Abstract
This study attempts to evaluate the prognostic role of PHYH for overall survival (OS) in clear cell renal cell carcinoma (ccRCC) by means of publicly available data from The Cancer Genome Atlas (TCGA). Clinical pathologic features and PHYH expression were downloaded from the TCGA database and relationships between them were analyzed by univariate and multivariate Cox regression analyses. Gene Set Enrichment Analysis (GSEA) and gene–gene interactions were also performed between tissues with different PHYH expression levels. PHYH expression levels were significantly lower in patient with ccRCC compared with normal tissues (p = 1.156e−19). Kaplan–Meier survival analysis showed that high expression of PHYH had a better prognosis than low expression (p = 9e−05). Moreover, PHYH expression was also significantly associated with high grade (G2-4, p = 0.025), high stage (StageIII & IV, p = 5.604e−05), and high level of stage_T (T3-4, p = 4.373e−05). Univariate and multivariate Cox regression analyses indicated that PHYH could be acted as an independent prognostic factor (p < 0.05). Nomogram including clinical pathologic features and PHYH expression were also provided. GSEA revealed that butanoate metabolism, histidine metabolism, propanoate metabolism, pyruvate metabolism, tryptophan metabolism, PPAR signalling pathway, and renin–angiotensin system were differentially enriched in PHYH high-expression phenotype. ICGC database was utilized to verify the expression level and survival benefit of PHYH (both p < 0.05). We suspect that elevated PHYH expression may be served as a potential prognostic molecular marker of better survival in ccRCC. Besides, alpha-oxidation was closely regulated by PHYH, and PPAR signalling, pyruvate metabolism, butanoate metabolism, and RAS might be the key pathways regulated by PHYH in CCRC.
Similar content being viewed by others
Background
Clear cell renal cell carcinoma (ccRCC) is a major type of kidney cancer accounting for 90–95% of cases [1]. It sporadically arises from proximal tubular epithelial cells of the renal cortex, characterized by malignant epithelial cells with typical clear cytoplasm. During the past decade, data have shown a 2–3% yearly increase in ccRCC incidence. Recent advances in scientific medical research have led to an increased perception of the underlying pathophysiological molecular mechanism of ccRCC [2, 3]. The most common and vital characteristic associated with ccRCC and cancer in general is hypoxia. A condition that initiates a cascade of molecular events including angiogenesis and involves cell-cycle control proteins, which are closely associated with tumor growth [4, 5]. With regards to renal cell carcinoma (RCC), past researchers have identified that the hypoxia inducing factors 1α (HIF-1α) and its linked pathways such as ubiquitin–proteasome and rapamycin pathways are major contributors in RCC tumorigenesis [6,7,8,9]. More recent gene expression studies have identified some genes that predicts ccRCC aggressiveness and progression [10,11,12,13]. Yet, despite our efforts, no molecular biomarkers have been verified and potentially applicable in a clinical setting to move toward precision medicine of RCC treatment.
Phytanoyl-CoA 2-Hydroxylase gene (PHYH) is gene of the PHYH family and critical in the formation of peroxisomal protein which in turn assists in the alpha-oxidation of 3-methyl branched fatty acids. As immune system evasion is the hallmark of cancer, peroxisomes have an emerging role in the regulation of cellular immune response with reports showing pro-tumorigenic functions of peroxisome. However, there exists a significant gap in knowledge in the role of peroxisome and its associated gene PHYH in the potential of tumor induction and development [14].
Thus, the objective of the current study was aimed to evaluate the prognostic value of PHYH expression in human ccRCC data obtained from TCGA. Indeed, gene set enrichment analysis (GSEA) was performed to gain a better understanding into the underlying pathophysiological pathway mechanisms associated with ccRCC pathogenesis and its relationship with PHYH regulatory network. Potentially, discovering links and mechanisms connected to tumorigenesis.
Methods
RNA-sequencing patient data and bioinformatics analysis
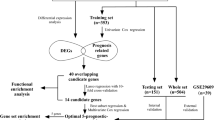
High-throughput sequencing of gene expression data (HTSeq-counts) and clinical information of 538 cases of ccRCC and 72 para-cancerous cases were downloaded from TCGA official website (https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga). Normal ccRCC samples were excluded, and boxplots and whiskers plot were used to visualize expression differences for discrete variables [15].
