Abstract
Background
Knee osteoarthritis is a common cause of musculoskeletal pain and a leading cause of disability and healthcare economic burden. The optimum treatment for knee osteoarthritis is still inconclusive. A network meta-analysis is required to assess the efficacy and safety of treatments and provide more scientific medical evidence.
Methods
Relevant studies were searched through PubMed, Embase, and Cochrane Library electronic databases from the inception to October 2018. Continuous outcomes such as pain, stiffness, physical function and total scores were expressed as the mean differences with 95% credible interval. Surface under the cumulative ranking curve illustrated the rank probability of each therapy under different outcomes.
Results
Nineteen studies were included in this study, with a total of 2395 patients. For knee pain, platelet-rich plasma (0.691) was ranked at the first place, followed by hyaluronic acid combined with platelet-rich plasma (0.670) and hyaluronic acid (0.402). In terms of stiffness, hyaluronic acid combined with platelet-rich plasma (0.743) enjoyed the highest value, platelet-rich plasma (0.603) was the next and hyaluronic acid (0.386) was the third. As for physical function, the rank was hyaluronic acid combined with platelet-rich plasma (0.772), platelet-rich plasma (0.608) and hyaluronic acid (0.343). For total scores, the order given by surface under the cumulative ranking was hyaluronic acid combined with platelet-rich plasma (0.765), platelet-rich plasma (0.624) and hyaluronic acid (0.37).
Conclusions
Hyaluronic acid combined with platelet-rich plasma showed the best efficacy in improving stiffness, physical function, and total scores, while platelet-rich plasma appeared the best in terms of pain reduction.
Similar content being viewed by others
Background
Knee osteoarthritis (OA) is a degenerative disease with clinical manifestations, including joint pain, tenderness, joint swelling restricted movement as well as joint deformities and a leading cause of disability and healthcare economic burden [1, 2]. More than 50% aged 60–75 years old people owned radiological evidence of knee OA and 80% of population over 75 years old suffered from knee OA [3, 4]. In recent years, the incidence of OA has increased and presents as a serious threat to human health and quality of life [5]. The current optional treatments for knee OA include oral anti-inflammatory drugs, physical therapy, topical anti-inflammatory gels and intra-articular injections [6]. The intra-articular injection consists of various drugs such as corticosteroid, hyaluronic acid (HA) and platelet-rich plasma (PRP).
Corticosteroids are commonly utilized as intra-articular injection agents [7]. Significant effects of corticosteroids have been reported by several studies for treating the knee OA [8,9,10]. The short-term benefits of intra-articular corticosteroid injection are well recognized, while the long-term benefits and the value of repetitive injections are still debatable [11, 12].
OA is characterized by degenerative of articular cartilage, osteophyte formation, changes in the synovial membrane, subchondral bone sclerosis, and reduced viscosity of synovial fluid [13]. HA is a widely distributed, liner glycosaminoglycan constituent of cartilage, synovial fluid, skin and aqueous humor [14]. It has been proved that synovial fluid from arthritic joints contains lower concentrations of HA than that from normal joint [15]. Since the elasticity and viscosity of synovial fluid are directly proportional to HA content and integrity, intra-articular injection of HA is a rational approach to the treatment of OA [14]. Although beneficial effects on pain, function and patient global assessment have been documented, the real entity of improvement and which of the many available HA products can offer the best results is not clear [16].
Platelet-rich plasma (PRP) has also been introduced as an alternative treatment for patients with knee OA [17]. PRP comprised a potent cellular milieu containing platelet concentrations above baseline, as well as an undifferentiated mixture of anti-inflammatory, pro-inflammatory, anabolic and catabolic mediators in an attempt to stimulate a supra-physiologic response and elicit the body’s natural healing potential [17,18,19]. The analysis of Zhang’s et al. review demonstrated that published literatures supported the efficacy of PRP in the treatment of tendinous, cartilaginous, ligamentous, and muscular injuries [12]. In a systematic review and meta-analysis, Laudy et al. found that patients treated with PRP injections reduced more pain than those receiving placebo injections [20]. Nevertheless, the level of evidence was limited since the risk of bias was high. Although intra-articular saline injections are used commonly as placebos in many trails, there has been increasing recognition that the application of intra-articular saline may have some effect [21, 22]. However, this result was drawn from clinically heterogeneous samples [22].
