Abstract
Ten isatin-based hydrazone derivatives were synthesized using two subseries, IA (isatin + acetophenone) and IB (isatin + benzaldehyde), and evaluated for their monoamine oxidases (MAOs) inhibitory activity. All the compounds showed stronger MAO-A inhibition than MAO-B, and the IB series showed more effective MAO-A inhibitory activity than IA series. Compound IB4 most potently inhibited MAO-A (half maximal inhibitory concentration IC50 = 0.015 µM), followed by IB3 (IC50 = 0.019 µM). On the contrary, compound IB3 showed the highest MAO-B inhibition (IC50 = 0.068 µM), followed by IB4 (IC50 = 1.87 µM). Compound IB3 and IB4 had low selectivity indices of 3.68 and 8.50, respectively. Structurally, the methyl group of IA series decreased the inhibition of both MAO-A and MAO-B. Among them, IB3 and IB4 (4-Cl and 4-Br in B-ring, respectively) showed higher MAO-A and MAO-B inhibition than the other substitutions. Inhibition constant Ki values of IB3 and IB4 for MAO-A were 0.0088 and 0.0063 µM, respectively, and those for MAO-B were 0.048 and 0.060 µM, respectively. IB3 and IB4 were competitive, reversible inhibitors of MAO-A and MAO-B. Molecular docking analysis predicted that IB3 and IB4 formed stable hydrogen bonds between Asn181 and the NH atom of isatin in the ligand-protein complex. Dynamic analysis revealed that IB3 and IB4 are stable with both MAO isoforms. These observations suggest IB3 and IB4 are potent and reversible MAO-A and MAO-B inhibitors and both compounds can be used as therapeutic agents for neurological disorders.
Similar content being viewed by others
Introduction
A fused diketopyrrole ring and a benzene ring compose the bicyclic heteroaromatic nucleus of isatin [1]. It is found naturally in several plant species and some animal tissues and is used as a versatile starting material in synthetic organic chemistry. Isatin demonstrates a wide range of reactivity owing to the presence of both electron-rich (benzene and non-bonding electrons of nitrogen atoms) and electron-deficient sites (diketo part of 2nd and 3rd positions) in its structure. It can undergo oxidation, substitution, spiroannulation, and condensation reactions to produce various derivatives with unique structural frameworks and characteristics [2,3,4]. In medicinal chemistry, isatin and its derivatives have attracted considerable attention because of their diverse biological profiles. These molecules exhibit a wide range of pharmacological activities including antiviral, antibacterial, anti-inflammatory, anticancer, and antidepressant effects. Derivatives of isatin have been studied as possible treatment agents for a number of illnesses, including infectious diseases, cancer, and neurological problems [5,6,7,8].
The brain and other peripheral human tissues have a class of enzymes called monoamine oxidases (MAOs) in their outer membranes of the mitochondria [9, 10]. They play a prominent role in the metabolism of monoamine neurotransmitters, which are signaling molecules that control mood, emotions, and thought processes. MAO has two primary isoforms: MAO-A and MAO-B. Although there was significant overlap, the substrate specificities and tissue distributions of each isoform varied. The main metabolites of the MAO-A isoform are dopamine, norepinephrine, and serotonin. MAO-A is a major player in the breakdown of serotonin and norepinephrine, which is why drugs are used to treat mood disorders, such as anxiety and depression conditions [11,12,13]. MAO-B inhibitors are used to treat Parkinson’s disease (PD), which is a neurodegenerative disorder accompanying with the loss of dopaminergic neurons owing to their unique function in the breakdown of dopamine [14, 15]. As specific inhibitors target only one type of MAO enzyme, they provide a tailored therapy with possibly fewer side effects. In contrast, nonselective inhibitors may work better in some circumstances and have wider therapeutic effects. Furthermore, in order to prevent potentially harmful interactions with foods containing tyramine, which can result in hypertensive crises, non-selective MAO inhibitors require stringent dietary restrictions [16, 17]. Both enzymes of MAO-A and MAO-B were inhibited by isatin and its derivatives. Isatin and its derivatives inhibits MAO-A and MAO-B with reversible type and with Ki values of 3 and 15 µM, respectively [18, 19]. This implies that the dysregulation of neurotransmitter levels is linked to illnesses, including depression, anxiety, and PD, for which isatin may have therapeutic potential. According to our group, the C-5 and C-6 positions have been more thoroughly reviewed than the N-1 and C-3 positions. Following this, our team investigated the C-3 location and discovered that IS7 (IC50 = 0.082µM), IHC3 (IC50 = 1.672µM), ISB1 (IC50 = 0.124µM), and ISFB1 (IC50 = 0.135µM) (Fig. 1) were all influential against MAO-B [20,21,22,23]. The chemical and physical properties of acyl hydrazones are typically attributed to their structural framework, which consist of two interconnected nitrogen atoms. Acyl hydrazones are widely employed in the synthesis of new compounds with MAO inhibition [24,25,26]. To improve the pharmacological characteristics of MAO inhibition, isatin-based hydrazones containing various aldehydes and ketones were incorporated in the current study.
