and cortical bone are reported to be the causes of age-related proximal humeral fractures, but bone loss mainly involves cortical bone given larger accessible areas for bone resorption in cortical versus trabecular bone [12]. Therefore, cortical bone has a great impact on the loss of bone mass and fracture risk [14]. It was reported that the associations between trabecular microstructure and prevalence of fracture were no longer apparent after adjustments for aBMD in older men with fractures [14]. Previous work has figured out that it is necessary to investigate the changes in cortical bone histological structure of femoral neck region [19]. Cortical thickness in the tibia was also reported to have an association with aBMD and fracture incidence in postmenopausal people [20]. Furthermore, with high-resolution peripheral quantitative computed tomography, Helfen reported that the mean thickness of cortical bone in the SNR was decreased, and cortical porosity, is an essential factor explaining bone fragility and fracture risk in old patients, increased during aging [12, 21]. In accordance with those studies, the results in our study demonstrated that the cortical thickness in this cortical change region decreased with aging. The cortical thinning process can be accounted by the fact that the conversion of compact to cancellous bone arising from disordered Haversian remodeling and the trabecularization of the endocortical region [22,23,24]. It was also reported that 80% of all fractures in older individuals are located at sites that are mainly cortical and occur after the age of 60 years when the rate of trabecular bone loss decelerates [25, 26]. However, the reason why the fracture was primarily obvious in this cortical changing region rather than other cortical regions, such as the diaphysis, is still not completely understood.
Furthermore, the height of this region decreased from Group I to Group III, but surprisingly increased from Group IV to Group V. The relationship between age and height of the cortical change region was subject to curve-fitting and exhibited a shape similar to a parabolic relation. The valid height increase in older years can be explained by the fact that it was associated with osteoporosis, but the decreasing trend at a young age is worthy of further study. Therefore, the height and cortical thickness changes in this region are more complicated than we traditionally imagined, and some other mechanisms will affect the process of bone thinning and the remodeling process. In previous research, it was considered that age-related bone loss was found after a series of bony structural changes, and bone structure is preserved in mechanically loaded regions. In contrast, less mechanically loaded regions are attenuated with age [27, 28]. For example, the superior cortex of the femoral neck has been exhibited to become thinner much faster with age compared with the inferior cortex [29]. As a non-weight-bearing bone, cortical bone changes in the proximal humerus were more suitable to reflect the normal regulation process of osteoporosis without obvious external bearing force. Based on the increasing height of the cortical change region found in elderly subjects, it was considered that the region was the early phase where osteoporosis appeared, similar to the distal radius. The coalescence located between the epiphysis and diaphysis (age 16–18 years), which is located at the same place as the cortical change region, involved the last part of epiphyseal closure compared with the epiphysis of the greater or lesser tuberosity [30]. The resting zone maintains the growth plate grown by expressing parathyroid hormone-related protein, and it has an interaction with Indian hedgehog (Ihh) which was released from the hypertrophic zone [25, 26, 31, 32]. It was also reported that skeletal stem cells steadily formed in PTHrP+ chondrocytes which located at the growth plate resting zone [33]. Therefore, the resting zone of the growth plate might still exist after closure, and some skeletal stem cells or residual chondrocytes continue to regulate bone maturation until the age of 30 years old [33]. Along with the special position of the cortical change region, we would like to consider that the cortical changing region was formed after the metaphysical epiphyseal plate region closed in adults.
As the connecting region of the diaphysis and humeral head, the cortical thickness value of the cortical change region found in our research was thinner than the diaphysis, and thicker than the greater tuberosity. To further identify the character of this connected region, subjects younger than 18 years were enrolled, and an obvious boundary was noted between the greater tuberosity and diaphysis region in people younger than 18 years, and the cortical changed region did not or partly existed in these individuals (Fig. 6). A region with significant cortical thickness changes was only found when the proximal epiphyseal plate was closed. A precise definition of the SNR is lacking, and most authors defined it as a transition region of trabecular bone to cortical bone with a relatively thinner diameter. The SNR was always considered to have a high rate of fracture, and it was notable that the description of the SNR had many similar characteristics to this cortical change region. In our previous experimental results, the main fracture lines clinically diagnosed as surgical neck fractures were all located in the cortical change region, so it was believed that the SNR coincided with the cortical change regions, an area that was formed after the proximal epiphyseal plate closed at 18 years old. Therefore, it was reasonable to conclude that the surgical neck was a region that originated from or formed after the proximal humeral epiphyseal plate closed.