Gene set enrichment analysis
GSEA is bioinformatics method aimed to identify whether prior sets of genes or proteins are significantly different between two phenotypes [16]. Our study applied GSEA to generate an order list of all genes according to their correlation with PHYH expression, and significant survival differences observed between high and low PHYH groups were elucidated. Gene set permutations were performed 1000 times for each analysis. The expression level of PHYH was used as a phenotype label. The nominal p value and normalized enrichment score (NES) were used to sort the pathways enriched in each phenotype.
Gene-network analysis
To investigate associated genes in performing different molecular function and biological pathway, gene interaction analysis was performed for the PHYH gene. Gene cards database (http://www.genecards.org) was used for searching gene–gene interaction network to identify gene–gene association, and then, we selected those that have a confidence value of 0.7 (high confidence) or higher. Furthermore, these set of genes were displayed using interactive gene view software (http://software.broadinstitute.org/software/igv).
Nomogram model analysis
R (v3.4.3) was used to perform all statistical related analysis. Relationship between clinical pathological features and PHYH expression were analyzed via Wilcoxon signed-rank test and logistic regression. Nomogram construction was performed according to the guidelines proposed by Iasonos [17]. To identify independent prognostic predictors, we used a Cox proportional hazard regression model for univariable and multivariable analyses by the “Enter” method. The nomogram was developed to predict the 3 and 5 year prognosis mainly based on the results of the multivariable Cox regression model. The performance of the nomogram was estimated regarding discrimination and calibration. The C-index was applied to evaluate discrimination [18], which refers to the models’ ability to accurately distinguish the outcomes. A higher C-index indicates more precise model predictions [19]. Calibration curves were performed by comparing the means of the nomogram-predicted outcomes with the actual outcomes estimated with Kaplan–Meier. The bootstrapping (1000 repetitions) method was applied to reduce the estimate bias. In addition, model validations were performed using the data of the validation ccRCC cases as follows. First, we calculated the total points of the patients in the validation group using the established nomogram. Next, we used the total points as a factor to perform Cox regression analysis. Finally, the C-index and calibration curves were developed with the results of regression analysis. Receiver-operating characteristics (ROCs) curve was used for the sensitivity and specificity of nomogram.
Statistical analysis
All statistical analyses were conducted using R (v.3.4.3). The relationship between clinical pathologic features and PHYH were analyzed with the Wilcoxon signed-rank test and logistic regression. Clinicopathologic characteristics associated with overall survival in TCGA patients using Cox regression and the Kaplan–Meier method. Multivariate Cox analysis was used to compare the influence of PHYH expression on survival along with other clinical characteristics (age, gender, race, grade, and Stage). The cut-off value of PHYH expression was determined by its median value.
Results
Association with PHYH expression and clinicopathologic variables
As shown in Fig. 1, expression of PHYH is significantly lower in patients with tumor (p = 1.156e−19 & p = 2.634e−10). Classic univariate ROC curve analysis was performed to assess true-positive rate and false-positive rate of the PHYH expression between adjacent non-neoplastic kidney tissue and tumor based on the are under the curve (AUC). The results revealed that PHYH expression had a reasonable AUC of 0.611. In addition, decreased expression of PHYH correlated significantly with grade of cancer cells (G1-2 vs. G2-4, p = 0.025), the Union for International Cancer Control (UICC) stage (Stage I&II vs. Stage III&IV, p = 5.604e−05), and size of primary tumor (T1-2 vs. T3-4, p = 4.373e−05) (Fig. 2a–c, Table 1).
