Abstract
The physiological changes caused by the decline in estrogen levels due to menopause are linked to an increased risk of depression. This study investigated the antidepressant effects of hyperoside (HYP), a natural flavonol glycoside, and its associated molecular mechanisms in primary hippocampal neurons and ovariectomized (OVX) mice. HYP treatment increased nitric oxide (NO) production and neuronal nitric oxide synthase (nNOS) expression in primary hippocampal neurons; additionally, it upregulated the expression of brain-derived neurotrophic factor (BDNF) and phosphorylated tropomyosin receptor kinase B (TrkB). In OVX mice, HYP treatment significantly improved depression-like behaviors in an open field test to a level comparable to estrogen treatment. Furthermore, HYP treatment upregulated OVX-induced decreased nNOS expression and BDNF-TrkB signaling in the hippocampus. Therefore, this study suggests that HYP exhibits antidepressant potential by addressing estrogen deficiency-induced alterations, specifically by restoring nNOS expression, promoting NO production, and concurrently enhancing BDNF-TrkB signaling in OVX mice.
Similar content being viewed by others
Introduction
The reduction in estrogen levels precipitated by menopause elicits noteworthy psychological and physiological alterations in women, notably manifesting as depression [1]. As a result, women going through menopause are at a higher risk of experiencing clinically significant depressive symptoms compared to those who are premenopausal [2]. Hormone replacement therapy (HRT) has been used as an efficacious intervention for menopausal depression [3, 4]. Nevertheless, owing to the association of HRT with significant adverse effects, notably an elevated risk of breast cancer [5], investigations into safer antidepressant alternatives, including phytochemicals and dietary supplements, are underway to address postmenopausal depressive symptoms [6, 7].
The hippocampus exhibits a close association with depression, and nitric oxide (NO), produced by neuronal nitric oxide synthase (nNOS) expressed in the hippocampus, is recognized for its pivotal involvement in depression-related behaviors [8]. Both excess and shortage of NO in the hippocampus appear to be associated with the pathology of depression, possibly because some sex hormones affect hippocampal nNOS expression, leading to sex-differentiated effects of NO levels on depression [9]. Studies indicate that a deficiency of estrogen in females may contribute to depression by reducing the expression of nNOS and the production of NO. Estrogen promotes nNOS expression via estrogen receptor β [10]. In female mice, stress impedes hippocampal nNOS expression by suppressing estrogen secretion, resulting in a deficit of NO and consequent depression. Conversely, 17β-estradiol (E2) upregulates nNOS to counteract ovariectomy (OVX)-induced hippocampal NO decrease [9]. Additionally, in postmenopausal women, estrogen replacement therapy has been associated with increased plasma levels of NO, whereas the discontinuation of estrogen replacement therapy significantly reduces NO levels [11].
Brain-derived neurotrophic factor (BDNF) is a crucial neurotrophic factor, and several studies have demonstrated that antidepressants exert their antidepressant effects via BNDF-dependent signaling. BNDF binds to its receptor tropomyosin receptor kinase B (TrkB), and is associated with diverse neural functions in the hippocampus [7, 12]. In animal studies, estrogen deprivation induced by OVX resulted in diminished levels of hippocampal and cortical BDNF levels [13], and this reduction is thought to be mediated by the signal transduction pathway involving nNOS and NO. Specifically, the nNOS-NO signaling pathway serves to upregulate BDNF gene expression [14]. Consequently, the decrease in nNOS-NO expression due to estrogen deprivation is anticipated to inhibit BDNF-TrkB signaling, thereby potentially contributing to depressive manifestations.
Hyperoside (quercetin-3-O-b-D-galactosidepyranose; HYP) is a flavonol glycoside compound, is predominantly present in natural plants, such as rosa multiflora fruit. Studies have shown that the diverse biological effects of HYP, including antioxidant [15, 16], anti-inflammatory [16, 17], anti-atherosclerotic [18], and anti-cancer [19]. Moreover, HYP has demonstrated neuroprotective effects in rodent models of Alzheimer’s disease [20], Parkinson’s disease [21], and diabetes mellitus-induced cognitive impairment [16]. Furthermore, in vitro and animal studies have reported results on the antidepressant effects of HYP [15, 22].
This antidepressant efficacy of HYP have been reported to be mediated by monoamine neurotransmitters [23]. However, there is limited research elucidating the definitive mechanisms underlying these effects. Certain investigations have documented the modulation of BDNF expression as a mechanism through which HYP exerts antidepressant effects. It has been reported that HYP upregulates the expression of BDNF and TrkB [17], exerting antidepressant effects by upregulating hippocampal BDNF in rodents with chronic mild stress-induced depression [15]. Limited research has been conducted on the effect of HYP on the expression of NOS and NO. HYP inhibited NOS activity in rat cerebral homogenates [24] and reduced NO production in brain tissue from male mice with Parkinson’s disease [25]. Given the sex-differentiated effect of NO levels on depression, it is anticipated that the effects of HYP on NO may vary during estrogen deprivation. Nevertheless, the effects of HYP on estrogen deficiency-induced depression, along with its underlying mechanisms, remain unexplored.