The cortical change region did not exist integrally in younger subjects. A cortical change region in an 8-year-old patient. B The cortical change region was partly formed in a 16-year-old male patient. C The SNR was completely formed in a 17-year-old female patient. Females were more inclined to mature than males
The limitation of the research was the number of subjects enrolled, and the morphology with different sexes, body anthropometric data, body weight, BMI were not compared. More volunteers will be enrolled in our following work, and the changing characteristics of the SNR will be observed more obvious in women than in men due to the relatively earlier maturation and menopause in adolescence or aging females respectively. In our subsequent research, a special molecular biological index that may exist in the resting zone of the epiphyseal plate will be searched. Further research will evaluate the changes in the biomechanical strength of the SNR and whether antiresorptive or gene treatment influence the bone remodeling processes or morphological characteristics of the identified SNR (proximal humeral epiphyseal plate region). Relative histopathology and gene editing technology will be conducted to test the hypothesis of the existence of skeletal stem cells in the closed epiphyseal plate region in adults.
In conclusion, this study identified an obvious decrease in cortical thickness with aging, and the height was curve fitted with aging in SNR. The characteristics of the SNR reflected that it might have potentially originated from the proximal epiphyseal plate closure, but need to be tested in further research.
Availability of data and materials
All relevant data are within the manuscript.
References
Pasco JA, et al. The epidemiology of incident fracture from cradle to senescence. Calcif Tissue Int. 2015;97:568–76.
Handoll HH, Brorson S. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2015;11:Cd000434.
Helfen T, Siebenbürger G, Fleischhacker E, Gleich J, Böcker W, Ockert B. Operative treatment of 2-part surgical neck type fractures of the proximal humerus in the elderly: cement augmented locking plate PHILOS™ vs. proximal humerus nail multiloc®. Injury. 2020;51:2245–52.
Chang CY, Tang CH, Chen KC, Huang KC, Huang KC. The mortality and direct medical costs of osteoporotic fractures among postmenopausal women in Taiwan. Osteoporos Int. 2016;27:665–76.
Mantila Roosa SM, Hurd AL, Xu H, Fuchs RK, Warden SJ. Age-related changes in proximal humerus bone health in healthy, white males. Osteoporos Int. 2012;23:2775–83.
Mayhew PM, et al. Relation between age, femoral neck cortical stability, and hip fracture risk. Lancet. 2005;366:129–35.
Poole KES, et al. Focal osteoporosis defects play a key role in hip fracture. Bone. 2017;94:124–34.
Neer CS. Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am. 1970;52:1077–89.
Sprecher CM, Schmidutz F, Helfen T, Richards RG, Blauth M, Milz S. Histomorphometric assessment of cancellous and cortical bone material distribution in the proximal humerus of normal and osteoporotic individuals: significantly reduced bone stock in the metaphyseal and subcapital regions of osteoporotic individuals. Medicine. 2015;94:e2043.
Bala Y, et al. Cortical porosity identifies women with osteopenia at increased risk for forearm fractures. J Bone Miner Res. 2014;29:1356–62.
Nishiyama KK, Macdonald HM, Buie HR, Hanley DA, Boyd SK. Postmenopausal women with osteopenia have higher cortical porosity and thinner cortices at the distal radius and tibia than women with normal aBMD: an in vivo HR-pQCT study. J Bone Miner Res. 2010;25:882–90.
Zebaze R, Seeman E. Cortical bone: a challenging geography. J Bone Miner Res. 2015;30:24–9.
Popp AW, et al. Prediction of bone strength at the distal tibia by HR-pQCT and DXA. Bone. 2012;50:296–300.
Sundh D, Mellström D, Nilsson M, Karlsson M, Ohlsson C, Lorentzon M. Increased cortical porosity in older men with fracture. J Bone Miner Res. 2015;30:1692–700.
Zebaze RM, et al. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet. 2010;375:1729–36.
Kamer L, Noser H, Blauth M, Lenz M, Windolf M, Popp AW. Bone mass distribution of the distal Tibia in normal, osteopenic, and osteoporotic conditions: an ex vivo assessment using HR-pQCT, DXA, and computational modelling. Calcif Tissue Int. 2016;99:588–97.
Poole KE, et al. Denosumab rapidly increases cortical bone in key locations of the femur: a 3D bone mapping study in women with osteoporosis. J Bone Miner Res. 2015;30:46–54.
Virtama P, Telkka A. Cortical thickness as an estimate of mineral content of human humerous and femur. Br J Radiol. 1962;35:632–3.
Jordan GR, Loveridge N, Bell KL, Power J, Rushton N, Reeve J. Spatial clustering of remodeling osteons in the femoral neck cortex: a cause of weakness in hip fracture? Bone. 2000;26:305–13.