a Boxplot showed that the PHYH expression in ccRCC tissues (n = 538) was different from that in para-cancerous tissues (n = 72) in TCGA dataset; b pairwise boxplot showed that the PHYH expression in ccRCC tissues (n = 72) was also different from that in matched para-cancerous tissues (n = 72) in TCGA dataset; c Impact of PHYH expression on overall survival in ccRCC patients in TCGA cohort. d ROC curve analysis of significantly PHYH expression between normal patients and ccRCC patients with tumor
Survival outcomes and multivariate analysis
The Kaplan–Meier survival analysis (Fig. 1c) showed that ccRCC with low expression of PHYH had a worse prognosis than that with high expression of PHYH ( p = 9e−5). The univariate analysis revealed that positive distant metastasis is correlated significantly with a poor overall survivability (hazard ratio [HR]: 2.1; 95% confidence interval [CI]: 1.661–2.655; p < 0.001). Other clinicopathologic variables associated with poor survival include age, grade, UICC stage, size of primary tumor, and PHYH expression (Fig. 3s, Table 2). At multivariate analysis, factors such as age, grade, stage, and PHYH expression remained associated with overall survival (Fig. 3b, Table 2). Classical univariate ROC curve analyses revealed that grade, stage, and size of primary tumor (T) showed a high AUC of 0.7, 0.779, and 0.723, respectively (Fig. 3c).
a Univariate Cox regression analysis of PHYH expression and clinicopathologic characteristics; b multivariate Cox regression analysis of PHYH expression and clinicopathologic characteristics; c ROC curves analysis of PHYH expression and clinicopathologic characteristics. d Nomogram of PHYH expression and clinicopathologic characteristics
A point ranking system was also developed to rank the association of each factor with survivability (Fig. 3d). The higher the point for a give factor, the lower the survivability. As the results show, grade (G2, G3, and G4) and stage (I, II, and IV) are significantly associated with low survivability. In addition, higher age and lower PHYH expression are also significant related to low survivability. Interestingly, ethnic group African and white have lower survivability compared to Asian.
Gene network
We also investigate gene network to identify their gene–gene interaction. Our results showed that PHYH in connected to 10 different genes in gene–gene interaction (Fig. 4a). Associations are meant to be specific and meaningful; this does not necessarily mean that they are physically binding each other (Additional file 1: Fig.S1). Among these genes, 5 are PEX genes that encode peroxin proteins (PEX2, PEX7, PEX10, PEX13, and PEX14) which suggest the existence of protein interactions with PHYH in ccRCC. Figure 4b shows the relationships between PHYH and microsatellite instability (MSI). The associations between PHYH and immune checkpoint inhibitors are also displayed in Fig. 4c. Figure 4d presents the relationships between PHYH and the methods of immunity.
GSEA identifies a PHYH-related signalling pathway
To identify signalling pathways that are differentially activated in ccRCC, we conducted Gene Set Enrichment Analysis (GSEA) between low and high TFAP2B expression data sets. GSEA reveal significant differences (FDR b 0.05, NOM p-val b 0.05) in enrichment of MSigDB Collection (c2.cp.biocarta and h.all. v6.1. symbols). We selected the most significantly enriched signalling pathways based on their normalized enrichment score (NES) (Fig. 5, Table 3). Figure 5 shows butanoate metabolism, histidine metabolism, propanoate metabolism, pyruvate metabolism, tryptophan metabolism, PPAR signalling pathway, and renin–angiotensin system are differentially enriched in high PHYH expression phenotype.
Enrichment plots from gene set enrichment analysis (GSEA). GSEA results showing a butanoate metabolism, b histidine metabolism, c propanoate metabolism, d pyruvate metabolism, e tryptophan metabolism, f PPAR signalling pathway, and g renin–angiotensin system are differentially enriched in PHYH-related ccRCC. h Comparison of enrichment plots for all significant pathways
Verification of PHYH in ccRCC
To further verify the expression level and survival benefit of PHYH in ccRCC, the GTEx, ICGC, and HPA databases were utilized, respectively. As displayed in Fig. 6a, the expression levels of PHYH in various cancers were shown including ccRCC with p < 0.001. In terms of ICGC database, the boxplot and survival analysis were consistent with in TCGA (p = 5.214e−18, p = 1.51e−03, respectively, Fig. 6b, c). The HPA database indicated the difference of immunohistochemistry in normal and kidney cancers (Fig. 6d, e).
Discussion
The expression of PHYH has been linked to multiple diseases such as Refsum Disease and Retinitis Pigmentosa [20]. Although there are no study associating human cancers to PHYH expression, The Human Protein Atlas have reported it to be a prognostic marker in renal cancer [21]. To our knowledge, expression of PHYH and its impact on ccRCC has not yet been explored. Therefore, the potential role of PHYH in ccRCC was the main focus point of our study.