Controversies remain regarding in the treatment of knee OA. Many articles compared HA and PRP to determine which therapy was better in terms of pain reduction. In 2016, a meta-analysis reported that PRP was more effective when compared with HA [23]. Cole et al. observed that there was no difference between HA and PRP in the primary outcome in terms of the patient-reported Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain score [6]. Similarly, studies of HA and corticosteroid injection in patients with knee OA have displayed various results [24, 25].
Therefore, a network meta-analysis (NMA) is required due to contradictions and to give more scientific evidence for the choice of treatments. We performed the first NMA to evaluate the efficacy and safety of five different treatments for patients with OA, including corticosteroid, HA, PRP, placebo and HA + PRP, in regard to their performance on pain, stiffness, physical function or total assessed with quantitative scores by WOMAC, in order to provide optimal treatments for knee OA patients.
Methods
Literature search
Relevant studies were searched through PubMed, Embase, and Cochrane Library electronic databases from the inception to October 2018. All searches were limited to randomized controlled trials (RCTs) in humans. These following search terms and their corresponding synonyms were applied for systematic searching: “knee osteoarthritis”, “platelet-rich plasma”, “hyaluronic acid”, “corticosteroid”, “placebo”, and “randomized controlled trial”. There were no limits on language and publication date. Two authors independently retrieved potential eligible articles.
Study selection
Criteria for eligibility of published studies were as follows: (1) study design should be double/triple-blinded RCTs; (2) follow-up period should be at least 1 month; (3) study subjects should be patients diagnosed with knee OA according to the American College of Rheumatology criteria [26], Ahlbäck’s criteria [27], or by the radiological assessment of Kellgren and Lawrence [28]; (4) studies contained at least two of the following treatments: corticosteroid, HA, PRP and HA + PRP; (5) outcomes included pain, stiffness, physical function or total assessed with quantitative scores by WOMAC.
The exclusion criteria included the following items: (1) patients receiving previous surgery or joint arthroplasties of the investigational knee; (2) studies with insufficient data, such as the article has only one study group or has not the main outcomes; (3) duplicated patients or studies; (4) systematic reviews, conference reports or comments; (5) cell or animal experiments.
Data extraction
The data were independently extracted by two reviewers. The following information was extracted from the included articles: first author’s name, publication year, country, blinding, treatments of each groups, samples size, the degree of disease, the number of injection, duration of follow-up, age, sex ratio and BMI. Any disagreement concerning the data extraction was resolved by discussion. The outcomes included WOMAC pain, stiffness, physical function and total scores. WOMAC is a 24-item questionnaire, which is divided into three subscales measuring pain, stiffness and physical function [29]. Pain contains 5 items and score ranges from 0 to 20. Stiffness contains 2 items and score ranges from 0 to 8. As for physical function, it includes 17 items and score ranges from 0 to 68 [29].
Statistical analysis
All data analysis was performed through a Bayesian model in WinBUGS (MRC Bio-statistics Unit, Cambridge, UK). Endnote software (version X8.0) was utilized to manage articles. Continuous outcomes such as pain, stiffness, physical function and total scores were expressed as the mean differences (MD) with 95% credible interval (CrI). A significant difference could be found by 95% CrI which did not contain 0 for MD. The net plot was used to show the connection between each treatment for four outcomes. The result of NMA was indicated by the slash table. Moreover, the comparison between direct and indirect data was demonstrated by the node-splitting table. Surface under the cumulative ranking curve (SUCRA) illustrated the rank probability of each therapy under different outcomes. The larger SUCRA value stood for the better rank of the intervention. Finally, the funnel plot was generated to assess the publication bias.
Results
Included studies
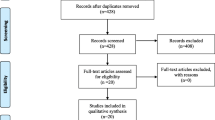
As shown in the flowchart (Fig. 1), 2974 literatures were searched from electronic database. After adding 3 studies from the citations of related review, 2977 studies were assessed for the next step. Then 696 studies were removed due to duplicates, leaving 2281 literatures assessed to be eligible. By analyzing the titles and abstracts, 2145 articles were excluded for irrelevant content (2114) and systematic review (31). No non-English article was included due to not being eligible for the inclusion criteria after analyzing the abstract. By reading the full articles, a total of 117 studies were excluded for not randomized controlled trial (26), insufficient data (29), low relevance (35) and unavailability of specific data (27). Ultimately, 19 double-blinded studies were involved in our analysis.