Sornay-Rendu E, Boutroy S, Munoz F, Delmas PD. Alterations of cortical and trabecular architecture are associated with fractures in postmenopausal women, partially independent of decreased BMD measured by DXA: the OFELY study. J Bone Miner Res. 2007;22:425–33.
Helfen T, et al. High-resolution tomography-based quantification of cortical porosity and cortical thickness at the surgical neck of the humerus during aging. Calcif Tissue Int. 2017;101:271–9.
Tong X, Burton IS, Isaksson H, Jurvelin JS, Kröger H. Cortical bone histomorphometry in male femoral neck: the investigation of age-association and regional differences. Calcif Tissue Int. 2015;96:295–306.
Power J, Loveridge N, Lyon A, Rushton N, Parker M, Reeve J. Bone remodeling at the endocortical surface of the human femoral neck: a mechanism for regional cortical thinning in cases of hip fracture. J Bone Miner Res. 2003;18:1775–80.
Power J, Doube M, van Bezooijen RL, Loveridge N, Reeve J. Osteocyte recruitment declines as the osteon fills in: interacting effects of osteocytic sclerostin and previous hip fracture on the size of cortical canals in the femoral neck. Bone. 2012;50:1107–14.
Kanis JA, Johnell O, Oden A, Dawson A, Laet CD, Jonsson B. Ten year probabilities of osteoporotic fractures according to BMD and diagnostic thresholds. Osteoporos Int. 2001;12:989–95.
Riggs BL, Wahner HW, Dunn WL, Mazess RB, Offord KP, Melton LJ. Differential changes in bone mineral density of the appendicular and axial skeleton with aging: relationship to spinal osteoporosis. J Clin Invest. 1981;67:328–35.
Thomas CD, et al. Femoral neck trabecular bone: loss with aging and role in preventing fracture. J Bone Miner Res. 2009;24:1808–18.
Poole KE, et al. Changing structure of the femoral neck across the adult female lifespan. J Bone Miner Res. 2010;25:482–91.
Johannesdottir F, et al. Similarities and differences between sexes in regional loss of cortical and trabecular bone in the mid-femoral neck: the AGES-Reykjavik longitudinal study. J Bone Miner Res. 2013;28:2165–76.
Ekizoglu O, et al. Applicability of T1-weighted MRI in the assessment of forensic age based on the epiphyseal closure of the humeral head. Int J Legal Med. 2019;133:241–8.
Laib A, Häuselmann HJ, Rüegsegger P. In vivo high resolution 3D-QCT of the human forearm. Technol Health Care. 1998;6:329–37.
Han ZH, Palnitkar S, Rao DS, Nelson D, Parfitt AM. Effect of ethnicity and age or menopause on the structure and geometry of iliac bone. J Bone Miner Res. 1996;11:1967–75.
Mizuhashi K, et al. Resting zone of the growth plate houses a unique class of skeletal stem cells. Nature. 2018;563:254–8.
Acknowledgements
The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Funding
The research was supported by National Natural Science Foundation of China (82002281, 82072523) and The Natural Science Foundation of Hebei (H2021206054, H2020206193), the main. Medical Scientific Research of Hebei (20210543) and China Postdoctoral Fund (2021M701785), Science and Technology Project of Hebei Education Department (SLRC2019046), Government-funded Clinical Medicine Outstanding Talent Training Project (2019) and the 14th Five-Year Clinical Medicine Innovation Research Team (2022). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Ethical approval was received from the Regional Ethics Committee of the Third Hospital of Hebei Medical University, and the study was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all patients enrolled in the study.
Author information
Authors and Affiliations
Contributions
Conceptualization: YZ. Data curation: MS. Formal analysis: WD. Investigation: YZ, WC. Methodology: JG. Project administration: JG, YZ. Resources: YZ, WD. Software: JG. Supervision: ZH, YZ. Validation: ZH, YZ. Visualization: ZH. Writing—original draft: JG. Writing—review and editing: JG. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval and consent to participate
Ethical approval was received from the Regional Ethics Committee of the Third Hospital of Hebei Medical University, and the study was conducted in accordance with the Declaration of Helsinki (NCT04523415). Informed consent was obtained from all patients enrolled in the study.
Consent for publication
All authors and participants are consent for publication.
Competing interests
The authors have declared that no competing interests exist.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Guo, J., Zhou, Y., Shang, M. et al. Morphological characteristics of the surgical neck region in the proximal humerus at different ages. Eur J Med Res 27, 102 (2022). https://doi.org/10.1186/s40001-022-00724-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-022-00724-w