Therefore, the primary objective of this study is to examine the potential antidepressant effects of HYP on estrogen-deprivation-induced depression in OVX mice, a model of menopausal depression. Additionally, we aim to elucidate the molecular mechanisms underlying these effects, with a focus on the modulation of nNOS expression, NO production, and BDNF-TrkB expression.
Materials and methods
Cell culture
The hippocampus of ICR mice at postnatal day 0 were dissected and immersed in Hanks’ balanced salt solution (HBSS) supplemented with 1 mM sodium pyruvate and 10 mM HEPES. After incubation, the hippocampal tissue underwent dissociation in HBSS with 2.5% trypsin. The dissociated tissue was triturated through repeated pipetting. Then supernatant was centrifuged at 16,000 rpm for 5 min. The pellet was resuspended in a Neurobasal medium A. Subsequently, the cells were plated on poly-L-ornithine-coated (5 mg/mL) 6-well plates. Following the incubation, the cells were exposed to 10 nM E2 and 0.1 or 1 µM HYP, and subjected to measurements of NO content, immunofluorescence analysis, and western blot analysis (IACUC number, KFRI-M-22059).
Measurement of NO contents
Primary hippocampal neurons were plated in 96-well plates at a density of 1 × 104 cells/well and cultured for 14 days. After a 24-h treatment with samples, NO levels were assessed. Intracellular NO levels were determined using DAF-FM, a photo-stable NO-specific indicator. Following a 24 h treatment of primary hippocampal neurons with samples, cells were rinsed with PBS. Subsequently, DMEM (phenol red-free) containing DAF-FM (10 µM) was added to the cells and incubated for 45 min at 37 °C. DAF-FM fluorescence was quantified using a fluorescence microplate reader (excitation: 495 nm and emission: 515 nm). Visualization of NO was conducted through fluorescence microscopy (Zeiss, Thornwood, NY, USA), and the count of cells exhibiting a positive NO signal was measured.
Immunofluorescence analysis
The quantification of nNOS intensity was conducted through an immunofluorescence assay as previously described [26]. Primary hippocampal neurons were seed in 12-well plates at a density of 1 × 105 cells/well. The cells were treated with samples for 24 h. Post-treatment, cells were rinsed with PBS and fixed with 4% paraformaldehyde. Subsequently, fixed cells were permeabilized and blocked through incubation with a mixture of 1% goat and donkey serum for 1 h at RT. The cells were then subjected to anti-nNOS antibody at RT for 2 h (Table 1). Following antibody incubation, the cells underwent three PBS washes and were subsequently incubated with secondary antibodies for 1 h at RT. Coverslips, used for mounting on slides, were contained a DAPI-containing mounting solution for nuclei staining. Immunofluorescence images were captured through a confocal microscope (Zeiss), and quantification of nNOS intensity was performed.
Animals
All animal-related procedures were performed with ethical approval from the Institutional Animal Care and Use Committee of the Korea Food Research Institute (IACUC number: KFRI-M-19037). Female C57BL/6 N mice (5 weeks) were obtained from KOATECH Animal Inc. (Pyeongtaek, Korea). The housing environment was maintained at 21 ± 2 °C, with a 12 h light, 12 h dark cycle. A minimum acclimatization period of one week was observed before commencing the experimental procedures.
Ovariectomy
After acclimatization period, 6 weeks aged mice were ovariectomized. Mice were anesthetized with 2% isoflurane, and the abdominal region was prepared for surgery. A midline abdominal incision was made, and the ovaries were carefully exposed. The ovarian vessels were tied off, severed, and both ovaries were removed via small bilateral dorsal flank incisions. The abdominal wall was sutured, and the skin incision was closed using surgical staples. Sham-operated mice underwent a similar anesthesia and surgical procedure, without removal of the ovaries. Mice received postoperative care, including administration of analgesics, to ensure their well-being during the recovery period (2-week).
Treatment
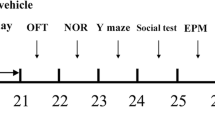
The OVX mice were divided into four groups (n = 9 per group): Sham-operated group (SHAM), OVX control group (OVX), OVX with E2 treatment (OVX + E2, 10 µg/kg), and OVX with HYP treatment (OVX + HYP30, 30 mg/kg). E2 was administered intraperitoneally (i.p.) in the abdominal cavity using a solution of 1% DMSO and 1% Tween 80 in saline. HYP dosages were based on previously published study and our preliminary experiments [27]. Throughout the 24-week experimental period, treatments were administered once daily. Behavioral assessments (open field test) were conducted after 24 weeks. After sacrifice, brain tissues were promptly isolated and kept at -70 °C for subsequent analysis. The experimental design is illustrated in Fig. 3A.