We applied bioinformatics analysis using high-throughput RNA-sequencing data from TCGA to examine PHYH expression in ccRCC patients and its association with various advanced pathologic characteristics. We demonstrated that a decrease PHYH expression is associated with presence of tumor, grade of cancer, stage of cancer, primary size of tumor, age, and presence of distant metastasis. To further investigate the functions of PHYH in ccRCC, we performed GSEA and gene–gene network using TCGA data. GSEA showed that butanoate metabolism, histidine metabolism, propanoate metabolism, pyruvate metabolism, tryptophan metabolism, PPAR signalling pathway, and renin–angiotensin system are differentially enriched in PHYH low-expression phenotype. Gene-gen network analysis revealed association of PHYH with multiple PEX genes. These evidences highlighted the potential of PHYH serving as a prognostic marker of prognosis and therapeutic target in ccRCC.
Results from our study showed a decreased expression of PHYH gene in patients diagnosed with ccRCC. The PHYH gene encodes the enzyme phytanoyl-CoA hydroxylase, which is required for the alpha-oxidation of branched chain [22, 23] and long-chain [24] fatty acids such as phytanic acid in peroxisomes [25]. Researchers suspect that phytanoyl-CoA hydroxylase potentially participates in determining the number of peroxisomes within cells and is involved in regulating their activities [26]. Peroxisomes are membrane bound organelle within the cytoplasm that is conserved across eukaryotic cells [27], and plays a vital role in peroxisomal fatty acid beta-oxidation metabolism and ROS (reactive oxygen species) conversion [28]. Diseases such as the Zellweger syndrome and other genetic diseases occurs due to implications in the fatty acid beta-oxidation [29, 30]. Study have found that many chemicals designated as peroxisome proliferators can induce peroxisome proliferation, resulting in increase in fatty acid oxidation in liver cells which leads to tumors’ growth in rodents [31,32,33]. A study has observed an absence of peroxisome in epithelial cells of proximal tubule in cancer cells of renal cell carcinoma [34]. AS phytanoyl-CoA hydroxylase is coded by the PHYH gene and key component in peroxisome regulation, results of the present study agree with the provided evidence and suggest that decreased expression of PHYH gene is associated with the absence of peroxisomes in ccRCC patients.
The gene–gene interactions form the results of our study have shown associations of multiple PEX genes (PEX2, PEX7, PEX10, PEX13, and PEX 14) with PHYH. PEX genes encode peroxins, a class machinery protein required for proper peroxisome assembly [35]. Autosomal recessive loss of function mutations in the PEX genes can result in peroxisome biogenesis disorders in the brain bone kidney and liver [36,37,38,39]. Overexpression of PEX genes such as PEX2 can result in accumulation of ubiquitinated PEX5 which can promote pexophagy (autophagosomal degradation of peroxisomes) [14]. Decreased PEX5 levels are associated with both the onset of cancer in vivo [40], and sensitivity to exogenous H2O2 addition in hepatocarcinoma model systems in vitro [41]. Identification of PEX14-containing vesicles has connected peroxisomes biogenesis to mitochondrial mediation [42]. PEX7 facilitate matrix protein import, which significantly contributes to peroxisome membrane growth [43]. Notably, PEX7 has primarily been documented to directly shuttle PHYH to the peroxisomal matrix [25]. Given the importance of peroxisomal matrix protein import in normal cells, it could be anticipated that the expression and/or function of peroxisome matrix proteins might become aberrant in tumor cells [14]. Combining the results from our analysis with the evidence presented, a clear association can be observed in which PHYH expression affects the expression of PEX genes. This in turn causes perturbations in peroxisomes biogenesis, function, and structure.
The abnormal expression of PHYH in our tumor cells activates the immune checkpoint. When the immune checkpoint is activated, the Antigen cannot be presented to T cells, blocking the presentation of Antigen in the Tumor Immune Ring, thus inhibiting the immune function of T cells, which allows the tumor cells to escape immune surveillance and survive. Through the change of immune checkpoint, we can infer the specific changes of immune pathway, which can be used to judge the therapeutic effect of targeted drugs in the future.