Study characteristics
All included studies were RCTs published between 2002 and 2018, with a total of 2395 patients. The sample size varied between 10 and 174 and the average age varied between 46.2 and 69.6 years old. Follow-up period ranged from 8 weeks to 2 years. The mean number of female ratio ranged from 27.3% to 100% of patients. The characteristics of 19 included studies are presented in Table 1. As for five treatments, the comparison between HA and placebo was the most frequently reported one and the pair of PRP and placebo was the second. In these 19 studies, 17 were two-arms, while the other 2 were three/four-arms, respectively. The number of injection and dosage of each treatment has also been presented. Figure 2 shows more details on direct comparison of different therapies.
Net plots of four outcomes: pain, stiffness, physical function and total (sum of the other 3 outcomes). Line width is proportional to the number of trials comparing treatments with numbers on the lines representing the exact number. Circle area represents the cumulative number of patients in each intervention. HA hyaluronic acid, PRP platelet-rich plasma
Efficacy and safety results
For knee pain, better improvement can be found in patients receiving PRP treatment compared with those receiving corticosteroid [PRP vs. Corticosteroid: MD = 2.8, 95% CrI (0.22, 5.3)]. Compared with placebo, there was statistical significance in the improvement of KOA in patients treated with HA [Placebo vs. HA: MD = − 1.7, 95% CrI (− 2.9, − 0.39)], PRP [Placebo vs. PRP: MD = − 3.1, 95% CrI (− 4.5, − 1.6)] and HA + PRP [HA + PRP vs. Placebo: MD = 3.1, 95% CrI (0.63, 5.4)]. These results are shown in Fig. 3 and Table 2. Similarly to the SUCRA value in Table 3, PRP (0.691) was ranked at the first place, followed by HA + PRP (0.670) and HA (0.402).
Forest plots of four outcomes: pain, stiffness, physical function and total (sum of the other 3 outcomes). The mean difference with 95% credible intervals (CrIs) indicates relative efficacy of treatment compared to another treatment. A hyaluronic acid, B triamcinolone, C platelet-rich plasma, D saline, E hyaluronic acid + platelet-rich plasma
In terms of stiffness, HA + PRP and PRP demonstrated its significant advantages over placebo [HA + PRP vs. Placebo: MD = 1.4, 95% CrI (0.30, 2.5), Placebo vs. PRP: MD = − 1.0, 95% CrI (− 1.8, − 0.28)]. No remarkable improvement was observed in other comparisons, as displayed in Table 2 and Fig. 3. Illustrated by the results of SUCRA in Table 3, HA + PRP (0.743) enjoyed the highest SUCRA value, PRP (0.603) was the next and HA (0.386) was the third.
As for physical function, HA + PRP exhibited a better effect than HA, corticosteroid and placebo with significant difference [(HA + PRP vs. HA: MD = 11.0, 95% CrI (2.6, 20.0), HA + PRP vs. Corticosteroid: MD = 14.0, 95% CrI (1.4, 26.0), HA + PRP vs. Placebo: MD = 15.0, 95% CrI (6.1, 24.0)]. Besides, PRP had a better efficacy than placebo in the treatment of KOA [Placebo vs. PRP: MD = − 10.0, 95% CrI (− 17.0, − 4.2)]. This was also verified by SUCRA values in Table 3. The rank was HA + PRP (0.772), PRP (0.608) and HA (0.343).
For total scores, HA + PRP performed better than corticosteroid, HA and placebo with statistical significance [HA + PRP vs. Corticosteroid: MD = 18.0, 95% CrI (4.1, 31.0), HA + PRP vs. HA: MD = 13.0, 95% CrI (2.9, 24.0), HA + PRP vs. Placebo: MD = 20.0, 95% CrI (8.4, 31.0)]. In addition, PRP functioned better than corticosteroid, HA and placebo with significant difference [PRP vs. Corticosteroid: MD = 13.0, 95% CrI (1.5, 24.0), PRP vs. HA: MD = 8.4, 95% CrI (0.55, 16.0), Placebo vs. PRP: MD = − 15.0, 95% CrI: (− 22.0, − 7.2)]. Moreover, HA was better than placebo for patients with KOA [Placebo vs. HA: MD = − 6.2, 95% CrI (− 12.0, − 0.016)]. The order given by SUCRA in Table 3 was HA + PRP (0.765), PRP (0.624) and HA (0.37).