Materials and methods
Synthetic procedure
To create the appropriate isatin hydrazones, a 2.5:1 mixture of hydrazine hydrate and isatin was dissolved in methanol (7.5 mL) and acetic acid (glacial, as the catalyst). A 1:1 combination of the intermediate and alternatively substituted aldehydes and ketones was then mixed in methanol (7.5 mL), with a catalytic amount of the acetic acid (Scheme 1) [21]. To finish the reaction, the mixture was placed in a microwave reactor (Monowave 50) for 10–20 min at 100 °C. Hexane and ethyl acetate were mixed in a 1:1 ratio and the progress of the reaction was checked by thin-layer chromatography. After filtering and air-drying, the product was cleaned and recrystallized from methanol. The 1H and 13C NMR data were included in Supplementary Information (Figs. S1 ~ S6).
Enzyme assays and kinetics
Enzyme activities of MAO-A and MAO-B activities were analyzed using the continuous assay method described [27, 28]. For enzyme kinetics, MAO-A and MAO-B activities were analyzed using five substrate concentrations, i.e., in the range of 0.0075–0.12 mM of kynuramine and 0.0375–0.6 mM of benzylamine, respectively. The Michaelis constant (Km) values of MAO-A and MAO-B were determined to be 0.045 and 0.139 mM, respectively, using Lineweaver (LB) plots.
Inhibition studies of MAO-A and MAO-B
In the primary screening, inhibitory activity against MAO-A and MAO-B at 10 µM concentration of ten compounds were evaluated. IC50 values, i.e., 50% inhibitory concentration were determined using the GraphPad Prism software 5 [29,30,31]. The curves were constructed with 3–9 points of duplicated inhibitory data. Selectivity index (SI) was calculated using the IC50 for MAO-B/IC50 for MAO-A. The inhibition characteristics of lead compounds IB3 and IB4 against both enzymes were analyzed using LB plots in the presence of the inhibitors at three concentrations, and the inhibition constants (Ki) were determined using a secondary plot derived from the LB plots [27, 28].
Reversibility studies
The reversibility of MAO-A or MAO-B inhibition by IB3 or IB4 was tested using the 6–8 kDa DiaEasy™ dialyzer, by comparing the residual activities of the undialyzed (AU) and dialyzed (AD) at ~ 2.0 ∼ IC50 after 30 min preincubation of inhibitor and enzyme, as previously described [29, 32]. Reference compounds were included for reversible and irreversible inhibitors, and their patterns were decided by comparing AU and AD of the compounds [29, 32].
Molecular docking
Using the Schrödinger suite [33], we conducted molecular docking investigations of IA3, IA4, IB3, and IB4 with the structures of human MAO-A (PDB: 2Z5Z) and MAO-B (PDB: 2V5Z) [34, 35]. The protein preparation wizard in the Schrödinger suite was used to improve and refine the crystal structures. The ligand preparations and docking simulations were from the previously reported literatures [21,22,23].
Molecular dynamic simulation
Molecular dynamics (MD) simulations were carried out using Schrödinger LLC’s Desmond simulation tool. Initially, the protein-ligand combination was conducted for the Desmond system builder panel, which targets MAO-A and MAO-B, by means of compounds IB3 and IB4. The simulation parameters were 100 ns at 300 K, 1.01325 bar of pressure, and 1000 frames [21,22,23].