Results from the network analysis also revealed that alteration of PHYH expression in ccRCC phenotype implicates the alpha-oxidation pathway. Genes HACL1 and SLC27A2 (shown to be associated with PHYH) are genes that code for protein 2-hydroxyacyl-CoA lyase 1 and very long-chain acyl-CoA synthetase, and both enzymes along with PHYH are critical enzymes in converting phytanic acid to pristanic acid. Recent review has highlighted that peroxisomal disorders affect phytanic acid and alpha-oxidation [44]. As most metabolism of phytanic acid occurs in the liver and kidney via alpha-oxidation, an alteration in PHYH expression will mostly likely implicate peroxisomal and subsequent alpha-oxidation. The highlights the alpha-oxidation as a target pathway for furfure studies in ccRCC.
GESA pathway analyses of TCGA data reveal multiple differentially expressed pathways in PHYH low-expression phenotype. Among these altered pathways, the key peroxisome proliferator-activated receptor gamma (PPARγ) pathway has been shown to be functionally expressed [45] in ccRCC and that increased PPARγ abundance correlates with reduced patient survival [46]. Gluconeogenesis associated pathways pyruvate and butanoate metabolism have also been shown to be downregulated in kidney cancer [47]. The renin–angiotensin system (RAS) was also demonstrated to be underexpressed in ccRCC. RAS is a hormone system known to maintain blood pressure and body fluids [48]. Recent literature has implicated a crucial role of the RAS in the development and maintenance of cancer, particularly its effects on cancer stem cells [49,50,51,52]. In addition, RAS deregulation was demonstrated as a renal cancer risk factor [53]. Collectively, evidences suggest that these altered pathways and metabolism are good association factors with ccRCC and starting points for understating in depth underlying pathophysiological mechanism of ccRCC phenotype.
In conclusion, PHYH expression may be a potential prognostic molecular marker of poor survival in ccRCC. Low PHYH expression in ccRCC patients is closely related with dysfunction, degradation, and absence of peroxides. This occurs alters alpha-oxidation pathway which may potentially be a targeted pathway for future studies, Moreover, PPAR signalling, pyruvate metabolism, butanoate metabolism, and RAS may be the key pathway regulated by PHYH in ccRCC. Further experimental validation should be performed to prove the biologic impact of PHYH.
Conclusion
Elevated PHYH expression could be served as a potential prognostic molecular marker of better survival in ccRCC. Besides, alpha-oxidation was closely regulated by PHYH, and PPAR signalling, pyruvate metabolism, butanoate metabolism, and RAS might be the key pathways regulated by PHYH in CCRC.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- OS:
-
Overall survival
- ccRCC:
-
Clear cell renal cell carcinoma
- TCGA:
-
The cancer genome atlas
- GSEA:
-
Gene set enrichment analysis
- PHYH:
-
Phytanoyl-CoA 2-hydroxylase
- PPAR:
-
Peroxisome proliferator-activated receptor
- ICGC:
-
International cancer genome consortium
- RCC:
-
Renal cell carcinoma
- HIF-1α:
-
Hypoxia inducing factors 1α
- NES:
-
Normalized enrichment score
- ROCs:
-
Receiver-operating characteristics
- AUC:
-
Are under the curve
- UICC:
-
Union for International Cancer Control
- MSI:
-
Microsatellite instability
- GTEx:
-
The genotype-tissue expression
- HPA:
-
Human protein atlas
- RAS:
-
Renin–angiotensin system
- PPARγ:
-
Peroxisome proliferator-activated receptor gamma
References
American Cancer Society. Facts & figures 2019. Atlanta: American Cancer Society; 2019.
Zhou Y, Lin L, Wang Y, Jin X, Zhao X, Liu D, et al. The association between hypoxia-inducible factor-1 α gene G1790A polymorphism and cancer risk: a meta-analysis of 28 case-control studies. Cancer Cell Int. 2014. https://doi.org/10.1186/1475-2867-14-37.