Inconsistency assessment and risk of bias
There was no evidence of inconsistency between direct and indirect estimates in the comparison among different studies, as seen in the heat plot and the node-splitting plot (Figs. 4 and 5). No evidence of publication bias is observed in Fig. 6.
Node-splitting plots of four outcomes: Pain, Stiffness, Physical Function and Total (sum of the other 3 outcomes). 95% mean difference credible intervals (CrIs) indicates relative efficacy of treatment compared to another treatment. A hyaluronic acid, B triamcinolone, C platelet-rich plasma, D saline, E hyaluronic acid + platelet-rich plasma
Comparison-adjusted funnel plots of treatments of 4 outcomes’ networks. The red line represents the null hypothesis that the study-specific effect sizes do not differ from the respective comparison-specific pooled effect estimates. A hyaluronic acid, B triamcinolone, C platelet-rich plasma, D saline, E hyaluronic acid + platelet-rich plasma
Discussion
Knee OA is a chronic progressive joint disease and the second main cause of disability, followed by a heavy social and economic burden [30]. Since the optimal treatment for knee OA patients remains inconclusive, the present NMA is performed to determine the best regime for patients with knee OA and provide a medical guideline. In the present study, the most important finding in this study is that HA + PRP showed the best efficacy in relieving stiffness, improving physical function and total scores, while PRP took the first place in terms of pain reduction.
As for stiffness, physical function and total scores, HA combined with PRP had the highest SUCRA value of 0.743, 0.772 and 0.765, respectively, which means that according to these three appraisal tools, HA combined with PRP is the first choice. This result was in accordance with the conclusions of some previous studies. The results of Yu et al. indicated that patients with knee OA who were treated with the combination of HA and PRP had more positive effects on body pain, and reduced arthralgia, bone damage and cartilage destruction, when compared with HA or PRP treatment alone [31]. Lana’s et al. study showed that HA and PRP combination treatment enjoyed better outcomes than HA alone up to 1 year and PRP alone up to 3 months [17]. Therefore, HA + PRP is the preferred therapy for knee OA patients.
Meanwhile, based on the SUCRA results of this NMA, PRP was another effective regime, as it ranked the first in pain reduction (0.691) and the second in stiffness (0.603), physical function (0.608), and total scores (0.624). A RCT study found that compared with therapeutic exercise alone, PRP injections and therapeutic exercise combination treatment could be more effective in reducing pain and improving stiffness as well as quality of life [32]. Another prospective, double-blind RCT supported that PRP injections had a better short-term effectiveness than placebos in the reduction of pain and stiffness and the improvement of knee function in early knee OA [33].
In the current study, we can see that irrespective of whether the HA was combined with PRP injection or the PRP injection alone, the key factor of these two treatments is PRP. Researches have indicated that inflammatory cytokines serve a critical function in the induction and development of OA [34, 35]. PRP is an autologous and multifunctional platelet concentrate of the blood that contains highly concentrated platelets and highly levels of cell growth factors. PRP promotes synovial cell proliferation and differentiation, as well as recovery of cartilage morphology [31]. That may be the key reason why the injection containing PRP shows an ideal outcome. Although plenty of studies have proven the efficacy of PRP in treatment of OA clinically, more mechanism research about OA and interaction research about the relationship between OA and PRP is required.
Although this NMA addressed many controversies on the clinical efficacy and safety of different treatments, there were still some limitations of the present study that require to be acknowledged. The limitations of our study include the following: (i) English studies were included, while non-English language articles without English abstract were omitted, which may lead to language bias; (ii) the study used self-reported questionnaire named WOMAC to evaluate outcomes, including pain, stiffness, physical function and total scores, which might limit the objectivity of results; (iii) due to insufficient data, the subgroup analysis in terms of severity of the disease, the frequency and dosage of the medication and the degree of activity has not been performed in this NMA. The fact of paucity of homogeneous studies also indicated to us that more high-quality and homogeneous studies should be carried out to provide us more strong evidence of this topic; (iv) the study sample size was relatively small, ranging from 10 to 174, which might have a negative effect on the conclusions. Hence, further high-quality RCTS are needed to address uncertainties regarding various treatments as well as qualify clinical efficacy.