In silico cytotoxicity and pharmacokinetics predictions of the compounds
The cytotoxicity and pharmacokinetics predictions of the compounds were analyzed using the ProTox-3.0 web tool (https://tox.charite.de/protox3/, accessed on June 5, 2024) for cytotoxicity, blood-brain barrier (BBB) permeability, and cytochrome P450 inhibition.
Results and discussion
Inhibition studies of MAO-A and MAO-B
Ten isatin-based derivatives of IA and IB subseries were evaluated for their MAO-A and MAO-B inhibitory activities. In this series, all compounds showed stronger MAO-A inhibition than MAO-B (Table 1). Compound IB4 exhibited the highest MAO-A inhibition (IC50 = 0.015 µM), followed by compound IB3 (IC50 = 0.019 µM). On the contrary, IB3 most greatly inhibited MAO-B (IC50 = 0.068 µM), followed by IB4 (IC50 = 0.127 µM) (Table 1).
The IC50 value of IB4 for MAO-A was lower than that of a sulfonyl isatin derivative 8 (IC50 = 4.31 µM) [36] and an isatin-based benzyloxybenzene derivative ISFB1 (IC50 = 0.678 µM) [22], but slightly higher than that of a 3-hydroxy-3-phenacyloxindole analog of isatin 18 (IC50 = 0.009) [37]. In addition, the IC50 value of IB3 for MAO-B were lower than those of a sulfonyl isatin derivative 5 (IC50 = 5.96 µM) [7], an isatin-based benzyloxybenzene derivative ISB1 (IC50 = 0.124 µM) [22], and a 3-hydroxy-3-phenacyloxindole analog of isatin 16 (IC50 = 0.111 µM) [37]. IB3 and IB4 had moderate SI values of 3.68 and 8.50 for MAO-A compared with those of MAO-B (Table 1).
Regarding the structure-activity relationship, IB series showed higher inhibition of MAO-A and MAO-B than did the IA series. However, IA series showed higher SI values for MAO-A than did the IB series. In IA series, IA5 (4-OH in B-ring) had 2.09- to 29.75-times higher MAO-A inhibition than did the other IA derivatives with substituents of -OH > -Br (IA4) > -F (IA2) > -Cl (IA3) > -H (IA1) at 4-position in B-ring in order. In addition, IA2 (4-F in B-ring) had 1.29- and 4.25-times higher MAO-B inhibition than did the other IA derivatives with substituents of -F > -OH > -Cl > -Br > -H at 4-position in B-ring in order. In IB series, IB4 (4-Br in B-ring) had 3.93- to 11.33-times higher MAO-A inhibition than did the other IB series, with substitutions of -Br > -Cl (IA3) > -F (IA2) > -H (IA1) > -OH (IA5) at 4-position in B-ring in order. Besides, IB3 (4-Cl in B-ring) had 1.87- to 24.94-times higher MAO-B inhibition than did the other IB series, with substitutions in order of -Cl > -Br > -F > -H > -OH (Fig. 2; Table 1). Collectively, IB3 and IB4 (4-Cl and 4-Br in B-ring, respectively) showed greater MAO-A and MAO-B inhibition than did the other derivatives. These results suggested that IB3 and IB4 are potent inhibitors of MAO-A and MAO-B.
Enzyme kinetics
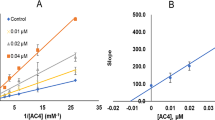
IB3 and IB4 were selected as the most potent MAO-B and MAO-A inhibitors, respectively. The kinetics of MAO-A in the presence of IB3 or IB4 were analyzed using five concentrations of kynuramine and three concentrations of inhibitor. In the LB plots, lines of IB3 or IB4 were coincided on the Y-axis with an increase in the Km values and observed to be competitive MAO-A inhibitors (Fig. 3A and C). Secondary plots, slope vs. inhibitor concentration, showed that the Ki values were 0.0088 ± 0.0015 and 0.0063 ± 0.0012 µM, respectively (Fig. 3B and D). These results suggest that IB3 and IB4 are competitive MAO-A inhibitors.