Open field test
Depressive-like behavior was assessed using the Open Field Test (OFT) following a previously described protocol [28]. Each mouse was individually placed in the center of the open field and allowed to move freely. The total distance traveled was observed for 5 min to analyze locomotor activity (SMART software, Panlab SL, Barcelona, Spain).
Western blotting
The brain tissue was homogenized in RIPA buffer with treatment with protease and phosphatase inhibitors. Equal amounts of proteins were loaded onto an 8–10% SDS-PAGE gel, and electrophoresis was conducted to separate the proteins. The proteins were then transferred to a PVDF membrane, which was blocked with 5% non-fat dry milk/TBST blocking solution. After overnight incubation with the primary antibody, the membrane was exposed to an HRP-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Visualization of the membrane was accomplished using the ECL solution (Bio-Rad, Mississauga, Ontario, Canada), and ImageJ software (National Institutes of Health, Bethesda, Maryland, USA) was employed for relative intensity analysis and quantification. β-actin served as a control for protein normalization.
Statistical analysis
The result is presented as mean ± standard error (SE) and analyzed using one-way analysis of variance, followed by Tukey’s post-hoc test using Prism 10 (GraphPad Software, Inc., San Diego, CA, USA). Statistical significance was set at P < 0.05.
Results
HYP increased NO generation and nNOS expression in primary hippocampal neurons
We investigated whether HYP affects NO production and nNOS expression. HYP treatment significantly increased intracellular NO levels in primary hippocampal neurons, i.e., the number of NO + cells in HYP-treated hippocampal neurons increased by 2.4-fold (0.1 µM) and 2.7-fold (1 µM) compared to controls, respectively, to levels not significantly different from those in E2-treated cells (P < 0.05) (Fig. 1B). In addition, expression of the NO-producing enzyme nNOS in hippocampal neurons was also significantly increased by HYP treatment, with nNOS expression in HYP 1 µM-treated cells increasing by 1.8-fold compared to controls (P < 0.01), similar to E2 treatment (Fig. 1C). Immunofluorescence analysis also showed that HYP (1 µM) treatment increased nNOS expression in hippocampal neurons (P < 0.05) (Fig. 2).
HYP upregulates the NO production and the expression of nNOS, BDNF, and phosphorylated TrkB in primary hippocampal neurons. (A) Chemical structure of HYP. (B) NO production in neuronal cells was assessed by treating cells with 10 nM (E2) and 0.1 or 1 µM HYP for 24 h. NO levels were measured using the indicator DAF-FM, and fluorescence quantification was done with a microplate reader (excitation: 495 nm, emission: 515 nm). Visualization and cell count of positive NO signals were performed using fluorescence microscopy. (C) Cultured hippocampal neuronal cells were treated with 10 nM E2 and 0.1 or 1 µM HYP for 24 h. Western blot showing the protein expression of nNOS, BDNF and phosphorylated TrkB. Results are presented as mean ± SE. *P < 0.05, **P < 0.01, *** P < 0.001 vs. CTL (vehicle-only treated cell). BDNF, brain-derived neurotrophic factor; E2, 17β-estradiol; HYP, hyperoside; nNOS, neuronal nitric oxide synthase; NO, nitric oxide; TrkB, tropomyosin receptor kinase B
Immunofluorescence analysis showing HYP increased nNOS expression in primary hippocampal neurons. Cultured hippocampal neuronal cells were treated with 10 nM E2 and 0.1 or 1 µM HYP for 24 h. Cells were incubated with primary nNOS antibody (red) and a neuronal marker neurofilament (NF, green). Cells were then incubated with secondary antibody, and nuclei were stained with DAPI (blue). Confocal microscopy captured immunofluorescence images, and nNOS intensity was quantified. The merged image shows the nNOS-positive cells. Results are presented as mean ± SE. *P < 0.05, **P < 0.01, ***P < 0.001 vs. CTL (vehicle-only treated cell). E2, 17β-estradiol; HYP, hyperoside; nNOS, neuronal nitric oxide synthase; NO, nitric oxide
HYP upregulated BDNF-TrkB signaling in primary hippocampal neurons
We investigated whether HYP affects BDNF-TrkB signaling. HYP treatment remarkably upregulated the expression of BDNF and phosphorylated TrkB, an activated form of the BDNF receptor, in primary hippocampal neurons (Fig. 1C). BDNF protein levels in HYP-treated cells increased dose-dependently and were significantly higher than those in E2-treated cells, 11.8% (HYP 0.1 µM) and 111.2% (HYP 1 µM) higher than those in E2-treated cells. Consistent with these results, the phosphorylated TrkB was significantly increased in HYP (1 µM) treated cells (P < 0.05), showing that HYP upregulated TrkB activation. Therefore, these results indicate that HYP treatment upregulated BDNF-TrkB signaling in hippocampal neurons as much or more than E2.