Conclusions
In conclusion, this NMA revealed that HA + PRP demonstrated the best efficacy in the improvement of stiffness, physical function, and total scores, while PRP had the best performance in terms of pain reduction. Ultimately, we hope that more and more researchers to compare the efficacy and safety of different regimes for patients with knee OA, and thus there will be more data and literatures to be referenced, which will provide more theoretical basis for clinical therapy of knee OA.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- CrI:
-
Credible interval
- MD:
-
Mean differences
- NMA:
-
Network meta-analysis
- OA:
-
Osteoarthritis
- PRP:
-
Platelet-rich plasma
- RCTs:
-
Randomized controlled trials
- SUCRA:
-
Surface under the cumulative ranking curve
- WOMAC:
-
Western Ontario and McMaster Universities Osteoarthritis Index
References
Krackow KA, Mandeville DS, Rachala SR, Bayers-Thering M, Osternig LR. Torsion deformity and joint loading for medial knee osteoarthritis. Gait Posture. 2011;33(4):625–9.
Onuora S. Osteoarthritis: molecular imaging detects activated macrophages. Nat Rev Rheumatol. 2016;12(6):313.
Bhandari M, Bannuru RR, Babins EM, Martel-Pelletier J, Khan M, Raynauld JP, Frankovich R, McLeod D, Devji T, Phillips M, et al. Intra-articular hyaluronic acid in the treatment of knee osteoarthritis: a Canadian evidence-based perspective. Therap Adv Musculoskelet Dis. 2017;9(9):231–46.
Wellsandt E, Golightly Y. Exercise in the management of knee and hip osteoarthritis. Curr Opin Rheumatol. 2018;30(2):151–9.
Zhang YQ, Jordan JM. Epidemiology of Osteoarthritis. Clin Geriatr Med. 2010;26(3):355–69.
Cole BJ, Karas V, Hussey K, Pilz K, Fortier LA. Hyaluronic acid versus platelet-rich plasma: a prospective, double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Am J Sports Med. 2017;45(2):339–46.
Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;2:CD005321.
Arroll B, Goodyear-Smith F. Corticosteroid injections for osteoarthritis of the knee: meta-analysis. Br Med J. 2004;328(7444):869A–70A.
Bain LS, Balch HW, Wetherly JM, Yeadon A. Intraarticular triamcinolone hexacetonide: double-blind comparison with methylprednisolone. Br J Clin Pract. 1972;26(12):559–61.
Cederlof S, Jonson G. Intraarticular prednisolone injection for osteoarthritis of the knee. A double blind test with placebo. Acta chirurgica Scand. 1966;132(5):532–7.
Askari A, Gholami T, NaghiZadeh MM, Farjam M, Kouhpayeh SA, Shahabfard Z. Hyaluronic acid compared with corticosteroid injections for the treatment of osteoarthritis of the knee: a randomized control trail. Springerplus. 2016;5:442.
Kon E, Filardo G, Drobnic M, Madry H, Jelic M, van Dijk N, Della Villa S. Non-surgical management of early knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2012;20(3):436–49.
Gerwin N, Hops C, Lucke A. Intraarticular drug delivery in osteoarthritis. Adv Drug Deliv Rev. 2006;58(2):226–42.
Altman RD, Akermark C, Beaulieu AD, Schnitzer T. Durolane International Study G: Efficacy and safety of a single intra-articular injection of non-animal stabilized hyaluronic acid (NASHA) in patients with osteoarthritis of the knee. Osteoarthritis Cartil. 2004;12(8):642–9.
Balazs EA, Denlinger JL. Viscosupplementation: a new concept in the treatment of osteoarthritis. J Rheumatol Suppl. 1993;39:3–9.
Colen S, van den Bekerom MP, Mulier M, Haverkamp D. Hyaluronic acid in the treatment of knee osteoarthritis: a systematic review and meta-analysis with emphasis on the efficacy of different products. BioDrugs Clin Immunotherap Biopharm Gene Therapy. 2012;26(4):257–68.