Pharoah PDP, Dunning AM, Ponder BAJ, Easton DF. Association studies for finding cancer-susceptibility genetic variants. Nat Rev Cancer. 2004. https://doi.org/10.1038/nrc1476.
Ruan K, Song G, Ouyang G. Role of hypoxia in the hallmarks of human cancer. J Cell Biochem. 2009. https://doi.org/10.1002/jcb.22214.
Harris AL. Hypoxia - A key regulatory factor in tumour growth. Nat Rev Cancer. 2002. https://doi.org/10.1038/nrc704.
Shen C, Kaelin WG. The VHL/HIF axis in clear cell renal carcinoma. Semin Cancer Biol. 2013;23:18–25. https://doi.org/10.1016/j.semcancer.2012.06.001.
Klatte T, Seligson DB, Riggs SB, Leppert JT, Berkman MK, Kleid MD, et al. Hypoxia-inducible factor 1α in clear cell renal cell carcinoma. Clin Cancer Res. 2007. https://doi.org/10.1158/1078-0432.CCR-07-0411.
Young AC, Craven RA, Cohen D, Taylor C, Booth C, Harnden P, et al. Analysis of VHL gene alterations and their relationship to clinical parameters in sporadic conventional renal cell carcinoma. Clin Cancer Res. 2009. https://doi.org/10.1158/1078-0432.CCR-09-2131.
Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor. J Biol Chem. 1995. https://doi.org/10.1074/jbc.270.3.1230.
Chen D, Gassenmaier M, Maruschke M, Riesenberg R, Pohla H, Stief CG, et al. Expression and prognostic significance of a comprehensive epithelial-mesenchymal transition gene set in renal cell carcinoma. J Urol. 2014. https://doi.org/10.1016/j.juro.2013.08.052.
Kosari F, Parker AS, Kube DM, Lohse CM, Leibovich BC, Blute ML, et al. Clear cell renal cell carcinoma: gene expression analyses identify a potential signature for tumor aggressiveness. Clin Cancer Res. 2005. https://doi.org/10.1158/1078-0432.CCR-05-0073.
Lane BR, Li J, Zhou M, Babineau D, Faber P, Novick AC, et al. Differential expression in clear cell renal cell carcinoma identified by gene expression profiling. J Urol. 2009;181:849–60. https://doi.org/10.1016/j.juro.2008.10.069.
Tan W, Hildebrandt MAT, Pu X, Huang M, Lin J, Matin SF, et al. Role of inflammatory related gene expression in clear cell renal cell carcinoma development and clinical outcomes. J Urol. 2011. https://doi.org/10.1016/j.juro.2011.06.049.
Dahabieh MS, Di Pietro E, Jangal M, Goncalves C, Witcher M, Braverman NE, et al. Peroxisomes and cancer: the role of a metabolic specialist in a disease of aberrant metabolism. Biochim Biophys Acta Rev Cancer. 2018. https://doi.org/10.1016/j.bbcan.2018.07.004.
Kruppa J, Jung K. Automated multigroup outlier identification in molecular high-throughput data using bagplots and gemplots. BMC Bioinformatics. 2017. https://doi.org/10.1186/s12859-017-1645-5.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005. https://doi.org/10.1073/pnas.0506580102.
Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008. https://doi.org/10.1200/JCO.2007.12.9791.
Harrell FE, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA J Am Med Assoc. 1982. https://doi.org/10.1001/jama.1982.03320430047030.
Huitzil-Melendez FD, Capanu M, O’Reilly EM, Duffy A, Gansukh B, Saltz LL, et al. Advanced hepatocellular carcinoma: which staging systems best predict prognosis? J Clin Oncol. 2010. https://doi.org/10.1200/JCO.2009.25.9895.
Jansen GA, Waterham HR, Wanders RJA. Molecular basis of refsum disease: sequence variations in Phytanoyl-CoA hydroxylase (PHYH) and the PTS2 receptor (PEX7). Hum Mutat. 2004. https://doi.org/10.1002/humu.10315.
Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Tissue-based map of the human proteome. Science. 2015. https://doi.org/10.1126/science.1260419.