The kinetics of MAO-B inhibition by IB3 or IB4 were also analyzed using benzylamine as a substrate. In the LB plots, IB3 and IB4 were observed to be competitive inhibitors of MAO-B (Fig. 4A and C), as similar to the MAO-A inhibition. Secondary plots showed that the Ki values were 0.048 ± 0.005 and 0.060 ± 0.017 µM, respectively (Fig. 4B and D). These results suggest that IB3 and IB4 are competitive MAO-B inhibitors.
Reversibility studies
Reversibility of the inhibition by the compound was evaluated using the dialysis method. In this study, concentrations of compounds IB3 and IB4 used were ~ 2.0 times of their IC50 values (0.040 and 0.030 µM for MAO-A, and 0.14 and 0.26 µM for MAO-B, respectively). Recovery types were determined by comparing the AU and AD values. MAO inhibition by IB3 and IB4 recovered from 22.66 to 84.07% and 27.17% and 82.65%, respectively (Fig. 5A). The recovery values of IB3 and IB4 were comparable to those of the reversible inhibitor toloxatone (from 38.01 to 77.97%) and different from those of clorgyline, the irreversible inhibitor (from 27.62 to 27.41%). In addition, MAO-B inhibition by IB3 and IB4 recovered from 30.81 to 85.67% and from 33.36 to 83.64%, respectively (Fig. 5B). The recovery values of IB3 and IB4 were similar to those of the reversible inhibitor safinamide (from 31.76 to 82.51%) and different from those of pargyline, the irreversible inhibitor (from 34.50 to 35.09%). These results indicate that IB3 and IB4 are reversible-type inhibitors of MAO-A and MAO-B, respectively.
Recovery of MAO-A (A) and MAO-B (B) inhibitions by IB3 and IB4 using the dialysis experiments. The concentrations of IB3 and IB4 used were 0.040 and 0.030 μM for MAO-A, respectively; 0.14 and 0.26 μM for MAO-B, respectively. After 30 min preincubation, the mixtures were dialyzed for 6 h with twice of buffer change
Molecular docking
Extensive molecular docking analyses were conducted to gain deeper insights into the binding mechanisms of lead compounds. Interactions were observed between IB3, IB4, IA3, and IA4 along with MAO-B and MAO-A. Docking was confirmed using native ligands. The compounds were listed in Table 2, with docking scores (XP mode) ranging from − 7.16 to -10.85 kcal/mol. Through docking evaluation, the lead molecules IB3 and IB4 received scores of -10.23 and − 9.95 kcal/mol, respectively. Safinamide, on the contrary, had a score of -10.85 kcal/mol. The docking scores indicated an affinity comparable to that of biological activity (IC50). The lead molecules (IB3 and IB4) exhibited binding interactions with the MAO-B-binding pocket via hydrophobic forces (Fig. 6).
The docking scores for MAO-A, IA3, IA4, IB3, IB4, and Harmine in XP mode ranged from − 7.19 kcal/mol to -3.15 kcal/mol (Table 2). All the molecules were aligned in the same manner as the original ligands. Extensive examination of IB3 and IB4 in the active site of MAO-A revealed their presence at various positions, including Tyr69, Leu97, Phe108, Ala111, Ile180, Asn181, Ile207, Phe208, Val210, Cys323, Ile325, Ile335, Leu337, Phe352, and Tyr407 (Fig. 7). By utilizing pi–pi stacking, the interaction between rings A and B of IB3 and IB4 with Tyr407 and Phe208 was established. A comparable interaction was observed for IA3 and IA4 except for IB4 and IB3, in which Asn181 interacted with NH atom of the isatin ring through hydrogen bonding. In general, molecular docking studies suggest that the interaction between the IB series of molecules and the MAO-A pocket is more stable when hydrogen bonding occurs with the NH atom.