HYP improved the depression-like behavior in OVX mice
Next, we examined whether HYP affects estrogen-deficiency induced depression in OVX mice, by conducting an OFT (Fig. 3B). In OVX group, the total distance of movements was significantly decreased compared to SHAM group (P < 0.01), which was significantly increased by E2 treatment (P < 0.05) in the OFT. The total distance of movements in the HYP-treated group was 29.6% higher than that in the OVX group (P < 0.05), demonstrating that HYP treatment can significantly improve depression-like behavior, comparable to E2 treatment in OVX mice.
HYP restored the depression like behavior and the protein expression of nNOS, BDNF, and phosphorylated TrkB in OVX mice. (A) Experimental design and schedule. OVX mice were orally administered E2 (10 µg/kg) or HYP (30 mg/kg) for 24 weeks. (B) Depression-like behaviors were assessed with the open field test. The locomotor activity was analyzed by recording the total distance traveled in 5 min. (C) Western blot showing the protein expression of nNOS, BDNF and phosphorylated TrkB. Results are presented as mean ± SE. ##P < 0.01, ###P < 0.001 vs. Sham mice; *P < 0.05, ***P < 0.001vs. OVX mice. BDNF, brain-derived neurotrophic factor; E2, 17β-estradiol; HYP, hyperoside; nNOS, neuronal nitric oxide synthase; NO, nitric oxide; OFT, open field test; OVX, ovariectomized; TrkB, tropomyosin receptor kinase B
HYP upregulated hippocampal nNOS expression and BDNF-TrkB signaling in OVX mice
To understand the mechanism of the antidepressant effect of HYP, we determined the protein expression of nNOS, BDNF, and phosphorylated TrkB in the hippocampus (Fig. 3C). The expression of nNOS, BDNF, and phosphorylated TrkB in OVX mice were significantly decreased compared to sham mice, and were significantly increased by E2 treatment. As expected, in OVX mice, we found similar results to the effects of HYP in primary hippocampal neurons. HYP treatment significantly upregulated the OVX-induced decrease in the expression of nNOS (12.9-fold, P < 0.001), BDNF (6.1-fold, P < 0.001), and phosphorylated TrkB (14.3-fold, P < 0.001) to levels comparable to those observed with E2 treatment. These results suggest that HYP treatment restored the decrease in hippocampal nNOS expression and BDNF-TrkB signaling induced by estrogen deprivation.
Discussion
In the present study, we investigated the effects of HYP treatment on depression-like behavior, as well as the modulation of nNOS-NO and BDNF-TrkB signaling in OVX mice. Our aim was to elucidate the potential effectiveness of HYP in alleviating estrogen deficiency-induced depression and its molecular mechanisms.
Our results showed that HYP treatment elevated locomotor movement in the OFT to a magnitude comparable to estrogen treatment, thereby mitigating the reduction induced by OVX, suggesting that HYP ameliorates depression-like behavior in OVX mice. Previous studies have reported results on the ameliorative efficacy of HYP on depression in vitro and animal studies. Specifically, HYP protected against corticosterone-induced neurotoxicity in PC12 cells [22]. Furthermore, HYP has been demonstrated to reduce chronic stress-induced increased immobility time in behavioral tests such as the forced swimming test [15, 29, 30] and tail-suspension test [30], restoring depression-like behavior in rodents. HYP has also demonstrated effectiveness in the recovery of cognitive impairment in rats subjected to chronic stress [29].
Additionally, HYP exhibited antidepressant effects in rodents by modulating monoamine neurotransmitters such as dopamine [31], serotonin and catecholamine in the central nervous system [23].