Poulos A, Sharp P, Fellenberg AJ, Johnson DW. Accumulation of pristanic acid (2, 6, 10, 14 tetramethylpentadecanoic acid) in the plasma of patients with generalised peroxisomal dysfunction. Eur J Pediatr. 1988. https://doi.org/10.1007/BF00442211.
Van Veldhoven PP. Biochemistry and genetics of inherited disorders of peroxisomal fatty acid metabolism. J Lipid Res. 2010. https://doi.org/10.1194/jlr.R005959.
Moser AE, Singh I, Brown FR, Solish GI, Kelley RI, Benke PJ, et al. The cerebrohepatorenal (Zellweger) syndrome: increased levels and impaired degradation of very-long-chain fatty acids and their use in prenatal diagnosis. N Engl J Med. 1984. https://doi.org/10.1056/NEJM198405033101802.
Mihalik SJ, Morrell JC, Kim D, Sacksteder KA, Watkins PA, Gould SJ. Identification of pahx, a refsum disease gene. Nat Genet. 1997. https://doi.org/10.1038/ng1097-185.
Kim EH, Kim GA, Taweechaipaisankul A, et al. Phytanic acid-derived peroxisomal lipid metabolism in porcine oocytes. Theriogenology. 2020;157:276–85. https://doi.org/10.1016/j.theriogenology.2020.07.007.
Islinger M, Voelkl A, Fahimi HD, Schrader M. The peroxisome: an update on mysteries 2.0. Histochem Cell Biol. 2018. https://doi.org/10.1007/s00418-018-1722-5.
Cooper GM. The cell: a molecular approach. The development and causes of cancer, 2nd edn. Sunderland (MA): Sinauer Associates; 2000. https://www.ncbi.nlm.nih.gov/books/NBK9963/
Reddy JK, Hashimoto T. Peroxisomal β-oxidation and peroxisome proliferator—activated receptor α: an adaptive metabolic system. Annu Rev Nutr. 2001. https://doi.org/10.1146/annurev.nutr.21.1.193.
Wanders RJA, Vreken P, Ferdinandusse S, Jansen GA, Waterham HR, van Roermund CWT, et al. Peroxisomal fatty acid α- and β-oxidation in humans: enzymology, peroxisomal metabolite transporters and peroxisomal diseases. Biochem Soc Trans. 2001. https://doi.org/10.1042/0300-5127:0290250.
Reddy JK, Krishnakantha TP. Hepatic peroxisome proliferation: Induction by two novel compounds structurally unrelated to clofibrate. Science. 1975. https://doi.org/10.1126/science.1198095.
Reddy JK, Azarnoff DL, Hignite CE. Hypolipidaemic hepatic peroxisome proliferators form a novel class of chemical carcinogens [22]. Nature. 1980. https://doi.org/10.1038/283397a0.
Reddy JK, Chu R. Peroxisome proliferator-induced pleiotropic responses: pursuit of a phenomenon. Ann N Y Acad Sci. 1996. https://doi.org/10.1111/j.1749-6632.1996.tb18616.x.
Frederiks WM, Bosch KS, Hoeben KA, van Marle J, Langbein S. Renal cell carcinoma and oxidative stress: the lack of peroxisomes. Acta Histochem. 2010. https://doi.org/10.1016/j.acthis.2009.03.003.
Waterham HR, Ebberink MS. Genetics and molecular basis of human peroxisome biogenesis disorders. Biochim Biophys Acta Mol Basis Dis. 2012. https://doi.org/10.1016/j.bbadis.2012.04.006.
Wanders RJA. Metabolic and molecular basis of peroxisomal disorders: a review. Am J Med Genet. 2004. https://doi.org/10.1002/ajmg.a.20661.
Wanders RJA, Waterham HR. Peroxisomal disorders I: biochemistry and genetics of peroxisome biogenesis disorders. Clin Genet. 2005. https://doi.org/10.1111/j.1399-0004.2004.00329.x.
Macdonald A, Baldwin E. Peroxisomal disorders. Clin Paediatr Diet Fourth Ed. 2014. https://doi.org/10.1002/9781118915349.ch20.
Gould SJ, Valle D. Peroxisome biogenesis disorders: genetics and cell biology. Trends Genet. 2000. https://doi.org/10.1016/S0168-9525(00)02056-4.