Molecular dynamic simulation
The binding mechanisms of IB3 and IB4 in the inhibitor-binding cavities (IBC) of MAO-A and MAO-B were monitored using MD simulations. The protein C-alpha and ligand remained within an acceptable range throughout the entire simulation duration of 100 ns, as determined by root mean square deviation (RMSD) analysis. The RMSD for the MAO-A protein is within the range of 1.06–7.58 Å for IB3 and 1.03–7.73 Å for IB4 (Fig. 8). The complex formed by IB3 and IB4 remained stable, with only a slight variation observed before 40 ns. For MAO-B, the range was 1.03–2.77 Å and 0.98–3.11 Å. It remained stable throughout the 100 ns simulation time. The RMSF for each amino acid residue in the protein was determined by the simulation in order to evaluate the system’s flexibility. High RMSF values indicate areas with flexibility, whereas low values suggest rigidity of the amino acids. The residues of MAO-A and -B exhibited significant fluctuations (Fig. 8). There were 17 interactions between IB4 and 13 interactions between IB3 and the amino acids of MAO-A. Nearly identical interactions were observed, except for a few specific residues. For MAO-B, there were 17 and 18 interactions for IB3 and IB4, respectively. Nearly identical interactions were observed, except for Tyr60, Pro102, Pro104, Trp119, and Leu328 (Table 3). There were similarities in the manner in which the ligand interacts in 2-D during docking and in the subsequent simulation, suggesting that the ligand might have the ability to inhibit MAO in a non-specific manner.
In silico cytotoxicity and pharmacokinetics predictions
The cytotoxicity and pahrmacokinetics of IB3 and IB4 using ProTox-3.0 web tool showed these two compounds had non-toxic and BBB permeability (Table 4). In addition, these compounds showed CYP450 inhibition including the CYP1A2 (Table 4). These results suggest that compounds IB3 and IB4 can be used as therapeutic drugs, but careful consideration of the dosage is required.
Ultimately, the synthesis of isatin-based hydrazone derivatives from two sub-series, IA and IB, was successfully performed, and their effects on MAOs were evaluated. Both series showed a higher level of prominence for MAO-A than MAO-B. IB3 and IB4 are reversible and competitive inhibitors against MAO-B (IC50 = 0.068 and 0.127 µM, respectively) with low selectivity (SI = 3.68 and 8.50, respectively). Furthermore, the stability of the complex was determined through docking analysis of MAO-A and MAO-B with IB3 and IB4. Stable hydrogen bonding between Asn181 and the NH atom of isatin forms a ligand-protein complex, similar to the work of a pharmacologist. It is possible to conclude from dynamic analysis that IB3 and IB4 are stable when both MAO isoforms are present. In addition, IB3 and IB4 were predicted to be non-toxic and to have BBB permeability using an in silico study. To sum up, the findings of this research indicate that IB3 and IB4 may find use in the management of depression and PD.
Data availability
All data generated or analyzed during the present study are included in this published article.
References
Bugalia S, Dhayal Y, Sachdeva H, Kumari S, Atal K, Phageria U, Saini P, Gurjar OP (2023) Review on Isatin- A Remarkable Scaffold for Designing PotentialTherapeutic complexes and its Macrocyclic complexes with Transition metals. J Inorg Organomet Polym Mater 33:1782–1801
Sadeghian Z, Bayat M (2022) Synthesis of heterocyclic compounds based on isatins. Curr Org Chem 26(8):756–770
Nath P, Mukherjee A, Mukherjee S, Banerjee S, Das S, Banerjee S (2021) Isatin: a scaffold with immense biodiversity. Mini Rev Med Chem 21(9):1096–1112
Singh GS, Desta ZY (2012) Isatins as privileged molecules in design and synthesis of spiro-fused cyclic frameworks. Chem Rev 112(11):6104–6155