Our results suggest that the antidepressant effects of HYP in OVX mice are associated with NO in the hippocampus; however, research findings regarding the relationship between NO and depression are controversial. The hippocampus, where nNOS is expressed, is closely related to major depressive disorder (MDD), and NO produced from the nNOS positive neurons plays an imperative role in MDD, with the nNOS-NO pathway recognized for its significance in depression-related behaviors [8]. Moreover, in human study, elevated NO levels have been observed in individuals with depression, and inhibitors of NOS have demonstrated antidepressant properties [32]. In animal study, increased NO production by nNOS in the hippocampus has been documented to exert an adverse effect on neurogenesis, consequently inducing depressive-like behavior [33]. NO has been reported to cause depressive-like behaviors by modulating the guanylate cyclase pathway [34]. While the pivotal role of NO in the pathology of depression is evident, on the contrary, human studies reveal diminished NO levels in individuals experiencing severe depression [32], and reduced levels of nNOS have been observed in the locus coeruleus of depressed patients, concomitant with alterations in excitatory neurotransmitters [35]. Both an excess and a shortage of NO in the hippocampus seem to be associated with the pathology of depression, which is inferred to be attributed to the effects of certain sex hormones on nNOS expression. According to Hu et al., in male mice, stress has been demonstrated to increase glucocorticoid secretion, subsequently promotes nNOS expression in the hippocampus, resulting in excess NO production, leading to depression. Conversely, in female mice, stress impedes estrogen secretion, subsequently inhibits hippocampal nNOS expression and resulting in a shortage of NO, which leads to depression [9].
Estradiol mediates estrogen receptor β to promote nNOS expression [10], consequently, estradiol has been demonstrated to upregulate nNOS, restoring the OVX-induced hippocampal NO decrease and exhibit antidepressant effects [9]. In addition, in postmenopausal women, estrogen replacement therapy has been associated with increased plasma levels of NO [11, 36]. Conversely, estrogen deprivation in female mice led to a reduction in hippocampal NO [9], and suspension of estrogen replacement therapy in postmenopausal women significantly decreased plasma NO levels [11]. Thus, in females, estrogen deprivation may contribute to depression due to diminished nNOS expression and NO production. In a preceding study, depressive-like behaviors were induced in OVX mice characterized by reduced hippocampal nNOS and NO expression, and treatment with 17β-estradiol (E2) ameliorated both [26]. Consistent with these findings, we observed depression-like behavior was observed in OVX mice, concomitant with a reduction in hippocampal nNOS expression. In addition, E2 treatment increased nNOS expression and NO production in primary hippocampal neurons, restoring the diminished nNOS expression induced by OVX in mice. Moreover, the findings of this study indicate that HYP demonstrated effects on nNOS expression and NO production akin to those of estrogen. Specifically, HYP treatment upregulated nNOS expression and NO production in primary hippocampal neurons, effectively normalizing the OVX-induced reduction in hippocampal nNOS expression in OVX mice to levels comparable to estrogen treatment. Collectively, these findings suggest that HYP alleviates depression-like behaviors by regulating nNOS-NO in the hippocampus. However, some prior studies have presented contradictory findings concerning the impact of HYP on NOS expression and the generation of NO. In vitro studies have shown inhibitory effects of HYP on inducible NOS (iNOS) expression in ischemic cortical neurons [37] and NOS activity in rat cerebral homogenate [24]. In addition, in male mice with induced Parkinson’s disease, HYP reduced NO production in brain tissue [25]. The discrepancies between these findings and the current study results may be attributed to variations in the type and site of NOS expression, as well as differences induced by the influence of sex hormones. Therefore, it is inferred that the effects of HYP on hippocampal nNOS expression under estrogen deficiency induced by OVX, the model employed in this study, are distinct.
The antidepressant efficacy of HYP, as observed in this study, were accompanied by an increase in BDNF. BDNF is a pivotal neurotrophic factor, and the efficacy of antidepressants in ameliorating depression mediated through BNDF-dependent signaling has been substantiated in several studies. BNDF, by binding to and activating its receptor TrkB is essential for the neural development and maintenance of normal brain function. In particular, BDNF-TrkB signaling promotes hippocampal neurogenesis and increases neuroplasticity, resulting in antidepressant effects [7, 12]. Polyphenols have been reported to exhibit antidepressant effects by upregulating BNDF-TrkB signaling, contributing to the restoration of plasticity in hippocampal nuclei and alleviation of injury [6]. In our study, a reduction in hippocampal BDNF expression was observed in OVX mice, and HYP treatment reversed this reduction. Similarly, previous research indicated that OVX resulted in reduced levels of BDNF in the hippocampus and cortex, and the activation of BDNF signaling ameliorated depression-like behavior in OVX mice [13]. The decrease in BDNF induced by OVX appears to be mediated by the signal transduction pathway of nNOS-NO. The activation of guanylyl cyclase by NO leads to the conversion of GTP to cGMP, subsequently triggering the activation of protein kinase G (PKG) and extracellular signal-regulated kinase (ERK). This cascade ultimately results in the activation of CREB, contributing to the upregulation of BDNF gene expression [14]. Therefore, it can be postulated that the antidepressant effects of HYP in OVX mice may stem from the restoration of the inhibition of the nNOS-NO pathway induced by estrogen deficiency in OVX mice, leading to the upregulation of BDNF-TrkB signaling. In line with our findings, a prior study showcased that the augmented ERK-CREB-BDNF signaling pathway confers antidepressant effects in stress hormone-treated mice [38], and the elevation of nNOS-NO and ERK-CREB-BDNF-TrkB signaling ameliorates OVX-induced depressive-like behaviors [26]. In line with our study, previous studies have reported the upregulation of BDNF expression by HYP. In an in vitro study, HYP demonstrated the inhibition of neuroinflammation by upregulating BDNF and TrkB expression [17], and it exhibited a neuroprotective effect by increasing brain BDNF expression in a mouse model of Parkinson’s disease [25]. Moreover, consistent with our findings, in vitro and animal studies have elucidated the antidepressant effects mediated by HYP’s BDNF signaling. HYP showed a protective effect against neurotoxicity in PC12 cells by increasing BDNF expression [22]. Furthermore, HYP exerted antidepressant effects by upregulating hippocampal BDNF in chronic mild stress-induced depression [15, 29, 30] and monoaminergic synthesis inhibition-induced depression [23] in rodents. Taken together, the antidepressant effects of HYP in OVX mice appear to be attributed to the restoration of reduced nNOS expression caused by estrogen deprivation, thereby increasing NO production, which in turn upregulated BDNF-TrkB signaling, promoting neurogenesis in the hippocampus.