Karlberg N, Karlberg S, Karikoski R, Mikkola S, Lipsanen-Nyman M, Jalanko H. High frequency of tumours in Mulibrey nanism. J Pathol. 2009. https://doi.org/10.1002/path.2538.
Wang W, Xia ZJ, Farré JC, Subramani S. TRIM37, a novel E3 ligase for PEX5-mediated peroxisomal matrix protein import. J Cell Biol. 2017. https://doi.org/10.1083/jcb.201611170.
Sugiura A, Mattie S, Prudent J, Mcbride HM. Newly born peroxisomes are a hybrid of mitochondrial and ER-derived pre-peroxisomes. Nature. 2017. https://doi.org/10.1038/nature21375.
Mast FD, Fagarasanu A, Rachubinski R. The peroxisomal protein importomer: a bunch of transients with expanding waistlines. Nat Cell Biol. 2010. https://doi.org/10.1038/ncb0310-203.
Wierzbicki AS. Peroxisomal disorders affecting phytanic acid α-oxidation: a review. Biochem Soc Trans. 2007. https://doi.org/10.1042/BST0350881.
Collet N, Théoleyre S, Rageul J, Mottier S, Jouan F, Rioux-Leclercq N, et al. PPARγ is functionally expressed in clear cell renal cell carcinoma. Int J Oncol. 2011. https://doi.org/10.3892/ijo.2010.891.
Zhu C, Wei J, Tian X, Li Y, Li X. Prognostic role of PPAR-γ and PTEN in the renal cell carcinoma. Int J Clin Exp Pathol. 2015;8(10):12668–77.
Perroud B, Lee J, Valkova N, Dhirapong A, Lin PY, Fiehn O, et al. Pathway analysis of kidney cancer using proteomics and metabolic profiling. Mol Cancer. 2006. https://doi.org/10.1186/1476-4598-5-64.
Peach MJ. Renin angiotensin system: Biochemistry and mechanisms of action. Physiol Rev. 1977. https://doi.org/10.1152/physrev.1977.57.2.313.
Bradshaw AR, Wickremesekera AC, Brasch HD, Chibnall AM, Davis PF, Tan ST, et al. Glioblastoma multiforme cancer stem cells express components of the renin-angiotensin system. Front Surg. 2016. https://doi.org/10.3389/fsurg.2016.00051.
Featherston T, Yu HH, Dunne JC, Chibnall AM, Brasch HD, Davis PF, et al. Cancer stem cells in moderately differentiated buccal mucosal squamous cell carcinoma express components of the renin-angiotensin system. Front Surg. 2016. https://doi.org/10.3389/fsurg.2016.00052.
Jokubaitis VJ, Sinka L, Driessen R, Whitty G, Haylock DN, Bertoncello I, et al. Angiotensin-converting enzyme (CD143) marks hematopoietic stem cells in human embryonic, fetal, and adult hematopoietic tissues. Blood. 2008. https://doi.org/10.1182/blood-2007-05-091710.
Sinka L, Biasch K, Khazaal I, Péault B, Tavian M. Angiotensin-converting enzyme (CD143) specifies emerging lympho-hematopoietic progenitors in the human embryo. Blood. 2012. https://doi.org/10.1182/blood-2010-11-314781.
Sobczuk P, Szczylik C, Porta C, Czarnecka AM. Renin angiotensin system deregulation as renal cancer risk factor (Review). Oncol Lett. 2017. https://doi.org/10.3892/ol.2017.6826.
Acknowledgements
We appreciate the International Cancer Genome Consortium Data and The Cancer Genome Atlas for the open data.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
QZ and AY conceived and designed the study; GZ and QH acquired and analyzed the data; JL and PJ interpreted the data and drafted the manuscript; QZ, AY, and GZ modified the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent for publication was obtained from all participants.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1.
Edges represent protein–protein associations
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhengqi, Q., Zezhi, G., Lei, J. et al. Prognostic role of PHYH for overall survival (OS) in clear cell renal cell carcinoma (ccRCC). Eur J Med Res 26, 9 (2021). https://doi.org/10.1186/s40001-021-00482-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-021-00482-1