Kakkar R (2019) Isatin and its derivatives: a survey of recent syntheses, reactions, and applications. MedChemComm 10(3):351–368
Brandao P, Marques C, Burke AJ, Pineiro M (2021) The application of isatin-based multicomponent-reactions in the quest for new bioactive and druglike molecules. Eur J Med Chem 211:113102
Nath R, Pathania S, Grover G, Akhtar MJ (2020) Isatin containing heterocycles for different biological activities: analysis of structure activity relationship. J Mol Struct 1222:128900
Kumar R (2023) Exploring the antimicrobial potential of isatin and derivatives: a comprehensive review. World J Pharm Res 12(3):1224–1246
Edmondson DE, Binda C (2018) Monoamine oxidases. Membrane protein complexes: Structure and function, pp.117–139
Edmondson DE, Binda C, Wang J, Upadhyay AK, Mattevi A (2009) Molecular and mechanistic properties of the membrane-bound mitochondrial monoamine oxidases. Biochemistry 48(20):4220–4230
Jones DN, Raghanti MA (2021) The role of monoamine oxidase enzymes in the pathophysiology of neurological disorders. J Chem Neuroanat 114:101957
Yeung AWK, Georgieva MG, Atanasov AG, Tzvetkov NT (2019) Monoamine oxidases (MAOs) as privileged molecular targets in neuroscience: research literature analysis. Front Mol Neurosci 12:143
Graves SM, Xie Z, Stout KA, Zampese E, Burbulla LF, Shih JC, Kondapalli J, Patriarchi T, Tian L, Brichta L, Greengard P (2020) Dopamine metabolism by a monoamine oxidase mitochondrial shuttle activates the electron transport chain. Nat Neurosci 23(1):15–20
Manzoor S, Hoda N (2020) A comprehensive review of monoamine oxidase inhibitors as Anti-alzheimer’s disease agents: a review. Eur J Med Chem 206:112787
Youdim MB, Bakhle YS (2006) Monoamine oxidase: isoforms and inhibitors in Parkinson’s disease and depressive illness. Br J Pharmacol 147(S1):S287–S296
Gillman PK (2016) Monoamine oxidase inhibitors: a review concerning dietary tyramine and drug interactions. Psycho Trop Commentaries 1:1–90
Suchting R, Tirumalaraju V, Gareeb R, Bockmann T, de Dios C, Aickareth J, Pinjari O, Soares JC, Cowen PJ, Selvaraj S (2021) Revisiting monoamine oxidase inhibitors for the treatment of depressive disorders: a systematic review and network meta-analysis. J Affect Disord 282:1153–1160
Prinsloo IF, Petzer JP, Cloete TT, Petzer A (2023) The evaluation of isatin analogues as inhibitors of monoamine oxidase. Chem Biol Drug Des 102(5):1067–1074
Tripathi RK, Goshain O, Ayyannan SR (2013) Design, synthesis, in vitro MAO-B inhibitory evaluation, and computational studies of some 6‐nitrobenzothiazole‐derived semicarbazones. ChemMedChem 8(3):462–474
Kumar S, Nair AS, Abdelgawad MA, Mathew B (2022) Exploration of the detailed structure–activity relationships of isatin and their isomers as monoamine oxidase inhibitors. ACS Omega 7(19):16244–16259
Manoharan A, Oh JM, Benny F, Kumar S, Abdelgawad MA, Ghoneim MM, Shaker ME, El-Sherbiny M, Almohaimeed HM, Gahtori P, Kim H (2023) Assembling a Cinnamyl Pharmacophore in the C3-Position of substituted isatins via microwave-assisted synthesis: development of a New Class of Monoamine Oxidase-B inhibitors for the treatment of Parkinson’s Disease. Molecules 28(16):6167
Benny F, Oh JM, Kumar S, Abdelgawad MA, Ghoneim MM, Abdel-Bakky MS, Kukerti N, Jose J, Kim H, Mathew B (2023) Isatin-based benzyloxybenzene derivatives as monoamine oxidase inhibitors with neuroprotective effect targeting neurogenerative disease treatment. RSC Adv 13(50):35240–35250
Kumar S, Oh JM, Prabhakaran P, Awasti A, Kim H, Mathew B (2024) Isatin-tethered halogen-containing acylhydrazone derivatives as monoamine oxidase inhibitor with neuroprotective effect. Scientific Reports, 14(1), p.1264
Raczuk E, Dmochowska B, Samaszko-Fiertek J, Madaj J (2022) Different Schiff bases—structure, importance and classification. Molecules, 27(3), p.787