In several studies, it has been demonstrated that monoaminergic antidepressants exert antidepressant effects through the BNDF-dependent pathway, and BDNF has been reported to influence serotonergic and dopaminergic neurotransmission [12, 13]. In addition, the antidepressant effects of HYP through BDNF signaling are reported to mediate the regulation of monoamine neurotransmission, including serotonin and catecholamine [23, 31]. Moreover, increased oxidative stress in neurodegenerative disorders has been reported to be associated with decreased BDNF expression [15], and HYP alleviated oxidative stress and elevated BDNF expression in the brain of mice with Parkinson’s disease [25]. However, this study has limitations in understanding the integrative neurophysiological mechanisms through which HYP improves depression induced by estrogen deficiency via BDNF-TrkB signaling, considering factors such as neurotransmitter modulation and neuroprotection through antioxidant effects. Therefore, further study is needed to elucidate the effects of HYP on the monoaminergic system and oxidative stress in depression induced by estrogen deficiency and to clarify the relationship between these factors and BDNF signaling, aiming to provide a clearer understanding of the antidepressant mechanisms of HYP. Figure 4 presents the schematic for the mechanism underlying the effect of HYP against OVX-induced depression observed in this study.
In conclusion, these findings provide evidence that HYP demonstrates antidepressant-like effects partially mediated by the restoration of diminished hippocampal nNOS expression caused by estrogen deficiency, thereby promoting NO production in estrogen deficiency-induced depressive mice. Moreover, the antidepressant activity of HYP might be mediated by the upregulation of BDNF-TrkB signaling by elevated NO in the hippocampus. Taken together, these research findings offer foundational data supporting the application of HYP as a novel candidate for improving menopausal depression.
A schematic diagram illustrating the mechanism underlying the antidepressant effects of HYP in OVX mice. HYP upregulates nNOS expression induced by estrogen deprivation, thereby restoring NO production in the hippocampus. Subsequently, it enhances BDNF-TrkB signaling, contributing to the amelioration of depression-like behavior in OVX mice. BDNF, brain-derived neurotrophic factor; HYP, hyperoside; nNOS, neuronal nitric oxide synthase; NO, nitric oxide; OVX, ovariectomized; TrkB, tropomyosin receptor kinase B
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Steiner M, Dunn E, Born L (2003) Hormones and mood: from menarche to menopause and beyond. J Affect Disord 74(1):67–83
Cohen LS, Soares CN, Vitonis AF, Otto MW, Harlow BL (2006) Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Arch Gen Psychiatry 63(4):385–390
Rasgon NL, Altshuler LL, Fairbanks LA, Dunkin JJ, Davtyan C, Elman S, Rapkin AJ (2002) Estrogen replacement therapy in the treatment of major depressive disorder in perimenopausal women. J Clin Psychiatry 63 Suppl 7:45–48
Pickar JH, Archer DF, Kagan R, Pinkerton JV, Taylor HS (2017) Safety and benefit considerations for menopausal hormone therapy. Expert Opin Drug Saf 16(8):941–954
Toffol E, Heikinheimo O, Partonen T (2015) Hormone therapy and mood in perimenopausal and postmenopausal women: a narrative review. Menopause 22(5):564–578
Wang Y-S, Shen C-Y, Jiang J-G (2019) Antidepressant active ingredients from herbs and nutraceuticals used in TCM: pharmacological mechanisms and prospects for drug discovery. Pharmacol Res 150:104520
Li J-M, Zhao Y, Sun Y, Kong L-D (2020) Potential effect of herbal antidepressants on cognitive deficit: pharmacological activity and possible molecular mechanism. J Ethnopharmacol 257:112830