Lana JF, Weglein A, Sampson SE, Vicente EF, Huber SC, Souza CV, Ambach MA, Vincent H, Urban-Paffaro A, Onodera CM, et al. Randomized controlled trial comparing hyaluronic acid, platelet-rich plasma and the combination of both in the treatment of mild and moderate osteoarthritis of the knee. J Stem Cells Regen Med. 2016;12(2):69–78.
Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91(1):4–15.
Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA. Platelet-rich plasma from basic science to clinical applications. Am J Sports Med. 2009;37(11):2259–72.
Laudy ABM, Bakker EWP, Rekers M, Moen MH. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med. 2015;49(10):17.
Miller LE, Block JE. US-approved intra-articular hyaluronic acid injections are safe and effective in patients with knee osteoarthritis: systematic review and meta-analysis of randomized, saline-controlled trials. Clin Med Insights Arthritis Musculoskeletal Disord. 2013;6:57–63.
Zou K, Wong J, Abdullah N, Chen X, Smith T, Doherty M, Zhang WY. Examination of overall treatment effect and the proportion attributable to contextual effect in osteoarthritis: meta-analysis of randomised controlled trials. Ann Rheum Dis. 2016;75(11):1964–70.
Sadabad HN, Behzadifar M, Arasteh F, Behzadifar M, Dehghan HR. Efficacy of platelet-rich plasma versus hyaluronic acid for treatment of knee osteoarthritis: a systematic review and meta-analysis. Electron Physician. 2016;8(3):2115–22.
Altman RD, Moskowitz R, Hyalgan Study G. Intraarticular sodium hyaluronate (Hyalgan (R)) in the treatment of patients with osteoarthritis of the knee: a randomized clinical trial. J Rheumatol. 1998;25(11):2203–12.
Creamer P, Sharif M, George E, Meadows K, Cushnaghan J, Shinmei M, Dieppe P. Intra-articular hyaluronic acid in osteoarthritis of the knee: an investigation into mechanisms of action. Osteoarthritis Cartil. 1994;2(2):133–40.
Altman RD. Criteria for the classification of osteo-arthritis of the knee and hip. Scand J Rheumatol. 1987;16:31–9.
Ahlback S. Osteoarthrosis of the knee. A radiographic investigation. Acta radiologica: diagnosis. 1968;277:7–72.
Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502.
Nadrian H, Moghimi N, Nadrian E, Moradzadeh R, Bahmanpour K, Iranpour A, Bellamy N. Validity and reliability of the Persian versions of WOMAC Osteoarthritis Index and Lequesne Algofunctional Index. Clin Rheumatol. 2012;31(7):1097–102.
Miller ME, Rejeski WJ, Messier SP, Loeser RF. Modifiers of change in physical functioning in older adults with knee pain: The observational arthritis study in seniors (OASIS). Arthritis Rheum Arthritis Care Res. 2001;45(4):331–9.
Yu W, Xu P, Huang G, Liu L. Clinical therapy of hyaluronic acid combined with platelet-rich plasma for the treatment of knee osteoarthritis. Exp Therap Med. 2018;16(3):2119–25.
Namazi H, Kayedi T. Investigating the effect of intra-articular platelet-rich plasma injection on union: pain and function improvement in patients with scaphoid fracture. J Hand Microsurg. 2016;8(3):140–4.
Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41(2):356–64.
Hodge JA, Kawabata TT, Krishnaswami S, Clark JD, Telliez JB, Dowty ME, Menon S, Lamba M, Zwillich S. The mechanism of action of tofacitinib—an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis. Clin Exp Rheumatol. 2016;34(2):318–28.
van der Goes MC, Jacobs JW, Bijlsma JW. Rediscovering the therapeutic use of glucocorticoids in rheumatoid arthritis. Curr Opin Rheumatol. 2016;28(3):289–96.
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
YZ participated in data analysis and collected field data; BL participated in the design of the study and drafted the manuscript; LB carried out the statistical analyses, conceived of the study and designed the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, B., Zhang, Y. & Bi, L. Comparative efficacy of treatments for patients with knee osteoarthritis: a network meta-analysis. Eur J Med Res 25, 27 (2020). https://doi.org/10.1186/s40001-020-00426-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-020-00426-1