El-Etrawy AAS, Sherbiny FF (2021) Design, synthesis, biological evaluation and molecular modeling investigation of new N’-(2-Thiouracil-5-oyl) hydrazone derivatives as potential anti-breast cancer and anti-bacterial agents. J Mol Struct 1232:129993
Tok F, Sağlık BN, Özkay Y, Ilgın S, Kaplancıklı ZA, Koçyiğit-Kaymakçıoğlu B (2021) Synthesis of new hydrazone derivatives and evaluation of their monoamine oxidase inhibitory activity. Bioorg Chem 114:105038
Lee HW, Ryu HW, Kang M-G, Park D, Oh S-R, Kim H (2016) Potent selective Monoamine Oxidase B inhibition by Maackiain, a Pterocarpan from the roots of Sophora Flavescens. Bioorg Med Chem Lett 26:4714–4719
Oh JM, Jang H-J, Kim WJ, Kang M-G, Baek SC, Lee JP, Park D, Oh S-R, Kim H (2020) Calycosin and 8-O-methylretusin isolated from Maackia amurensis as potent and selective reversible inhibitors of human monoamine oxidase-B. Int J Biol Macromol 151:441–448
El-Damasy AK, Oh JM, Kim HJ, Mun SK, Al-Karmalawy AA, Alnajjar R, Choi YJ, Kim JJ, Nam G, Kim H, Keum G (2024) Novel coumarin benzamides as potent and reversible monoamine oxidase-B inhibitors: design, synthesis, and neuroprotective effects. Bioorg Chem 142:106939
Baek SC, Park MH, Ryu HW, Lee JP, Kang M-G, Park D, Park CM, Oh S-R, Kim H (2019) Rhamnocitrin isolated from Prunus Padus Var. Seoulensis: a potent and selective reversible inhibitor of human monoamine oxidase A. Bioorg Chem 83:317–325
Lee HW, Ryu HW, Kang M-G, Park D, Lee H, Shin HM, Oh S-R, Kim H (2017) Potent inhibition of Monoamine Oxidase A by Decursin from Angelica Gigas Nakai and by Wogonin from Scutellaria baicalensis Georgi. Int J Biol Macromol 97:598–605
Oh JM, Jang HJ, Kang MG, Mun SK, Park D, Hong SJ, Kim MH, Kim SY, Yee ST, Kim H (2022) Medicarpin and homopterocarpin isolated from Canavalia lineata as potent and competitive reversible inhibitors of human monoamine oxidase-B. Molecules 28:258
Shaw DE (2021) Desmond molecular dynamics system. MaestroDesmond interoperability tools. Research, Schrodinger Release
Binda C, Wang J, Pisani L, Caccia C, Carotti A, Salvati P, Edmondson DE, Mattevi A (2007) Structures of human monoamine oxidase B complexes with selective noncovalent inhibitors: safinamide and coumarin analogs. J Med Chem 50:5848–5852
Binda C, Li M, Hubálek F, Restelli N, Edmondson DE, Mattevi A (2003) Insights into the mode of inhibition of human mitochondrial monoamine oxidase B from high-resolution crystal structures. Proc Natl Acad Sci USA 100:9750–9755
Tavari M, Malan SF, Joubert J (2016) Design, synthesis, biological evaluation and docking studies of sulfonyl isatin derivatives as monoamine oxidase and caspase-3 inhibitor. Med Chem Commun 7:1628–1639
Tripathi RK, Krishnamurthy S, Ayyannan SR (2016) Discovery of 3-hydroxy-3-phenacyloxindole analogues of isatin as potential monoamine oxidase inhibitors. Chem Med Chem 11:119–132
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research and Graduate Studies at King Khalid University for funding this work through Large Research Project under the grant number RGP2/473/45.
Funding
This study was supported by the Deanship of Scientific Research and Graduate Studies at King Khalid University under the grant number RGP2/473/45.
Author information
Authors and Affiliations
Contributions
Conceptualization, B.M., H.K., N.M.; Chemical synthesis, N.M., S.K.; Biological evaluation, J.M.O., H.K.; Computational studies, P.G., S.K., A.A.B., B.M.; Writing—original draft preparation, J.M.O., N.M., B.M., S.K.; Writing—review and editing, B.M., H.K.; Supervisions, B.M., H.K. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maliyakkal, N., Oh, J.M., Kumar, S. et al. Synthesis, biochemistry, and in silico investigations of isatin-based hydrazone derivatives as monoamine oxidase inhibitors. Appl Biol Chem 67, 63 (2024). https://doi.org/10.1186/s13765-024-00917-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-024-00917-3