Zhou Q-G, Zhu X-H, Nemes AD, Zhu D-Y (2018) Neuronal nitric oxide synthase and affective disorders. IBRO Rep 5:116–132
Hu Y, Wu D-L, Luo C-X, Zhu L-J, Zhang J, Wu H-Y, Zhu D-Y (2012) Hippocampal nitric oxide contributes to sex difference in affective behaviors. Proc Natl Acad Sci U S A 109(35):14224–14229
Gingerich S, Krukoff TL (2005) Estrogen modulates endothelial and neuronal nitric oxide synthase expression via an estrogen receptor beta-dependent mechanism in hypothalamic slice cultures. Endocrinology 146(7):2933–2941
Cicinelli E, Ignarro LJ, Matteo MG, Galantino P, Schonauer LM, Falco N (1999) Effects of estrogen replacement therapy on plasma levels of nitric oxide in postmenopausal women. Am J Obstet Gynecol 180(2 Pt 1):334–339
Colucci-D’Amato L, Speranza L, Volpicelli F (2020) Neurotrophic factor BDNF, physiological functions and therapeutic potential in depression, neurodegeneration and brain cancer. Int J Mol Sci 21(20):7777
Samy DM, Mostafa DK, Saleh SR, Hassaan PS, Zeitoun TM, Ammar GAG, Elsokkary NH (2023) Carnosic acid mitigates depression-like behavior in ovariectomized mice via activation of Nrf2/HO-1 pathway. Mol Neurobiol 60(2):610–628
Kourosh-Arami M, Hosseini N, Mohsenzadegan M, Komaki A, Joghataei MT (2020) Neurophysiologic implications of neuronal nitric oxide synthase. Rev Neurosci 31(6):617–636
Li X, Wu T, Yu Z, Li T, Zhang J, Zhang Z, Cai M, Zhang W, Xiang J, Cai D (2018) Apocynum Venetum leaf extract reverses depressive-like behaviors in chronically stressed rats by inhibiting oxidative stress and apoptosis. Biomed Pharmacother 100:394–406
Chen X, Famurewa AC, Tang J, Olatunde OO, Olatunji OJ (2022) Hyperoside attenuates neuroinflammation, cognitive impairment and oxidative stress via suppressing TNF-α/NF-κB/caspase-3 signaling in type 2 diabetes rats. Nutr Neurosci 25(8):1774–1784
Huang J, Zhou L, Chen J, Chen T, Lei B, Zheng N, Wan X, Xu J, Wang T (2021) Hyperoside attenuate inflammation in HT22 cells via upregulating SIRT1 to activities Wnt/β-Catenin and sonic hedgehog pathways. Neural Plast 2021:8706400
Shi Y, Jiang M, Zhang Y, Diao Y, Li N, Liu W, Qiu Z, Qiu Y, Jia A (2023) Hyperoside nanomicelles alleviate atherosclerosis by modulating the lipid profile and intestinal flora structure in high-fat-diet-fed apolipoprotein-E-deficient mice. Molecules 28(13):5088
Zhu X, Ji M, Han Y, Guo Y, Zhu W, Gao F, Yang X, Zhang C (2017) PGRMC1-dependent autophagy by hyperoside induces apoptosis and sensitizes ovarian cancer cells to cisplatin treatment. Int J Oncol 50(3):835–846
Chen L, Zhou Y-P, Liu H-Y, Gu J-H, Zhou X-F, Yue-Qin Z (2021) Long-term oral administration of hyperoside ameliorates AD-related neuropathology and improves cognitive impairment in APP/PS1 transgenic mice. Neurochem Int 151:105196
Fan H, Li Y, Sun M, Xiao W, Song L, Wang Q, Zhang B, Yu J, Jin X, Ma C, Chai Z (2021) Hyperoside reduces rotenone-induced neuronal injury by suppressing autophagy. Neurochem Res 46(12):3149–3158
Zheng M, Liu C, Pan F, Shi D, Zhang Y (2012) Antidepressant-like effect of hyperoside isolated from Apocynum Venetum leaves: possible cellular mechanisms. Phytomedicine 19(2):145–149
Orzelska-Górka J, Szewczyk K, Gawrońska-Grzywacz M, Kędzierska E, Głowacka E, Herbet M, Dudka J, Biała G (2019) Monoaminergic system is implicated in the antidepressant-like effect of hyperoside and protocatechuic acid isolated from Impatiens glandulifera Royle in mice. Neurochem Int 128:206–214
Luo L, Sun Q, Mao YY, Lu YH, Tan RX (2004) Inhibitory effects of flavonoids from Hypericum perforatum on nitric oxide synthase. J Ethnopharmacol 93(2–3):221–225
Xu X-J, Pan T, Fan H-J, Wang X, Yu J-Z, Zhang H-F, Xiao B-G, Li Z-Y, Zhang B, Ma C-G, Chai Z (2023) Neuroprotective effect of hyperoside in MPP(+)/MPTP-induced dopaminergic neurodegeneration. Metab Brain Dis 38(3):1035–1050
Lim DW, Kim M, Yoon M, Lee J, Lee C, Um MY (2021) 1,3-Dicaffeoylquinic acid as an active compound of Arctium lappa root extract ameliorates depressive-like behavior by regulating hippocampal nitric oxide synthesis in ovariectomized mice. Antioxid (Basel) 10(8):1281
Fan Q, He R, Li Y, Gao P, Huang R, Li R, Zhang J, Li H, Liang X (2023) Studying the effect of hyperoside on recovery from cyclophosphamide induced oligoasthenozoospermia. Syst Biol Reprod Med 69(5):333–346
Lim DW, Park J, Han D, Lee J, Kim YT, Lee C (2020) Anti-inflammatory effects of Asian fawn lily (Erythronium japonicum) extract on lipopolysaccharide-induced depressive-like behavior in mice. Nutrients 12(12):3809
Gong Y, Yang Y, Chen X, Yang M, Huang D, Yang R, Zhou L, Li C, Xiong Q, Xiong Z (2017) Hyperoside protects against chronic mild stress-induced learning and memory deficits. Biomed Pharmacother 91:831–840
Song A, Wu Z, Zhao W, Shi W, Cheng R, Jiang J, Ni Z, Qu H, Qiaolongbatu X, Fan G, Lou Y (2022) The role and mechanism of hyperoside against depression-like behavior in mice via the NLRP1 inflammasome. Med (Kaunas) 58(12):1749
Haas JS, Stolz ED, Betti AH, Stein AC, Schripsema J, von Poser GL, Rates SMK (2011) The anti-immobility effect of hyperoside on the forced swimming test in rats is mediated by the D2-like receptors activation. Planta Med 77(4):334–339
Dhir A, Kulkarni SK (2011) Nitric oxide and major depression. Nitric Oxide 24(3):125–131
Ignácio ZM, Réus GZ, Abelaira HM, de Moura AB, de Souza TG, Matos D, Goldim MP, Mathias K, Garbossa L, Petronilho F, Quevedo J (2017) Acute and chronic treatment with quetiapine induces antidepressant-like behavior and exerts antioxidant effects in the rat brain. Metab Brain Dis 32(4):1195–1208
Kaster MP, Ferreira PK, Santos ARS, Rodrigues ALS (2005) Effects of potassium channel inhibitors in the forced swimming test: possible involvement of L-arginine-nitric oxide-soluble guanylate cyclase pathway. Behav Brain Res 165(2):204–209
Karolewicz B, Szebeni K, Stockmeier CA, Konick L, Overholser JC, Jurjus G, Roth BL, Ordway GA (2004) Low nNOS protein in the locus coeruleus in major depression. J Neurochem 91(5):1057–1066
Hilal Balci, Altunyurt S, Acar B, Fadiloglu M, Kirkali G, Onvural B (2005) Effects of transdermal estrogen replacement therapy on plasma levels of nitric oxide and plasma lipids in postmenopausal women. Maturitas 50(4):289–293
Liu R-L, Xiong Q-J, Shu Q, Wu W-N, Cheng J, Fu H, Wang F, Chen J-G, Hu Z-L (2012) Hyperoside protects cortical neurons from oxygen-glucose deprivation-reperfusion induced injury via nitric oxide signal pathway. Brain Res 1469:164–173
Kim M, Kwon S, Cho S, Um MY (2022) Ishige foliacea ameliorates depressive-like behaviors in stress hormone treated mice. Appl Biol Chem 65:86
Acknowledgements
Not applicable.
Funding
This research was supported by the Main Research Program of the Korea Food Research Institute (KFRI) funded by the Ministry of Science and ICT (E0210201-03 and ER160700).
Author information
Authors and Affiliations
Contributions
Conceptualization, MYU; formal analysis, KHH; investigation, JJ and MK; writing—original draft preparation: KHH; writing—review and editing: KHH and MYU; funding acquisition: MYU. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hong, K.H., Jung, J., Kim, M. et al. Hyperoside ameliorates depression-like behavior in ovariectomized mice. Appl Biol Chem 67, 41 (2024). https://doi.org/10.1186/s13765-024-00897-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-024-00897-4